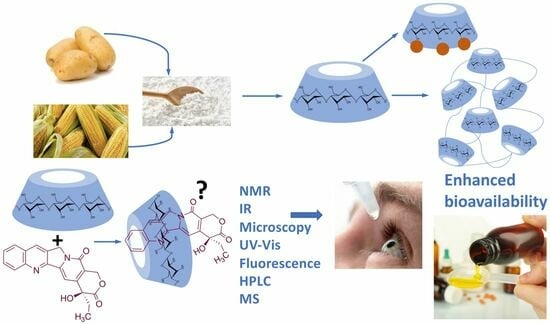
Cyclodextrin Inclusion Complexes for Improved Drug Bioavailability and Activity: Synthetic and Analytical Aspects
Abstract
:1. Introduction
2. Cyclodextrins for Increased Bioavailability
3. Cyclodextrins for Improved Drug Solubility
4. Cyclodextrins for Improved Drug Stability
5. Cyclodextrins for Solving Formulation Problems
6. Cyclodextrins for Targeted Drug Delivery
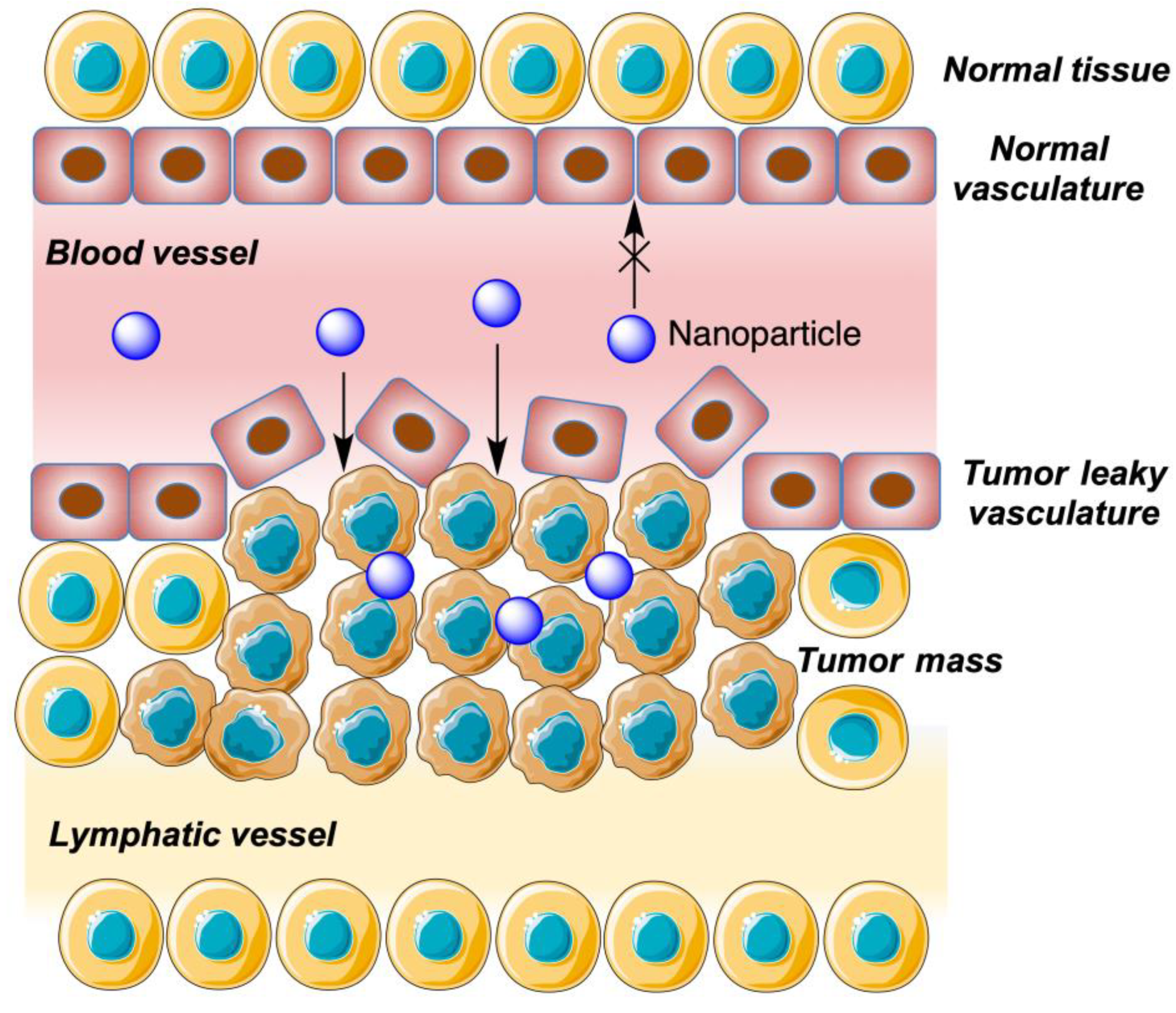
6.1. Introduction: Cyclodextrin-Derived Nanoparticles and the EPR Effect
6.2. Cyclodextrin-Based Nanoparticles for Combined Photothermal/Chemotherapy
7. Cyclodextrins for Increasing the Bioavailability of Diet Components
8. Cyclodextrin Nanofibers
9. Functionalization of Cyclodextrins: Synthetic Aspects
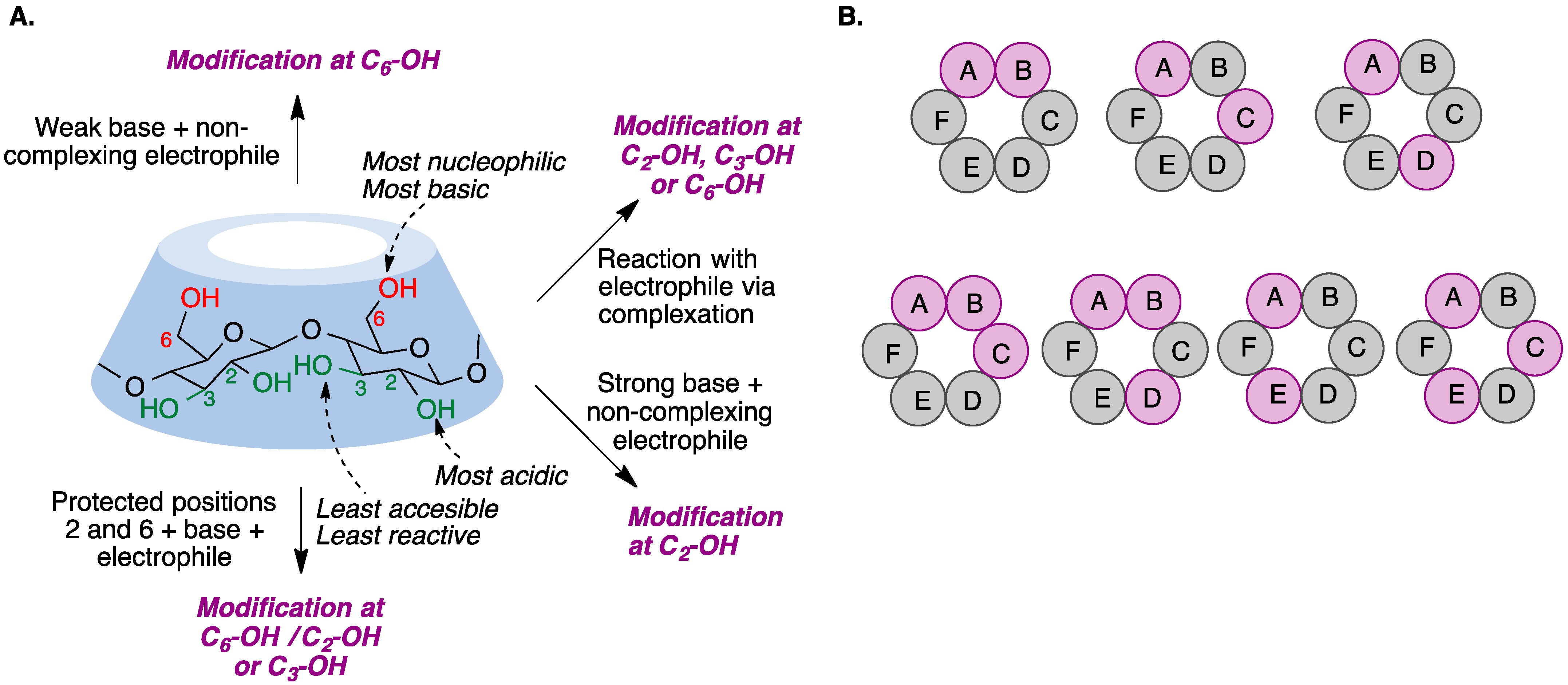
9.1. Introduction
- (a)
- Straightforward selective modification of the desired positions. This is chemically challenging due to the competition between the hydroxyl groups at positions 2, 3, and 6 of the glucopyranose.
- (b)
- Protection–deprotection strategies which involve taking advantage of the different reactivity of the hydroxyl groups toward protection and deprotection conditions. Deprotected hydroxyl groups can then undergo the desired modifications.
- (c)
- Non-selective modification of hydroxyl groups to provide a mixture of derivatives that is then purified to isolate the desired one. This method generates a large number of undesired by-products, requires tedious purifications, and is not chemically efficient.
9.2. Selective Modification of the Primary Rim
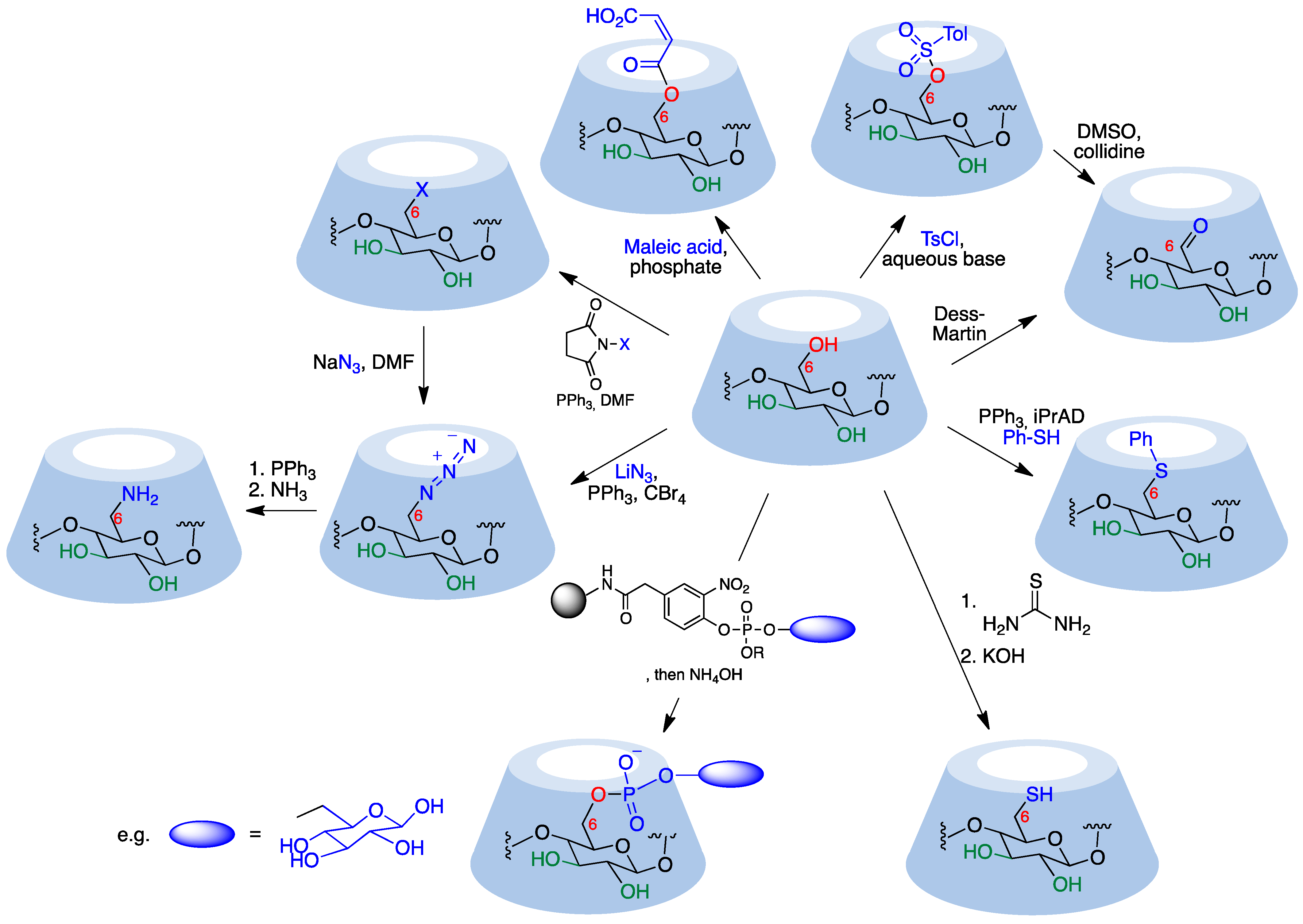
9.2.1. Monosubstitution at C6-OH
9.2.2. Disubstitution at C6-OH
9.2.3. Other C6-OH Substitution Patterns
9.3. Selective Modification of the Secondary Rim
9.3.1. Monosubstitution at C2-OH
9.3.2. Disubstitution at C2-OH
9.3.3. Persubstitution at C2-OH
9.3.4. Monosubstitution at C3-OH
9.3.5. Di- and Trisubstitution at C3-OH
9.3.6. Persubstitution at C3-OH
9.4. Persubstitution of the Primary and Secondary Rims
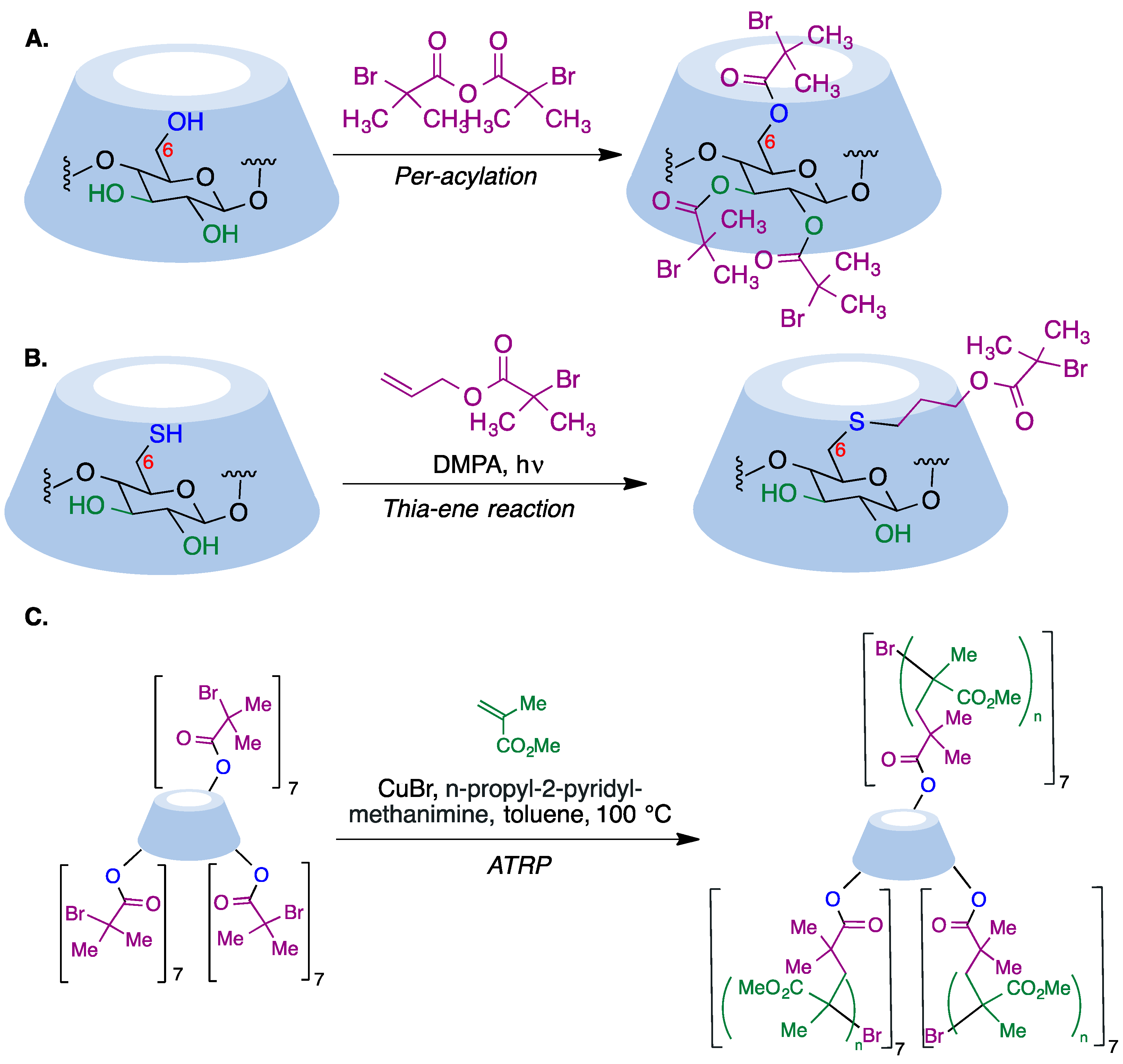
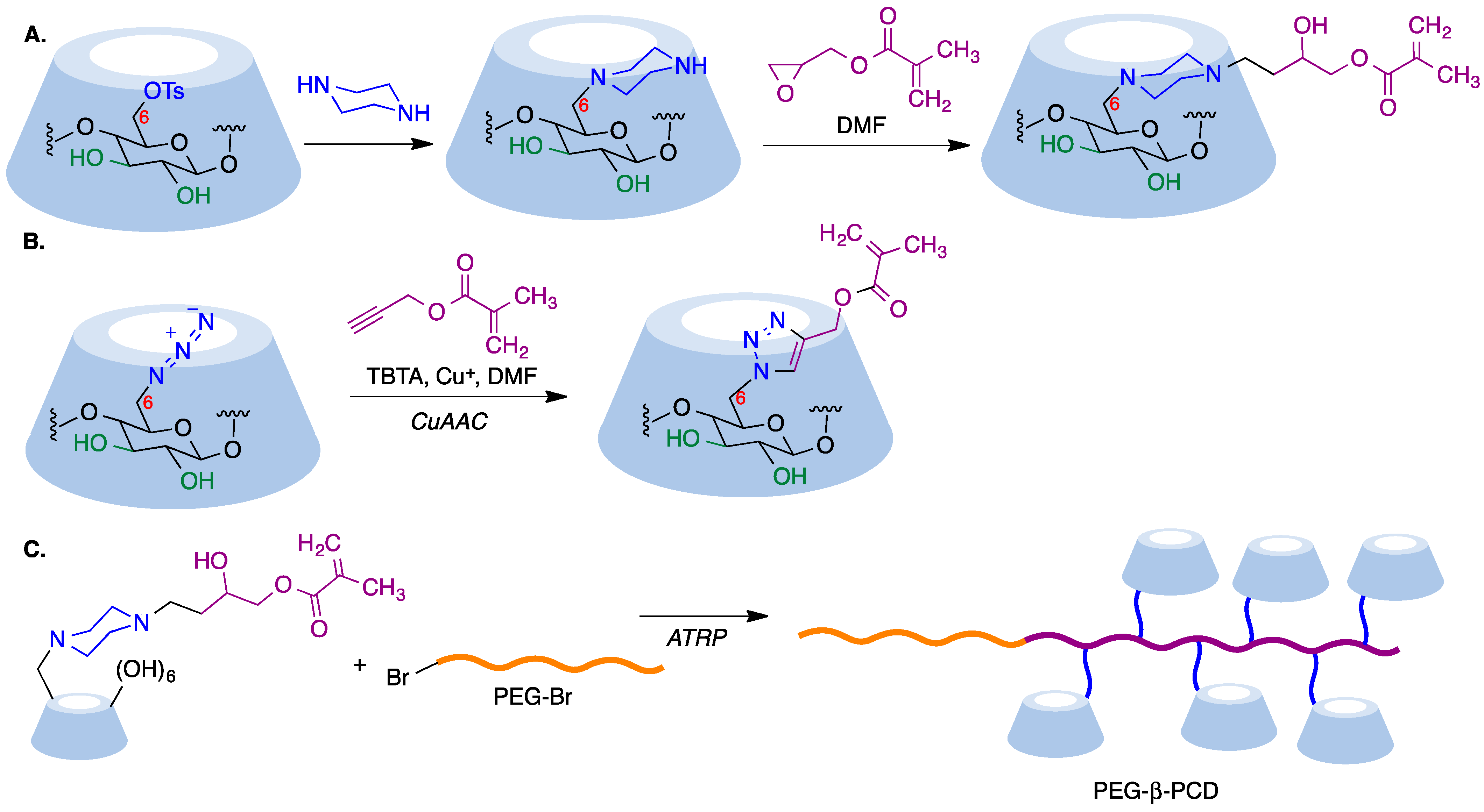
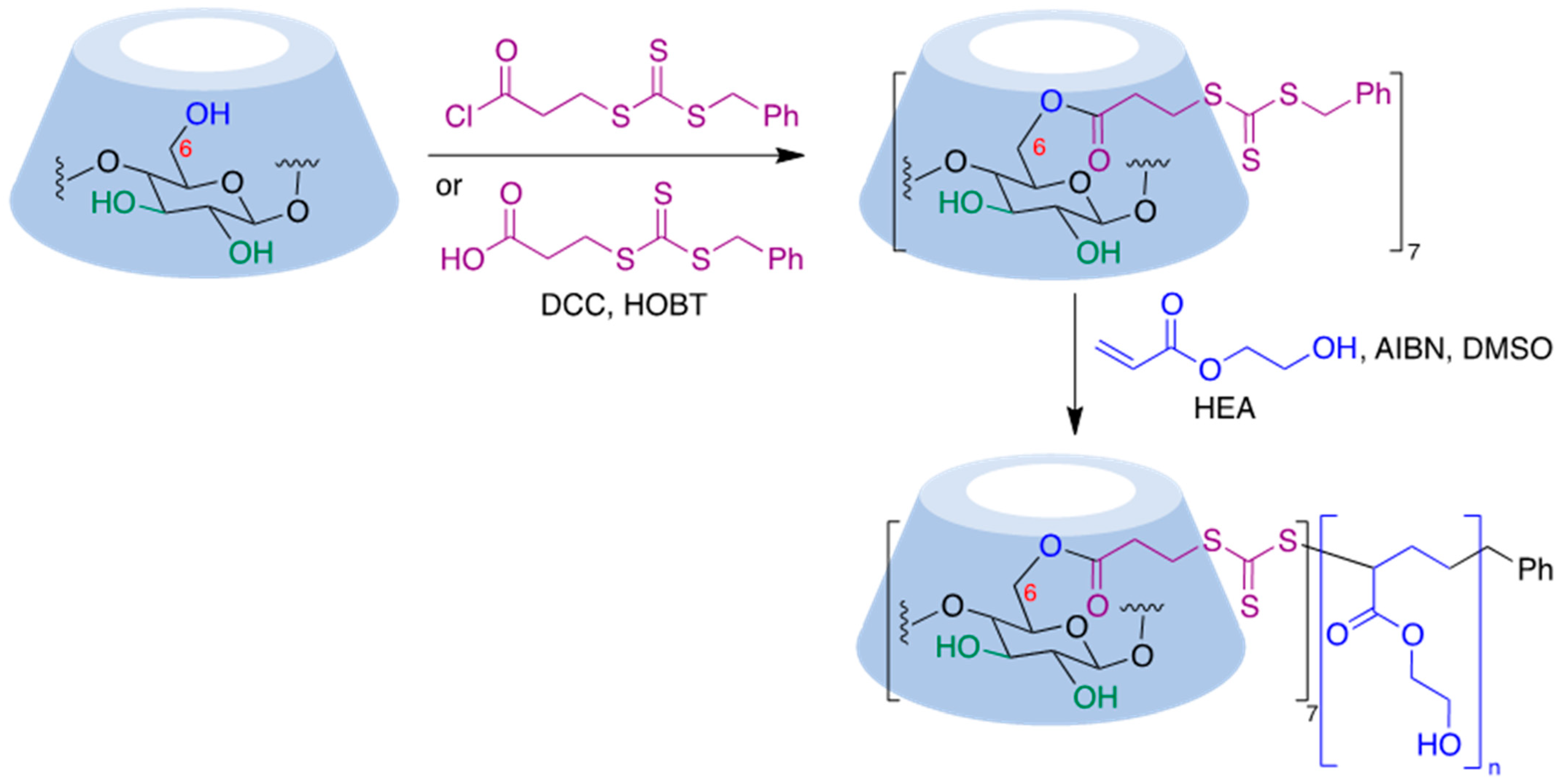
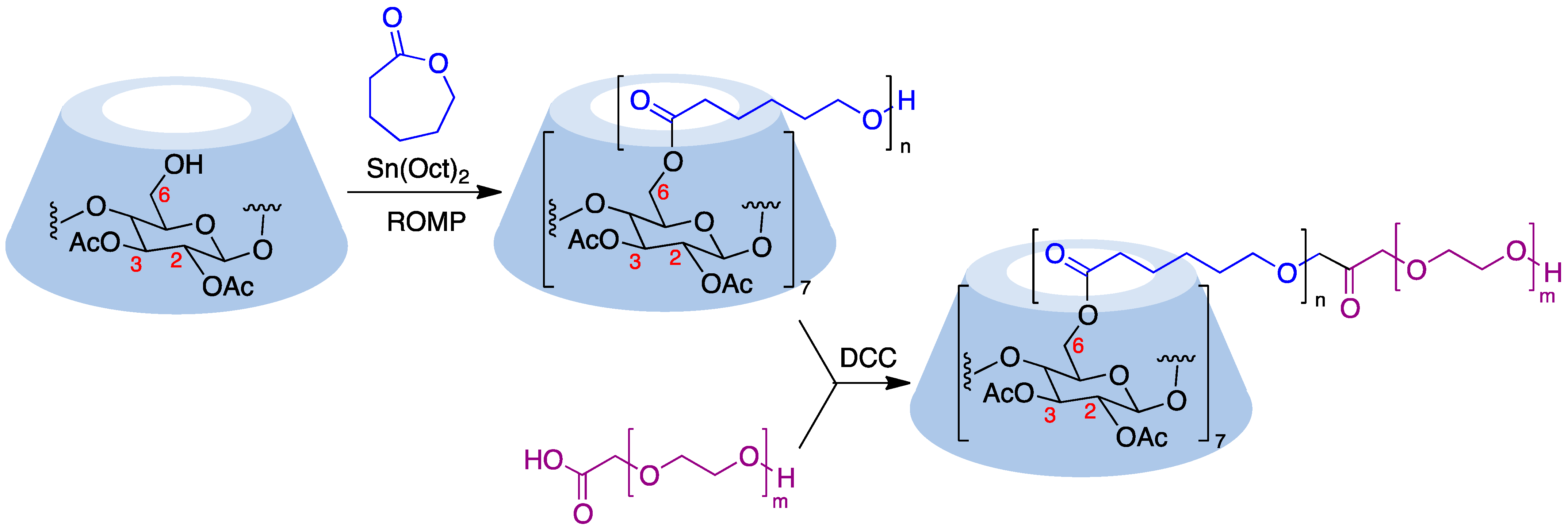
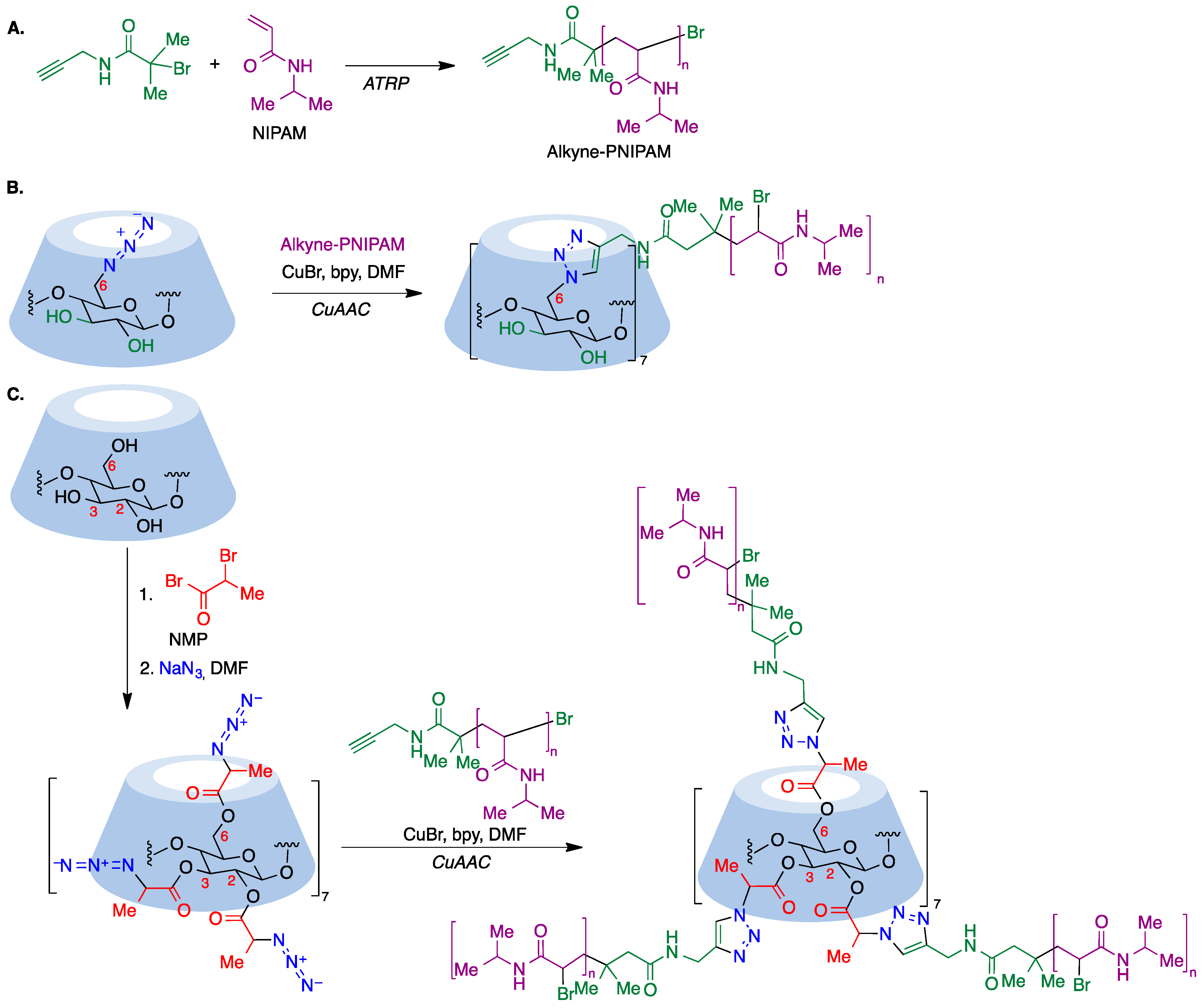
9.5. Cyclodextrin-Based Polymers
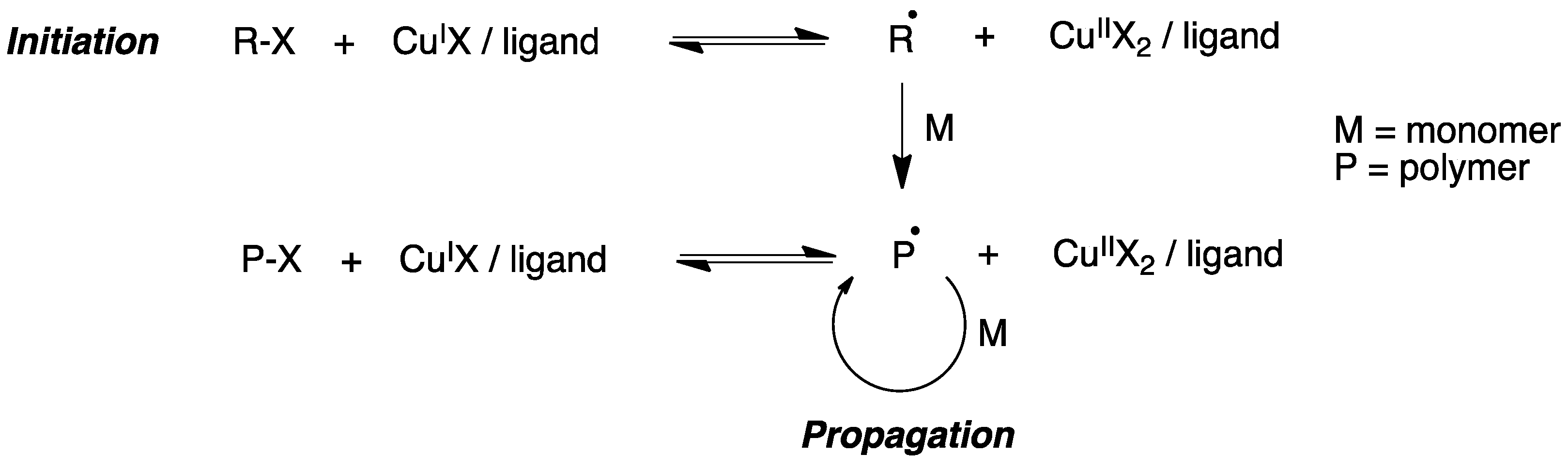
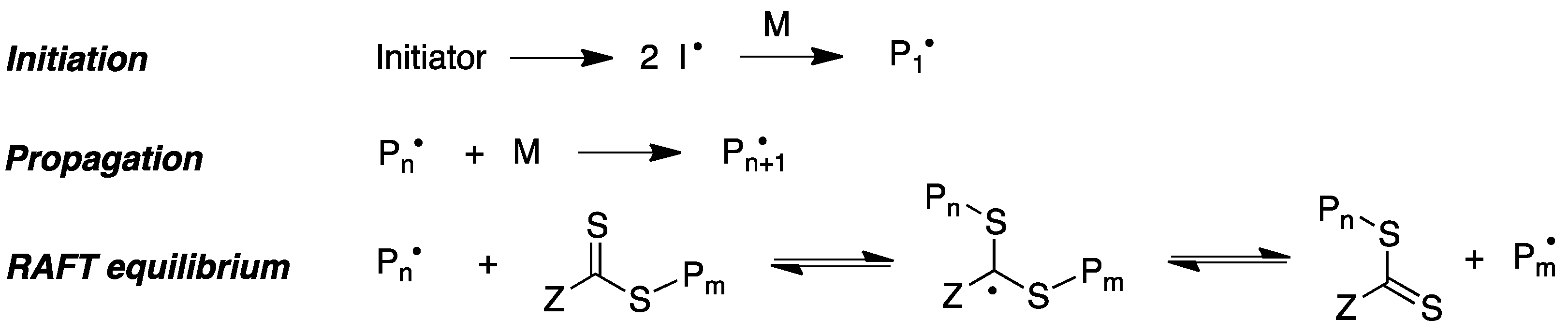
9.5.1. Radical Polymerization Reactions
9.5.2. Ring-Opening Polymerization (ROP)
9.5.3. CuAAC-Based Polymerization
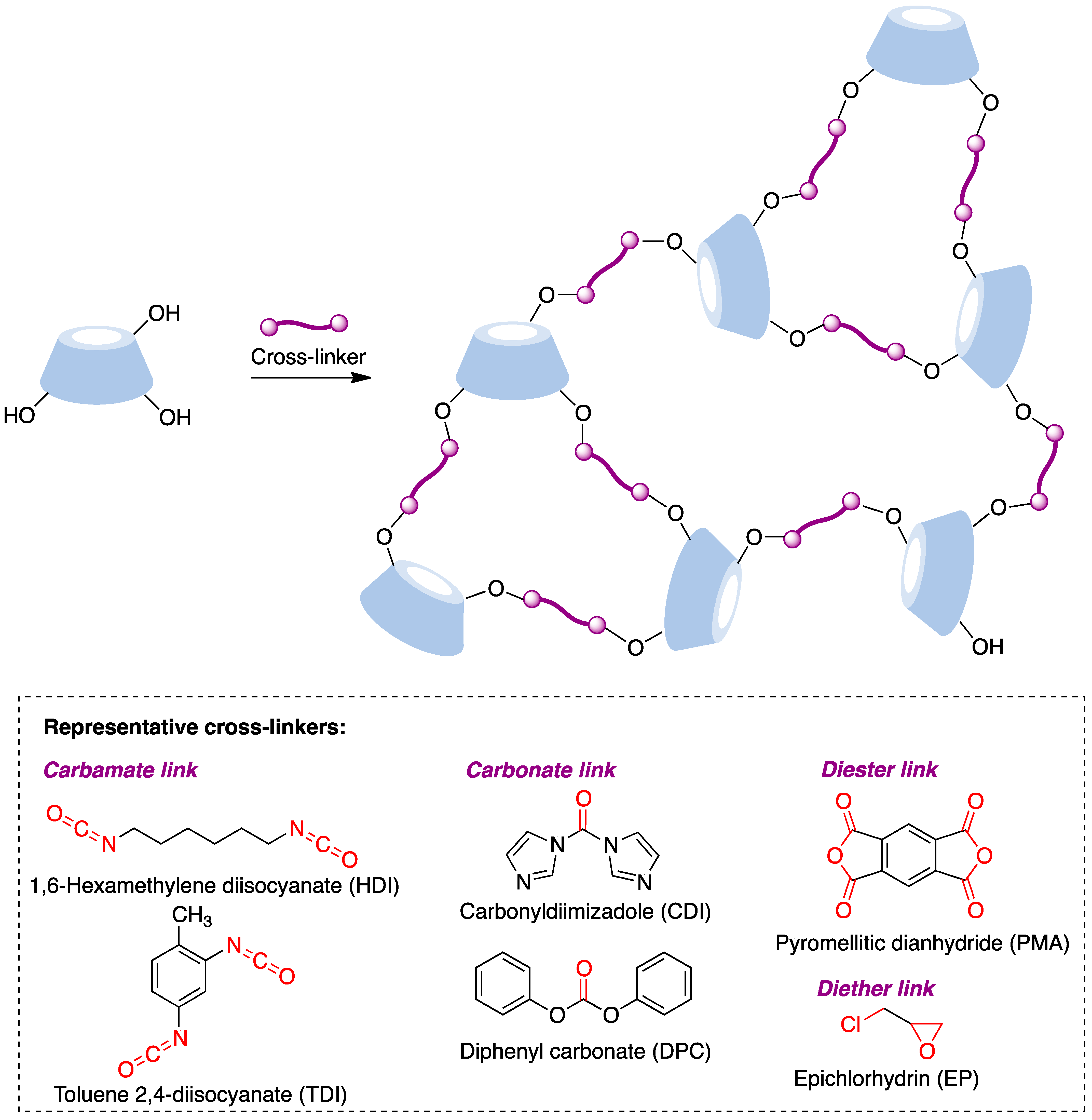
9.6. Cyclodextrin-Embedded Covalently Crosslinked Networks
10. Analytical Aspects
10.1. Introduction
10.2. Analytical Techniques for Studying Inclusion Complexes in the Solid State
10.2.1. X-ray Diffraction
10.2.2. Thermal Analytical Techniques
10.2.3. Electron Microscopy
10.2.4. Spectroscopic Techniques
10.3. Analytical Techniques for Studying Inclusion Complexes in Solution
10.3.1. UV-Vis Absorption Spectroscopy
10.3.2. Fluorescence Spectroscopy
10.3.3. Nuclear Magnetic Resonance Spectrometry (NMR)
10.3.4. Separation Techniques
10.3.5. Other Techniques
10.4. Analytical Techniques for Determining Drug Loading and Encapsulation Efficiency
11. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Mazurek, A.H.; Szeleszczuk, Ł. Current status of quantum chemical studies of cyclodextrin host–guest complexes. Molecules 2022, 27, 3874. [Google Scholar] [CrossRef] [PubMed]
- Kfoury, M.; Landy, D.; Fourmentin, S. Characterization of cyclodextrin/volatile inclusion complexes: A review. Molecules 2018, 23, 1204. [Google Scholar] [CrossRef] [PubMed]
- Loftsson, T.; Sigurdsson, H.H.; Jansook, P. Anomalous properties of cyclodextrins and their complexes in aqueous solutions. Materials 2023, 16, 2223. [Google Scholar] [CrossRef]
- Duchêne, D. (Ed.) New Trends in Cyclodextrins and Derivatives; Editions de Santé: Paris, France, 1991. [Google Scholar]
- Duchêne, D.; Bochot, A. Thirty years with cyclodextrins. Int. J. Pharmaceut. 2016, 514, 58–72. [Google Scholar] [CrossRef] [PubMed]
- Szejtli, J.; Osa, T. Cyclodextrins. In Comprehensive Supramolecular Chemistry; Atwood, J.L., Davies, J.E.D., MacNicol, D.D., Vögtle, F., Eds.; Pergamon: Exeter, UK, 1996; Volume 3. [Google Scholar]
- Szejtli, J. Introduction and general overview of cyclodextrin chemistry. Chem. Rev. 1998, 98, 1743–1753. [Google Scholar] [CrossRef] [PubMed]
- Fourmentin, S.; Crini, G.; Lichtfouse, E. (Eds.) Cyclodextrin Fundamentals, Reactivity and Analysis; Springer: Berlin/Heidelberg, Germany, 2018. [Google Scholar]
- Kurkov, S.V.; Loftsson, T. Cyclodextrins. Int. J. Pharmaceut. 2013, 453, 167–180. [Google Scholar] [CrossRef] [PubMed]
- Santos-Braga, S. Cyclodextrin superstructures for drug delivery. J. Drug. Deliv. Sci. Technol. 2022, 75, 103650. [Google Scholar] [CrossRef]
- Kawano, S.; Kida, T.; Miyawaki, K.; Noguchi, Y.; Kato, E.; Nakano, T.; Akashi, M. Cyclodextrin polymers as highly effective adsorbents for removal and recovery of polychlorobiphenyl (PCB) contaminants in insulating oil. Environ. Sci. Technol. 2014, 48, 8094–8100. [Google Scholar] [CrossRef]
- Zhou, Y.; Cheng, G.; Chen, K.; Lu, J.; Lei, J.; Pu, S. Adsorptive removal of bisphenol A, chloroxylenol, and carbamazepine from water using a novel β-cyclodextrin polymer. Ecotoxicol. Environ. Saf. 2019, 170, 278–285. [Google Scholar] [CrossRef]
- Muñoz-Botella, S.; Martín, M.A.; del Castillo, B.; Lerner, D.A.; Menéndez, J.C. Differentiating geometrical isomers of retinoids and controlling their photo-isomerization by complexation with cyclodextrins. Anal. Chim. Acta 2002, 468, 161–170. [Google Scholar] [CrossRef]
- Martín, L.; León, A.; Olives, A.I.; del Castillo, B.; Martín, M.A. Spectrofluorimetric determination of stoichiometry and association constants of the complexes of harmane and harmine with β-cyclodextrin and chemically modified β-cyclodextrins. Talanta 2003, 60, 493–503. [Google Scholar] [CrossRef] [PubMed]
- González-Ruiz, V.; León, A.G.; Olives, A.I.; Martín, M.A.; Menéndez, J.C. Eco-friendly liquid chromatographic separations based on the use of cyclodextrins as mobile phase additives. Green Chem. 2011, 13, 115–126. [Google Scholar] [CrossRef]
- González-Ruiz, V.; León, A.G.; Olives, A.I.; Martín, M.A. A down-scaled fluorimetric determination of the solubility properties of drugs to minimize waste generation. Green Chem. 2013, 15, 2558–2565. [Google Scholar] [CrossRef]
- Hoti, G.; Appleton, S.L.; Pedrazzo, A.R.; Cecone, C.; Matencio, A.; Trotta, F.; Caldera, F. Strategies to develop cyclodextrin-based nanosponges for smart drug delivery. In Smart Drug Delivery; Ahmad, U., Haider, M.F., Akhtar, J., Eds.; Intech Open: London, UK, 2021; pp. 1–22. [Google Scholar]
- Danish, K.A.; Lubhan, S. Various techniques of bioavailability enhancement: A review. J. Drug Deliv. Ther. 2016, 6, 34–41. [Google Scholar]
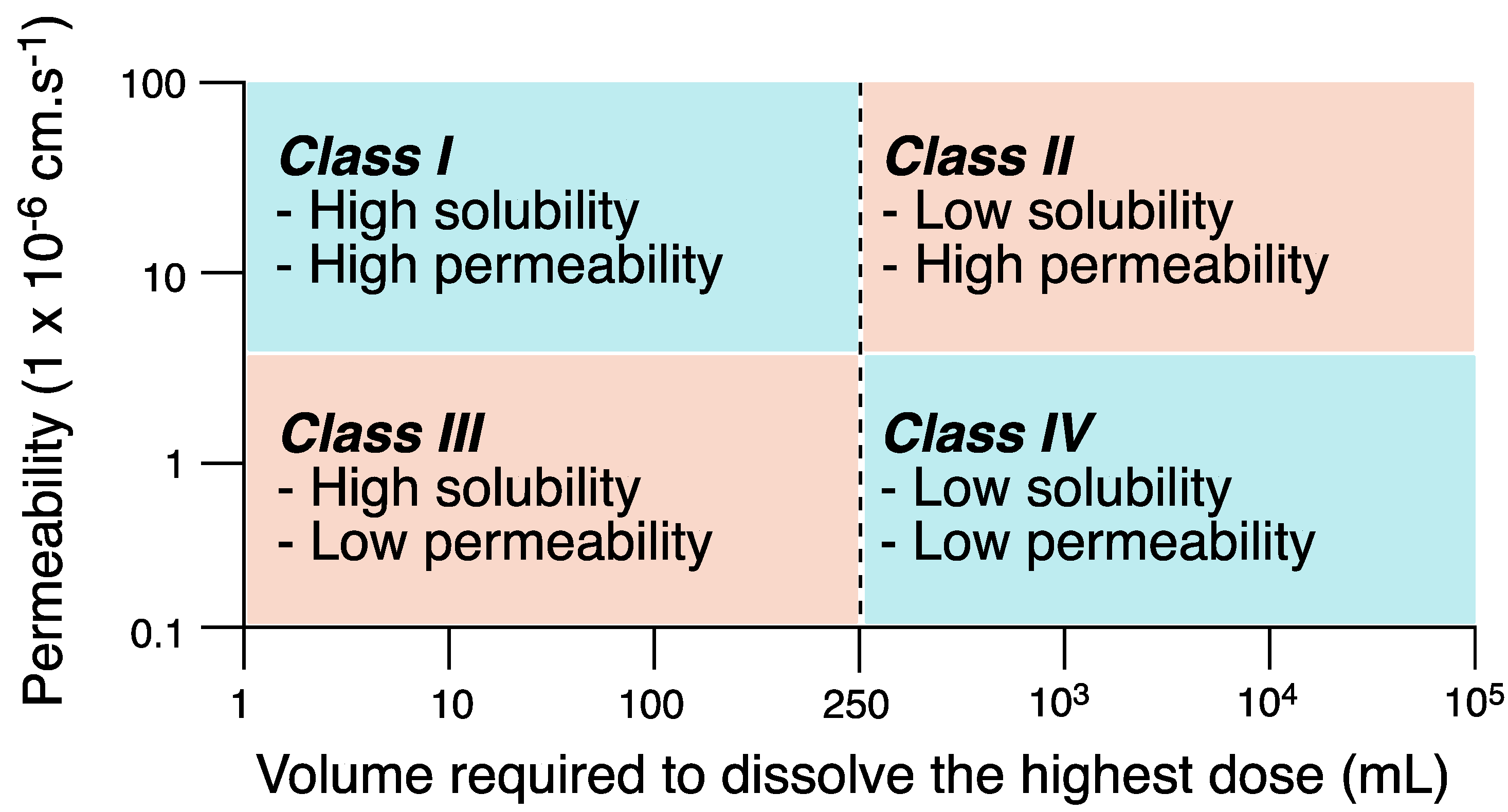
- Chavda, H.V.; Patel, C.N.; Anand, I.S. Biopharmaceutics classification system. Sys. Rev. Pharm. 2010, 1, 62–69. [Google Scholar] [CrossRef]
- Yasir, M.; Asif, M.; Kumar, A.; Aggarval, A. Biopharmaceutical classification system: An account. Int. J. PharmaTech. Res. 2010, 2, 1681–1690. [Google Scholar]
- Cook, J.; Addicks, W.; Wu, Y.H. Application of the biopharmaceutical classification system in clinical drug development—An industrial view. AAPS J. 2008, 10, 306–310. [Google Scholar] [CrossRef] [PubMed]
- Ku, M.S. Use of the biopharmaceutical classification system in early drug development. AAPS J. 2008, 10, 208–212. [Google Scholar] [CrossRef]
- Vyas, A.; Saraf, S.; Saraf, S. Cyclodextrin based novel drug delivery systems. J. Incl. Phenom. Macrocycl. Chem. 2008, 62, 23–42. [Google Scholar] [CrossRef]
- Arun, R.; Ashok-Kumar, C.K.; Sravanthi, V.V.N.S.S. Cyclodextrins as drug carrier molecules: A review. Sci. Pharm. 2008, 76, 567–598. [Google Scholar] [CrossRef]
- Vikas, Y.; Sandeep, K.; Braham, D.; Manjusha, C.; Budhwar, V. Cyclodextrin Complexes: An approach to improve the physicochemical properties of drugs and applications of cyclodextrin complexes. Asian J. Pharm. 2018, 12, S394–S409. [Google Scholar]
- Kim, D.-H.; Lee, S.-E.; Pyo, Y.-C.; Tran, P.; Park, J.-S. Solubility enhancement and application of cyclodextrins in local drug delivery. J. Pharm. Inv. 2020, 50, 17–27. [Google Scholar] [CrossRef]
- Topuz, F.; Uyar, T. Advances in the development of cyclodextrin-based nanogels/microgels for biomedical applications: Drug delivery and beyond. Carbohydr. Polym. 2022, 297, 120033. [Google Scholar] [CrossRef] [PubMed]
- Jansook, P.; Ogawa, N.; Loftsson, T. Cyclodextrins: Structure, physicochemical properties and pharmaceutical Applications. Int. J. Pharmaceut. 2018, 535, 272–284. [Google Scholar] [CrossRef]
- Morina, D.; Sessevmez, M.; Sinani, G.; Mülazımoğlu, L.; Cevher, E. Oral tablet formulations containing cyclodextrin complexes of poorly water soluble cefdinir to enhance its bioavailability. J. Drug Deliv. Sci. Technol. 2020, 57, 101742. [Google Scholar] [CrossRef]
- Amruta, T.; Nancy, P.; Prashant, K.; Niteshkumar, S. Encapsulation of boswellic acid with β- and hydroxypropyl-β-cyclodextrin: Synthesis, characterization, in vitro drug release and molecular modelling studies. J. Mol. Struct. 2018, 1154, 504–510. [Google Scholar]
- Liao, Y.; Zhang, X.; Li, C.; Huang, Y.; Lei, M.; Yan, M.M.; Zhou, Y.; Zhao, C. Inclusion complexes of HP-β-cyclodextrin with agomelatine: Preparation, characterization, mechanism study and in vivo evaluation. Carbohydr. Polym. 2016, 147, 415–425. [Google Scholar] [CrossRef]
- Gabra, M.M.; Mortada, S.M.; Sallam, M.A. Carboxylate cross-linked cyclodextrin: A nanoporous scaffold for enhancement of rosuvastatin oral bioavailability. Eur. J. Pharm. Sci. 2018, 111, 1–12. [Google Scholar] [CrossRef]
- Lahiani-Skib, M.; Hallouard, F.; Bounoure, F.; Milon, N.; Karrout, Y.; Skiba, M. Enhanced dissolution and oral bioavailability of cyclosporine A: Microspheres based on αβ-cyclodextrins polymers. Pharmaceutics 2018, 10, 285. [Google Scholar] [CrossRef]
- Real, D.A.; Bolaños, K.; Priotti, J.; Yutronic, N.; Kogan, M.J.; Sierpe, R.; Donoso-González, O. Cyclodextrin-modified nanomaterials for drug delivery: Classification and advances in controlled release and bioavailability. Pharmaceutics 2021, 13, 2131. [Google Scholar] [CrossRef]
- Saokham, P.; Muankaew, C.; Jansook, P.; Loftsson, T. Solubility of cyclodextrins and drug/cyclodextrin complexes. Molecules 2018, 23, 1161. [Google Scholar] [CrossRef] [PubMed]
- Fan, G.; Yu, Z.; Tang, J.; Dai, R.; Xu, Z. Preparation of gallic acid-hydroxypropyl-β-cyclodextrin inclusion compound and study on its effect mechanism on Escherichia coli in vitro. Mater. Express 2021, 11, 655–662. [Google Scholar] [CrossRef]
- Mady, F.M.; Mohamed Ibrahim, S.R. Cyclodextrin-based nanosponge for improvement of solubility and oral bioavailability of ellagic acid. Pak. J. Pharm. Sci. 2018, 31, 2069–2076. [Google Scholar] [PubMed]
- Maw, P.D.; Jansook, P. Cyclodextrin-based Pickering nanoemulsions containing amphotericin B: Part I. Evaluation of oil/cyclodextrin and amphotericin B/cyclodextrin inclusion complexes. J. Drug Deliv. Sci. Technol. 2022, 68, 103118. [Google Scholar] [CrossRef]
- Manne, A.S.N.; Hegde, A.R.; Raut, S.Y.; Rao, R.R.; Kulkarni, V.I.; Mutalik, S. Hot liquid extrusion assisted drug-cyclodextrin complexation: A novel continuous manufacturing method for solubility and bioavailability enhancement of drugs. Drug Deliv. Transl. Res. 2021, 11, 1273–1287. [Google Scholar] [CrossRef]
- Gidwani, B.; Vyas, A. A comprehensive review on cyclodextrin-based carriers for delivery of chemotherapeutic cytotoxic anticancer drugs. Biomed. Res. Int. 2015, 2015, 198268. [Google Scholar] [CrossRef]
- Yang, J.; Jia, L.; He, Z.; Wang, Y. Recent advances in SN-38 drug delivery system. Int. J. Pharmaceut. 2023, 637, 122886. [Google Scholar] [CrossRef]
- Qiu, N.; Li, X.; Liu, J. Application of cyclodextrins in cancer treatment. J. Incl. Phenom. Macrocycl. Chem. 2017, 89, 229–246. [Google Scholar] [CrossRef]
- Wang, X.; Guo, Z. Targeting and delivery of platinum-based anticancer drugs. Chem. Soc. Rev. 2013, 42, 202–224. [Google Scholar] [CrossRef]
- Yee, E.M.H.; Hook, J.M.; Bhadbhade, M.M.; Vittorio, O.; Kuchel, R.P.; Brandl, M.B.; Tilley, R.D.; Black, D.S.C.; Kumar, N. Preparation, characterization and in vitro biological evaluation of (1:2)phenoxodiol-β-cyclodextrin complex. Carbohydr. Polym. 2017, 165, 444–454. [Google Scholar] [CrossRef]
- Popielec, A.; Loftsson, T. Effects of cyclodextrins on the chemical stability of drugs. Int. J. Pharmaceut. 2017, 531, 532–542. [Google Scholar] [CrossRef] [PubMed]
- Popielec, A.; Agnes, M.; Yannakopoulou, K.; Fenyvesi, É.; Loftsson, T. Effect of β- and γ-cyclodextrins and their methylated derivatives on the degradation rate of benzylpenicillin. J. Incl. Phenom. Macrocycl. Chem. 2018, 91, 199–209. [Google Scholar] [CrossRef]
- Maw, P.D.; Pienpinijtham, P.; Pruksakorn, P.; Jansook, P. Cyclodextrin-based Pickering nanoemulsions containing amphotericin B: Part II. Formulation, antifungal activity, and chemical stability. J. Drug Deliv. Sci. Technol. 2022, 69, 103174. [Google Scholar] [CrossRef]
- Diniz, T.C.; Pinto, T.C.C.; Menezes, P.D.P.; Silva, J.C.; Teles, R.B.D.A.; Ximenes, R.C.C.; Guimarães, A.G.; Serafini, M.R.; Araújo, A.A.S.; Quintans, L.J.; et al. Cyclodextrins improving the physicochemical and pharmacological properties of antidepressant drugs: A patent review. Expert Opin. Ther. Pat. 2018, 28, 81–92. [Google Scholar] [CrossRef]
- González-Ruiz, V.; Cores, Á.; Martín-Cámara, O.; Orellana, K.; Cervera-Carrascón, V.; Michalska, P.; Olives, A.I.; León, R.; Martín, M.A.; Menéndez, J.C. Enhanced stability and bioactivity of natural anticancer topoisomerase I inhibitors through cyclodextrin complexation. Pharmaceutics 2021, 13, 1609. [Google Scholar] [CrossRef]
- Ünal, H.; Öztürk, N.; Bilensoy, E. Formulation development, stability and anticancer efficacy of core-shell cyclodextrin nanocapsules for oral chemotherapy with camptothecin. Beilstein J. Org. Chem. 2015, 11, 204–212. [Google Scholar] [CrossRef]
- Çirpanli, Y.; Bilensoy, E.; Doğan, A.L.; Çaliş, S. Comparative evaluation of polymeric and amphiphilic cyclodextrin nanoparticles for effective camptothecin delivery. Eur. J. Pharm. Biopharm. 2009, 73, 82–89. [Google Scholar] [CrossRef]
- Xiong, T.; Guo, T.; He, Y.; Cao, Z.; Xu, X.; Wu, W.; Wu, L.; Zhu, W.; Zhang, J. Lactone stabilized by crosslinked cyclodextrin metal-organic frameworks to improve local bioavailability of topotecan in lung cancer. Pharmaceutics 2022, 15, 142. [Google Scholar] [CrossRef]
- Einafshar, E.; Asl, A.H.; Nia, A.H.; Mohammadi, M.; Malekzadeh, A.; Ramezani, M. New cyclodextrin-based nanocarriers for drug delivery and phototherapy using an irinotecan metabolite. Carbohydr. Polym. 2018, 194, 103–110. [Google Scholar] [CrossRef]
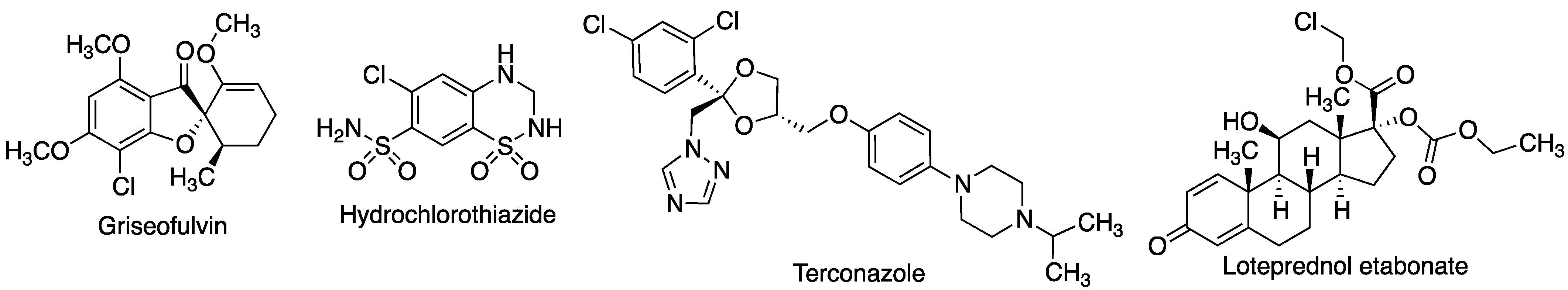
- Omar, S.M.; Ibrahim, F.; Ismail, A. Formulation and evaluation of cyclodextrin-based nanosponges of griseofulvin as pediatric oral liquid dosage form for enhancing bioavailability and masking bitter taste. Saudi Pharm. J. 2020, 28, 349–361. [Google Scholar] [CrossRef]
- Cirri, M.; Mennini, N.; Maestrelli, F.; Mura, P.; Ghelardini, C.; Mannelli, L.D.C. Development and in vivo evaluation of an innovative “hydrochlorothiazide-in cyclodextrins-in solid lipid nanoparticles” formulation with sustained release and enhanced oral bioavailability for potential hypertension treatment in pediatrics. Int. J. Pharmaceut. 2017, 521, 73–83. [Google Scholar] [CrossRef] [PubMed]
- Jansook, P.; Loftsson, T. Self-assembled γ-cyclodextrin as nanocarriers for enhanced ocular drug bioavailability. Int. J. Pharmaceut. 2022, 618, 121654. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Zhang, A.; Zhu, L.; Yang, X.; Fang, G.; Tang, B. Cyclodextrin-based ocular drug delivery systems: A comprehensive review. Coord. Chem. Rev. 2023, 476, 214919. [Google Scholar] [CrossRef]
- Zaghloul, N.; El Hoffy, N.M.; Mahmoud, A.A.; Elkasabgy, N.A. Cyclodextrin stabilized freeze-dried silica/chitosan nanoparticles for improved terconazole ocular bioavailability. Pharmaceutics 2022, 14, 470. [Google Scholar] [CrossRef] [PubMed]
- Soliman, O.A.E.A.; Mohamed, E.A.M.; El-Dahan, M.S.; Khatera, N.A.A. Potential use of cyclodextrin complexes for enhanced stability, anti-inflammatory efficacy, and ocular bioavailability of loteprednol etabonate. AAPS PharmSciTech 2017, 18, 1228–1241. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Yu, P.; Sollogoub, M.; Zhang, Y. Functionalized cyclodextrins and their applications in biodelivery. In Handbook of Macrocyclic Supramolecular Assembly; Liu, Y., Chen, Y., Zhang, H.Y., Eds.; Springer: Singapore, 2019. [Google Scholar]
- Rizzo, L.Y.; Theek, B.; Storm, G.; Kiessling, F.; Lammers, T. Recent progress in nanomedicine: Therapeutic, diagnostic and theranostic applications. Curr. Opin. Biotechnol. 2013, 24, 1159–1166. [Google Scholar] [CrossRef]
- Agüeros, M.; Zabaleta, V.; Espuelas, S.; Campanero, M.A.; Irache, J.M. Increased oral bioavailability of paclitaxel by its encapsulation through complex formation with cyclodextrins in poly(anhydride) nanoparticles. J. Control. Release 2010, 145, 2–8. [Google Scholar]
- Zhang, Y.; Yang, D.; Chen, H.; Lim, W.Q.; Phua, F.S.Z.; An, G.; Yang, P.; Zhao, Y. Reduction-sensitive fluorescence enhanced polymeric prodrug nanoparticles for combinational photothermal-chemotherapy. Biomaterials 2018, 163, 14–24. [Google Scholar] [CrossRef]
- Wüpper, S.; Lüersen, K.; Rimbach, G. Cyclodextrins, natural compounds, and plant bioactives—A nutritional perspective. Biomolecules 2021, 11, 401. [Google Scholar]
- Uekaji, Y.; Terao, K. Bioavailability enhancement of hydrophobic nutraceuticals using γ-cyclodextrin. J. Incl. Phenom. Macrocycl. Chem. 2019, 93, 3–15. [Google Scholar] [CrossRef]
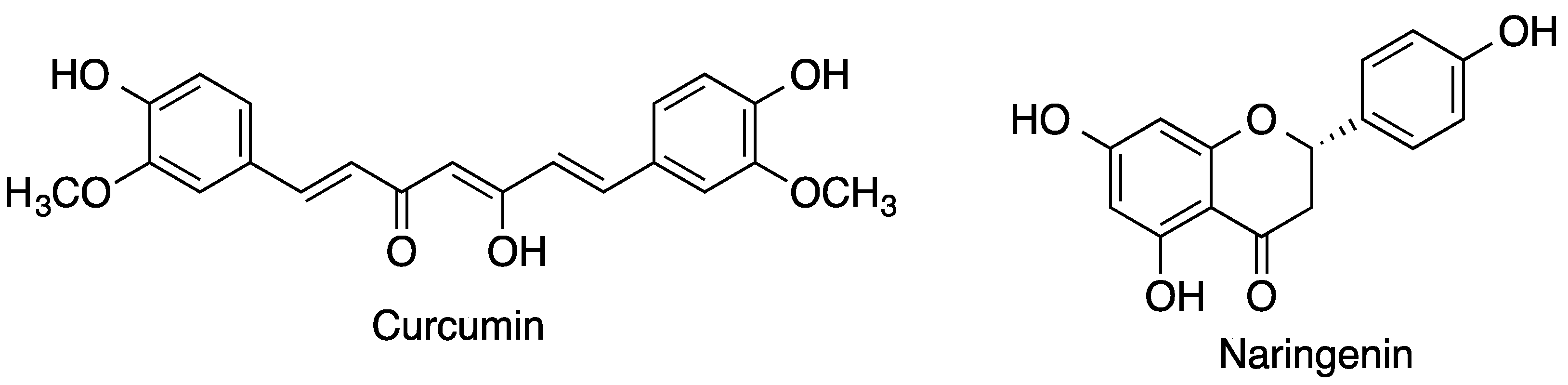
- Li, N.; Wang, N.; Wu, T.; Qiu, C.; Wang, X.; Jiang, S.; Zhang, Z.; Liu, T.; Wei, C.; Wang, T. Preparation of curcumin-hydroxypropyl-β-cyclodextrin inclusion complex by cosolvency-lyophilization procedure to enhance oral bioavailability of the drug. Drug Dev. Ind. Pharm. 2018, 44, 1966–1974. [Google Scholar] [CrossRef]
- Shulman, M.; Cohen, M.; Soto-Gutierrez, A.; Yagi, H.; Wang, H.; Goldwasser, J.; Lee-Parsons, C.W.; Benny-Ratsaby, O.; Yarmush, M.L.; Nahmias, Y. Enhancement of naringenin bioavailability by complexation with hydroxypropoyl-β-cyclodextrin. PLoS ONE 2011, 6, e18033. [Google Scholar] [CrossRef] [PubMed]
- Zidan, M.F.; Ibrahim, H.M.; Afouna, M.I.; Ibrahim, E.A. In vitro and in vivo evaluation of cyclodextrin-based nanosponges for enhancing oral bioavailability of atorvastatin calcium. Drug Devel. Industr. Pharm. 2018, 44, 1243–1253. [Google Scholar] [CrossRef] [PubMed]
- Abou-Taleb, H.A.; Fathalla, Z.; Abdelkader, H. Comparative studies of the effects of novel excipients amino acids with cyclodextrins on enhancement of dissolution and oral bioavailability of the non-ionizable drug carbamazepine. Eur. J. Pharm. Sci. 2020, 155, 105562. [Google Scholar] [CrossRef]
- Purpura, M.; Lowery, R.P.; Wilson, J.M.; Mannan, H.; Münch, G.; Razmovski-Naumovski, V. Analysis of different innovative formulations of curcumin for improved relative oral bioavailability in human subjects. Eur. J. Nutr. 2018, 57, 929–938. [Google Scholar] [CrossRef] [PubMed]
- Hundshammer, C.; Schön, C.; Kimura, M.; Furune, T.; Terao, K.; Elgeti, D.; Mohr, R. Enhanced metabolic bioavailability of tetrahydrocurcumin after oral supplementation of a γ-cyclodextrin curcumin complex. J. Funct. Foods 2021, 79, 104410. [Google Scholar] [CrossRef]
- Grimaudo, M.A.; Nicoli, S.; Santi, P.; Concheiro, A.; Alvarez-Lorenzo, C. Cyclosporine-loaded cross-linked inserts of sodium hyaluronan and hydroxypropyl-β-cyclodextrin for ocular administration. Carbohydr. Polym. 2018, 201, 308–316. [Google Scholar] [CrossRef] [PubMed]
- Borandeh, S.; Abdolmaleki, A.; Abolmaali, S.S.; Tamaddon, A.M. Synthesis, structural and in-vitro characterization of β-cyclodextrin grafted L-phenylalanine functionalized graphene oxide nanocomposite: A versatile nanocarrier for pH-sensitive doxorubicin delivery. Carbohydr. Polym. 2018, 201, 151–161. [Google Scholar] [CrossRef]
- Li, J.; Liu, P. One-pot fabrication of pH/reduction dual-stimuli responsive chitosan-based supramolecular nanogels for leakage-free tumor-specific DOX delivery with enhanced anti-cancer efficacy. Carbohydr. Polym. 2018, 201, 583–590. [Google Scholar] [CrossRef]
- Joardar, A.; Meher, G.; Bag, B.P.; Chakraborty, H. Host-guest complexation of eugenol in cyclodextrins for enhancing bioavailability. J. Mol. Liq. 2020, 319, 114336. [Google Scholar] [CrossRef]
- Dos Santos Lima, B.; Shanmugam, S.; de Souza Siqueira Quintans, J.; Quintans-Junior, L.J.; de Souza Araujo, A.A. Inclusion complex with cyclodextrins enhances the bioavailability of flavonoid compounds: A systematic review. Phytochem. Rev. 2019, 18, 1337–1359. [Google Scholar] [CrossRef]
- Fang, G.; Wang, Q.; Yang, X.; Qian, Y.; Zhang, G.; Tang, B. γ-Cyclodextrin-based polypseudorotaxane hydrogels for ophthalmic delivery of flurbiprofen to treat anterior uveitis. Carbohydr. Polym. 2022, 277, 118889. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Kong, L.; Zhu, P.; Liu, Q.; Liao, X.; Si, T.; Yang, B. Preparation, characterization and anticancer activity of inclusion complexes between genistein and amino-appended β-cyclodextrins. ChemistrySelect 2022, 7, e202201125. [Google Scholar] [CrossRef]
- Li, H.; Zhang, G.; Wang, W.; Chen, C.; Jiao, L.; Wu, W. Preparation, characterization, and bioavailability of host-guest inclusion complex of ginsenoside Re with gamma-cyclodextrin. Molecules 2021, 26, 7227. [Google Scholar] [CrossRef] [PubMed]
- Cheng, J.-G.; Yu, H.-J.; Chen, Y.; Liu, Y. Selective binding and controlled release of anticancer drugs by polyanionic cyclodextrins. Bioorg. Med. Chem. 2018, 26, 2287–2290. [Google Scholar] [CrossRef]
- Michalska, P.; Wojnicz, A.; Ruiz-Nuño, A.; Abril, S.; Buendia, I.; León, R. Inclusion complex of ITH12674 with 2-hydroxypropyl-β-cyclodextrin: Preparation, physical characterization and pharmacological effect. Carbohydr. Polym. 2017, 157, 94–104. [Google Scholar] [CrossRef]
- De Carvalho, L.B.; Burusco, K.K.; Jaime, C.; Venâncio, T.; de Carvalho, A.F.S.; Murgas, L.D.S.; Pinto, L.D.M.A. Complexes between methyltestosterone and β-cyclodextrin for application in aquaculture production. Carbohydr. Polym. 2018, 179, 386–393. [Google Scholar] [CrossRef]
- Li, Z.; Zhang, B.; Jia, S.; Ma, M.; Hao, J. Novel supramolecular organogel based on β-cyclodextrin as a green drug carrier for enhancing anticancer effects. J. Mol. Liq. 2018, 250, 19–25. [Google Scholar] [CrossRef]
- Dos Santos Lima, B.; de Alcântara Campos, C.; da Silva Santos, A.C.R.; Santos, V.C.N.; Trindade, G.D.G.G.; Shanmugam, S.; Pereira, E.W.M.; Mareto, R.M.; Duarte, M.C.; da Silva Almeida, J.R.G.; et al. Development of morin/hydroxypropyl-β-cyclodextrin inclusion complex: Enhancement of bioavailability, antihyperalgesic and anti-inflammatory effects. Food Chem. Toxicol. 2019, 126, 15–24. [Google Scholar] [CrossRef]
- Cerutti, J.P.; Quevedo, M.A.; Buhlman, N.; Longhi, M.R.; Zoppi, A. Synthesis and characterization of supramolecular systems containing nifedipine, β-cyclodextrin and aspartic acid. Carbohydr. Polym. 2019, 205, 480–487. [Google Scholar] [CrossRef]
- Vaidya, B.; Shukla, S.K.; Kolluru, S.; Huen, M.; Mulla, N.; Mehra, N.; Kanabar, D.; Palakurthi, S.; Ayehunie, S.; Muth, A.; et al. Nintedanib-cyclodextrin complex to improve bio-activity and intestinal permeability. Carbohydr. Polym. 2019, 204, 68–77. [Google Scholar] [CrossRef] [PubMed]
- Mendes, C.; Meirelles, G.C.; Barp, C.G.; Assreuy, J.; Silva, M.A.; Ponchel, G. Cyclodextrin based nanosponge of norfloxacin: Intestinal permeation enhancement and improved antibacterial activity. Carbohydr. Polym. 2018, 195, 586–592. [Google Scholar] [CrossRef] [PubMed]
- Aree, T.; Jongrungruangchok, S. Structure–antioxidant activity relationship of β-cyclodextrin inclusion complexes with olive tyrosol, hydroxytyrosol and oleuropein: Deep insights from X-ray analysis, DFT calculation and DPPH assay. Carbohydr. Polym. 2018, 199, 661–669. [Google Scholar] [CrossRef]
- Recio, R.; Elhalem, E.; Benito, J.M.; Fernández, I.; Khiar, N. NMR study on the stabilization and chiral discrimination of sulforaphane enantiomers and analogues by cyclodextrins. Carbohydr. Polym. 2018, 187, 118–125. [Google Scholar] [CrossRef]
- Erdoğar, N.; Nemutlu, E.; İskit, A.B.; Kara, S.C.; Teksin, Z.Ş.; Bilensoy, E. Improved oral bioavailability of anticancer drug tamoxifen through complexation with water soluble cyclodextrins: In vitro and in vivo evaluation. J. Incl. Phenom. Macrocycl. Chem. 2020, 96, 81–91. [Google Scholar] [CrossRef]
- Roy, N.; Ghosh, B.; Roy, D.; Bhaumik, B.; Roy, M.N. Exploring the inclusion complex of a drug (umbelliferone) with α-cyclodextrin optimized by molecular docking and increasing bioavailability with minimizing the doses in human body. ACS Omega 2020, 5, 30243–30251. [Google Scholar] [CrossRef]
- Madruga, L.Y.C.; Kipper, M.J. Expanding the repertoire of electrospinning: New and emerging biopolymers, techniques, and applications. Adv. Healthc. Mater. 2022, 11, 2101979. [Google Scholar] [CrossRef] [PubMed]
- Topuz, F.; Uyar, T. Electrospinning of Cyclodextrin Nanofibers: The Effect of Process Parameters. J. Nanomater. 2020, 2020, 7529306. [Google Scholar] [CrossRef]
- Celebioglu, A.; Uyar, T. Cyclodextrin nanofibers by electrospinning. Chem. Commun. 2010, 46, 6903–6905. [Google Scholar] [CrossRef] [PubMed]
- Topuz, F.; Uyar, T. Electrospinning of Cyclodextrin Functional Nanofibers for Drug Delivery Applications. Pharmaceutics 2019, 11, 6. [Google Scholar] [CrossRef]
- Dodero, A.; Schlatter, G.; Hebraud, A.; Vicini, S.; Castellano, M. Polymer-free cyclodextrin and natural polymer-cyclodextrin electrospun nanofibers: A comprehensive review on current applications and future perspectives. Carbohydr. Polym. 2021, 264, 118042. [Google Scholar] [CrossRef] [PubMed]
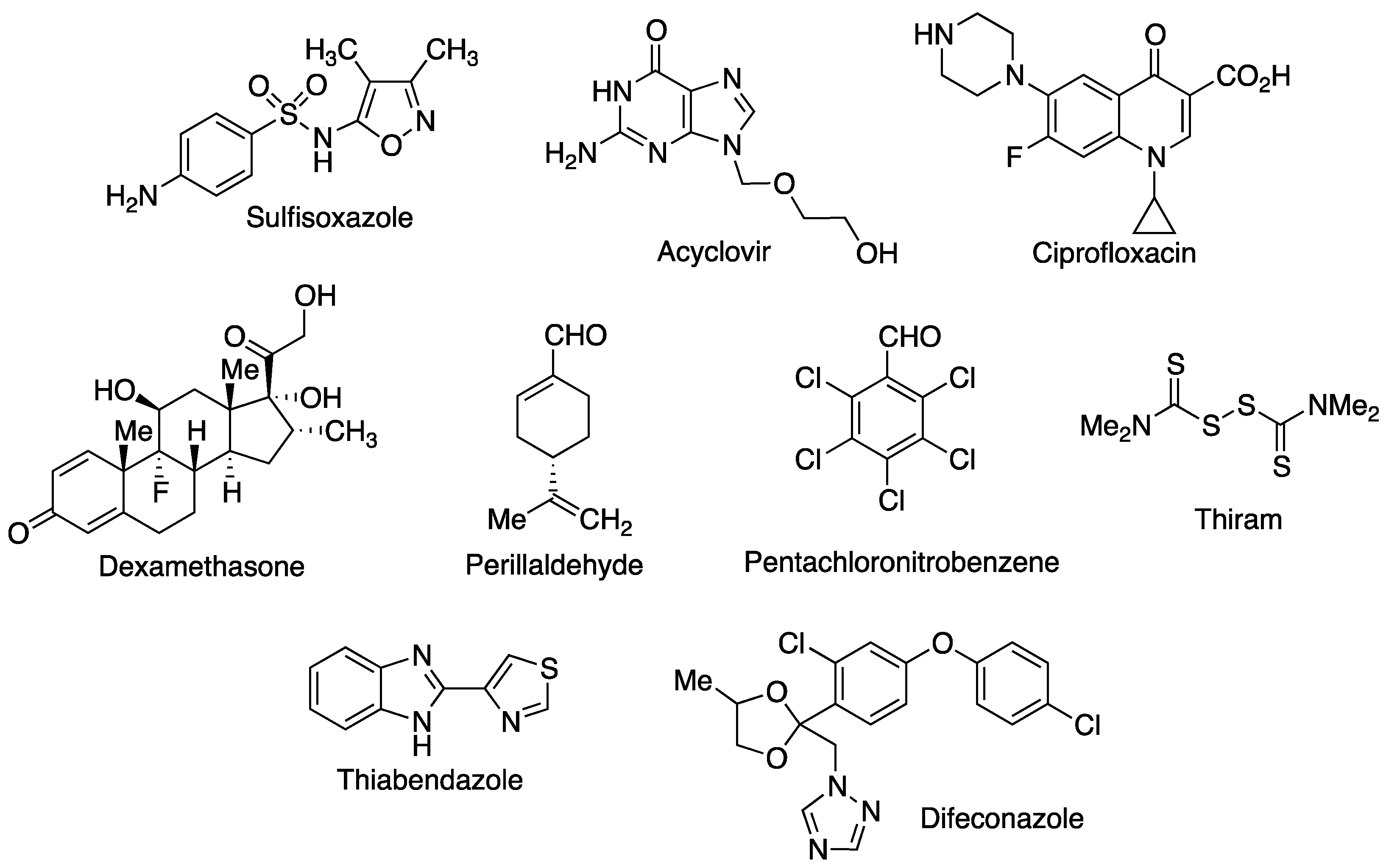
- Aytac, Z.; Sen, H.S.; Durgun, E.; Uyar, T. Sulfisoxazole/cyclodextrin inclusion complex incorporated in electrospun hydroxypropyl cellulose nanofibers as drug delivery system. Colloid Surf. B 2015, 128, 331–338. [Google Scholar] [CrossRef] [PubMed]
- Celebioglu, A.; Uyar, T. Electrospun formulation of acyclovir/cyclodextrin nanofibers for fast-dissolving antiviral drug delivery. Mater. Sci. Eng. C 2021, 118, 111514. [Google Scholar] [CrossRef]
- Hussain, Z.; Ullah, I.; Wang, Z.; Ding, P.; Ullah, S.; Zhang, Y.; Zhang, Z.; Yan, J.; Luo, B.; Pei, R. Electrospun nanofibrous membrane functionalized with dual drug-cyclodextrin inclusion complexes for the potential treatment of otitis externa. Colloid Surf. A 2022, 651, 129742. [Google Scholar] [CrossRef]
- Celebioglu, A.; Uyar, T. Hydrocortisone/cyclodextrin complex electrospun nanofibers for a fast-dissolving oral drug delivery system. RSC Med. Chem. 2020, 11, 245–258. [Google Scholar]
- Topuz, F. Rapid sublingual delivery of piroxicam from electrospun cyclodextrin inclusion complex nanofibers. ACS Omega 2022, 7, 35083–35091. [Google Scholar] [CrossRef]
- Gao, S.; Li, X.; Yang, G.; Feng, W.; Zong, L.; Zhao, L.; Ye, F.; Fu, Y. Antibacterial perillaldehyde/hydroxypropyl-γ-cyclodextrin inclusion complex electrospun polymer-free nanofiber: Improved water solubility, thermostability, and antioxidant activity. Ind. Crop. Prod. 2022, 176, 114300. [Google Scholar] [CrossRef]
- Gao, S.; Zong, L.; Zhang, Y.; Zhang, Y.; Guo, X.; Guo, G.; Zhao, L.; Ye, F.; Fu, Y. Antifungal pentachloronitrobenzene/hydroxypropyl-beta-cyclodextrin inclusion complex nanofibers by electrospun with no polymer: Fabrication and characterization. J. Clean. Prod. 2023, 413, 137499. [Google Scholar] [CrossRef]
- Gao, S.; Liu, Y.; Jiang, J.; Li, X.; Ye, F.; Fu, Y.; Zhao, L. Thiram/hydroxypropyl-β-cyclodextrin inclusion complex electrospun nanofibers for a fast dissolving water-based drug delivery system. Colloid Surf. B 2021, 201, 111625. [Google Scholar] [CrossRef]
- Gao, S.; Liu, Y.; Jiang, J.; Li, X.; Zhao, L.; Fu, Y.; Ye, F. Encapsulation of thiabendazole in hydroxypropyl-β-cyclodextrin nanofibers via polymer-free electrospinning and its characterization. Pest Manag. Sci. 2020, 76, 3264–3272. [Google Scholar] [CrossRef]
- Gao, S.; Jiang, J.; Li, X.; Ye, F.; Fu, Y.; Zhao, L. Electrospun polymer-free nanofibers incorporating hydroxypropyl-β-cyclodextrin/difenoconazole via supramolecular assembly for antifungal activity. J. Agric. Food Chem. 2021, 69, 5871–5881. [Google Scholar] [CrossRef] [PubMed]
- Xu, W.; Li, X.; Wang, L.; Li, S.; Chu, S.; Wang, J.; Li, Y.; Hou, J.; Luo, Q.; Liu, J. Design of cyclodextrin-based functional systems for biomedical applications. Front. Chem. 2021, 9, 635507. [Google Scholar] [CrossRef] [PubMed]
- Khan, A.R.; Forgo, P.; Stine, K.J.; D’Souza, V.T. Methods for selective modifications of cyclodextrins. Chem. Rev. 1998, 98, 1977–1996. [Google Scholar] [CrossRef]
- Liu, J.Y.; Zhang, X.; Tian, B.R. Selective modifications at the different positions of cyclodextrins: A review of strategies. Turk. J. Chem. 2020, 44, 261–278. [Google Scholar] [CrossRef] [PubMed]
- Řezanka, M. Synthesis of substituted cyclodextrins. Environ. Chem. Lett. 2019, 17, 49–63. [Google Scholar] [CrossRef]
- Martin, K.A.; Czarnik, A.W. Facile preparation of the β-cyclodextrinyl aldehyde. Tetrahedron Lett. 1994, 35, 6781–6782. [Google Scholar] [CrossRef]
- Tripodo, G.; Wischke, C.; Neffe, A.T.; Lendlein, A. Efficient synthesis of pure monotosylated beta-cyclodextrin and its dimers. Carbohydr. Res. 2013, 381, 59–63. [Google Scholar] [CrossRef]
- Nielsen, T.T.; Wintgens, V.; Amiel, C.; Wimmer, R.; Larsen, K.L. Facile synthesis of β-cyclodextrin-dextran polymers by “click” chemistry. Biomacromolecules 2010, 11, 1710–1715. [Google Scholar] [CrossRef]
- Zhong, N.; Byun, H.S.; Bittman, R. An improved synthesis of 6-O-monotosyl-6-deoxy-β-cyclodextrin. Tetrahedron Lett. 1998, 39, 2919–2920. [Google Scholar] [CrossRef]
- Cornwell, M.J.; Huff, J.B.; Bieniarz, C. A one-step synthesis of cyclodextrin monoaldehydes. Tetrahedron Lett. 1995, 36, 8371–8374. [Google Scholar] [CrossRef]
- Yoon, J.; Hong, S.; Martin, K.A.; Czarnik, A.W. A general method for the synthesis of cyclodextrinyl aldehydes and carboxylic acids. J. Org. Chem. 1995, 60, 2792–2795. [Google Scholar] [CrossRef]
- Sallas, F.; Leroy, P.; Marsura, A.; Nicolas, A. First selective synthesis of thio-β-cyclodextrin derivatives by a direct mitsunobu reaction on free β-cyclodextrin. Tetrahedron Lett. 1994, 35, 6079–6082. [Google Scholar] [CrossRef]
- Di Fabio, G.; Malgieri, G.; Isernia, C.; D’Onofrio, J.; Gaglione, M.; Messere, A.; Zarrelli, A.; De Napoli, L. A novel synthetic strategy for monosubstituted cyclodextrin derivatives. Chem. Commun. 2012, 48, 3875–3877. [Google Scholar] [CrossRef]
- Hanessian, S.; Benalil, A.; Laferriere, C. The synthesis of functionalized cyclodextrins as scaffolds and templates for molecular diversity, catalysis, and inclusion phenomena. J. Org. Chem. 1995, 60, 4786–4797. [Google Scholar] [CrossRef]
- Guieu, S.; Sollogoub, M. Regiospecific tandem azide-reduction/ deprotection to afford versatile amino alcohol-functionalized α- and β-cyclodextrins. Angew. Chem. Int. Ed. 2008, 47, 7060–7063. [Google Scholar] [CrossRef]
- Ashton, P.R.; Königer, R.; Fraser Stoddart, J.; Alker, D.; Harding, V.D. Amino acid derivatives of β-cyclodextrin. J. Org. Chem. 1996, 61, 903–908. [Google Scholar] [CrossRef]
- Chmurski, K.; Defaye, J. An improved synthesis of per(6-deoxyhalo)cyclodextrins using N-halosuccinimides—Triphenylphosphine in dimethylformamide. Supramol. Chem. 2000, 12, 221–224. [Google Scholar] [CrossRef]
- Li, Y.-F.; Ha, Y.-M.; Guo, Q.; Li, Q.-P. Synthesis of two β-cyclodextrin derivatives containing a vinyl group. Carbohydr. Res. 2015, 404, 55–62. [Google Scholar] [CrossRef]
- Tian, S.; D’Souza, V.T. Selective protection of the secondary side of β-cyclodextrin. Tetrahedron Lett. 1994, 35, 9339–9342. [Google Scholar] [CrossRef]
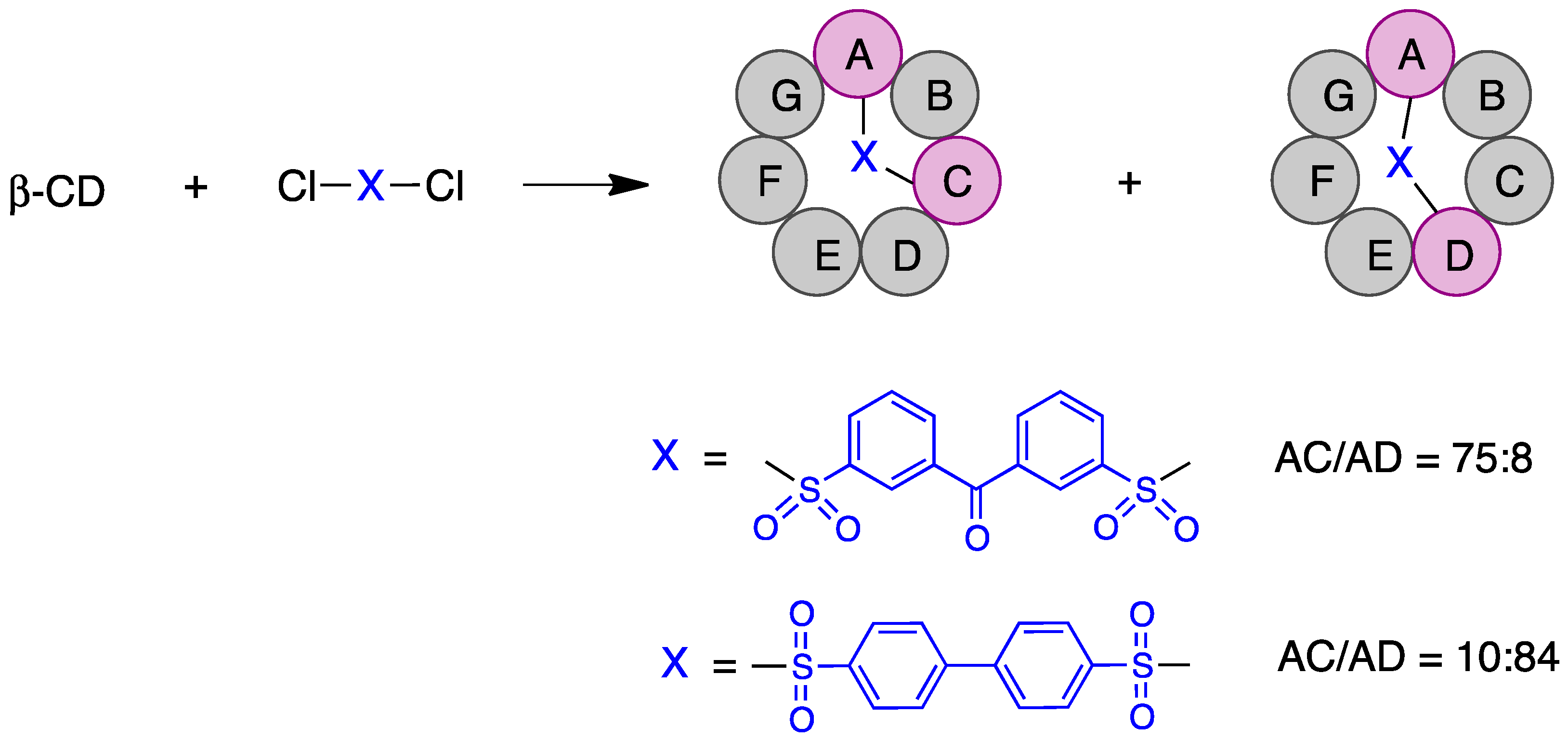
- Tabushi, I.; Yamamura, K.; Nabeshima, T. Characterization of regiospecific A,C- and A,D-disulfonate capping of β-cyclodextrin. Capping as an efficient production technique. J. Am. Chem. Soc. 1984, 106, 5267–5270. [Google Scholar] [CrossRef]
- Breslow, R.; Canary, J.W.; Varney, M.; Waddell, S.T.; Yang, D. Artificial transaminases linking pyridoxamine to binding cavities: Controlling the geometry. J. Am. Chem. Soc. 1990, 112, 5212–5219. [Google Scholar] [CrossRef]
- Benkovics, G.; Bálint, M.; Fenyvesi, É.; Varga, E.; Béni, S.; Yannakopoulou, K.; Malanga, M. Homo- and hetero-difunctionalized β-cyclodextrins: Short direct synthesis in gram scale and analysis of regiochemistry. Beilstein J. Org. Chem. 2019, 15, 710–720. [Google Scholar] [CrossRef] [PubMed]
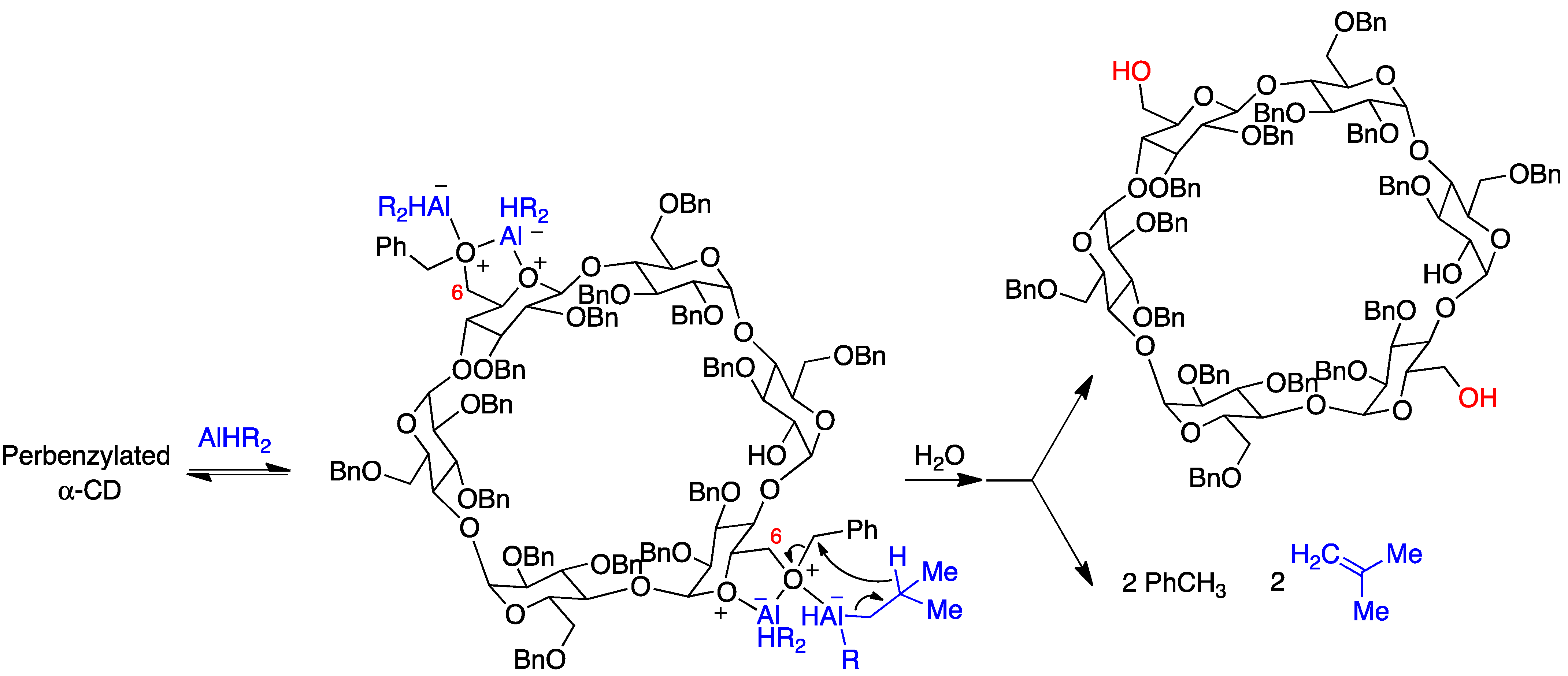
- Pearce, A.J.; Sinaÿ, P. Diisobutylaluminum-promoted regioselective de-O-benzylation of perbenzylated cyclodextrins: A powerful new strategy for the preparation of selectively modified cyclodextrins. Angew. Chem. Int. Ed. 2000, 39, 3610–3612. [Google Scholar] [CrossRef]
- Lecourt, T.; Herault, A.; Pearce, A.J.; Sollogoub, M.; Sinaÿ, P. Triisobutylaluminium and diisobutylaluminium hydride as molecular scalpels: The regioselective stripping of perbenzylated sugars and cyclodextrins. Chem. Eur. J. 2004, 10, 2960–2971. [Google Scholar] [CrossRef]
- Guieu, S.; Sollogoub, M. Multiple homo- and hetero-functionalizations of α-cyclodextrin through oriented deprotections. J. Org. Chem. 2008, 73, 2819–2828. [Google Scholar] [CrossRef] [PubMed]
- Wang, B.; Zaborova, E.; Guieu, S.; Petrillo, M.; Guitet, M.; Blériot, Y.; Ménand, M.; Zhang, Y.; Sollogoub, M. Site-selective hexa-hetero-functionalization of α-cyclodextrin an archetypical C6-symmetric concave cycle. Nat. Commun. 2014, 5, 5354. [Google Scholar] [CrossRef]
- Bistri, O.; Sinaÿ, P.; Sollogoub, M. Expeditious selective synthesis of primary rim tri-differentiated α-cyclodextrin. Tetrahedron Lett. 2006, 47, 4137–4139. [Google Scholar] [CrossRef]
- Fujita, K.; Ohta, K.; Masunari, K.; Obe, K.-I.; Yamamura, H. Selective preparation of hexakis (6-O-arenesulfonyl) -α-cyclodextrin. Tetrahedron Lett. 1992, 33, 5519–5520. [Google Scholar]
- Yamamura, H.; Fujita, K. Preparation of heptakis(6-O-(p-tosyl))-β-cyclodextrin and heptakis(6-O-(p-tosyl))-2-O-(p-tosyl)-β-cyclodextrin and their conversion to heptakis(3, 6-anhydro)-β-cyclodextrin. Chem. Pharm. Bull. 1991, 39, 2505–2508. [Google Scholar] [CrossRef]
- Yamamura, H.; Yoshitaka, K.; Masao, K.; Yasuo, B. Preparation of polytosylated γ-cyclodextrins. Bull. Chem. Soc. Jpn. 1993, 66, 585–588. [Google Scholar] [CrossRef]
- Gadelle, A.; Defaye, J. Selective halogenation at primary positions of cyclomaltooligosaccharides and a synthesis of per-3,6-anhydro cyclomaltooligosaccharides. Angew. Chem. Int. Ed. Engl. 1991, 30, 78–80. [Google Scholar] [CrossRef]
- Chmurski, K.; Defaye, J. An improved synthesis of 6-deoxyhalo cyclodextrins via halomethylenemorpholinium halides Vilsmeier-Haack type reagents. Tetrahedron Lett. 1997, 38, 7365–7368. [Google Scholar] [CrossRef]
- Jicsinszky, L.; Caporaso, M.; Martina, K.; Gaudino, E.C.; Cravotto, G. Efficient mechanochemical synthesis of regioselective persubstituted cyclodextrins. Beilstein J. Org. Chem. 2016, 12, 2364–2371. [Google Scholar] [CrossRef] [PubMed]
- Takeo, K.-I.; Ueraura, K.; Mitoh, H. Derivatives Of α-Cyclodextrin and the Synthesis of 6-O-α-D-glucopyranosyl-α-cyclodextrin. J. Carbohydr. Chem. 1988, 7, 293–308. [Google Scholar] [CrossRef]
- Boger, J.; Corcoran, R.J.; Lehn, J.-M. Cyclodextrin chemistry. Selective modification of all primary hydroxyl groups of α- and β-cyclodextrins. Helv. Chim. Acta 1978, 61, 2190–2218. [Google Scholar] [CrossRef]
- Letort, S.; Balieu, S.; Erb, W.; Gouhier, G.; Estour, F. Interactions of cyclodextrins and their derivatives with toxic organophosphorus compounds. Beilstein J. Org. Chem. 2016, 12, 204–228. [Google Scholar] [CrossRef]
- Ueno, A.; Breslow, R. Selective sulfonation of a secondary hydroxyl group of β-cyclodextrin. Tetrahedron Lett. 1982, 23, 3451–3454. [Google Scholar] [CrossRef]
- Law, H.; Baussanne, I.; García-Fernández, J.M.; Defaye, J. Regioselective sulfonylation at O-2 of cyclomaltoheptaose with 1-(p-tolylsulfonyl)-(1H)-1,2,4-triazole. Carbohydr. Res. 2003, 338, 451–453. [Google Scholar] [CrossRef]
- Teranishi, K.; Tanabe, S.; Hisamatsu, M.; Yamada, T. Efficient regioselective synthesis of mono-2-O-sulfonyl-cyclodextrins by the combination of sulfonyl imidazole and molecular sieves. J. Carbohydr. Chem. 1998, 17, 489–494. [Google Scholar] [CrossRef]
- Menuel, S.; Doumert, B.; Saitzek, S.; Ponchel, A.; Delevoye, L.; Monflier, E.; Hapiot, F. Selective secondary face modification of cyclodextrins by mechanosynthesis. J. Org. Chem. 2015, 80, 6259–6266. [Google Scholar] [CrossRef]
- Jindrich, J.; Pitha, J.; Lindberg, B.; Seffers, P.; Harata, K. Regioselectivity of alkylation of cyclomaltoheptaose (β-cyclodextrin) and synthesis of its mono-2-O-methyl, -ethyl, -allyl, and -propyl derivatives. Carbohydr. Res. 1995, 266, 75–80. [Google Scholar] [CrossRef] [PubMed]
- Du Roizel, B.; Baltaze, J.-P.; Sinaÿ, P. Diisobutylaluminum-promoted secondary rim selective de-O-methylation of permethylated cyclodextrins. Tetrahedron Lett. 2002, 43, 2371–2373. [Google Scholar] [CrossRef]
- Xiao, S.; Yang, M.; Yu, F.; Zhang, L.; Zhou, D.; Sinaÿ, P.; Zhang, Y. Synthesis of four mono-functionalized α-cyclodextrin derivatives for further confirming DIBAL-H-promoted bis-de-O-methylation mechanism. Tetrahedron 2013, 69, 4053–4060. [Google Scholar] [CrossRef]
- Teranishi, K. Regiospecific 2A,2C-disulfonation of β-cyclodextrin. Tetrahedron Lett. 2000, 41, 933–936. [Google Scholar] [CrossRef]
- Teranishi, K. Practical and convenient modifications of the A,C-secondary hydroxyl face of cyclodextrins. Tetrahedron 2003, 59, 2519–2538. [Google Scholar] [CrossRef]
- Teranishi, K. Regioselective 2A-2D-disulfonylations of cyclodextrins for practical bifunctionalization on the secondary hydroxyl face. J. Incl. Phenom. Macrocycl. Chem. 2002, 44, 313–316. [Google Scholar] [CrossRef]
- Coleman, A.W.; Zhang, P.; Parrot-Lopez, H.; Ling, C.-C.; Miocque, M.; Mascrier, L. The first selective per-tosylation of the secondary OH-2 of β-cyclodextrin. Tetrahedron Lett. 1991, 32, 3997–3998. [Google Scholar] [CrossRef]
- Rong, D.; D’Souza, V.T. A convenient method for functionalization of the 2-position of cyclodextrins. Tetrahedron Lett. 1990, 31, 4275–4278. [Google Scholar] [CrossRef]
- Yu, H.; Teramoto, A.; Fukudome, M.; Xie, R.-G.; Yuan, D.-Q.; Fujita, K. A facile sulfonylation method enabling direct syntheses of per(2-O-sulfonyl)-β-cyclodextrins. Tetrahedron Lett. 2006, 47, 8837–8840. [Google Scholar] [CrossRef]
- Ashton, P.R.; Boyd, S.E.; Gattuso, G.; Hartwell, E.Y.; Koeniger, R.; Spencer, N.; Fraser Stoddart, R. A novel approach to the synthesis of some chemically-modified cyclodextrins. J. Org. Chem. 1995, 60, 3898–3903. [Google Scholar] [CrossRef]
- Dittmann, H.; Scharwächter, K.; König, W.A. Synthesis and silica-based immobilization of monofunctionalized cyclomaltoheptaose derivatives for enantioselective HPLC. Carbohydr. Res. 2000, 324, 75–96. [Google Scholar] [CrossRef] [PubMed]
- Junge, M.; König, W.A. Selectivity tuning of cyclodextrin derivatives by specific substitution. J. Sep. Sci. 2003, 26, 1607–1614. [Google Scholar] [CrossRef]
- Yuan, D.-Q.; Tahara, T.; Chen, W.-H.; Okabe, Y.; Yang, C.; Yagi, Y.; Nogami, Y.; Fukudome, M.; Fujita, K. Functionalization of cyclodextrins via reactions of 2,3-anhydrocyclodextrins. J. Org. Chem. 2003, 68, 9456–9466. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Lu, R. Facile direct acylation and acyl migration of β-cyclodextrin on the secondary hydroxyl face. J. Incl. Phenom. Macrocycl. Chem. 2009, 63, 373–378. [Google Scholar] [CrossRef]
- Fujita, K.; Tahara, T.; Egashira, Y.; Imoto, T.; Koga, T. A complete set of 3A,6X-di-O-sulfonylated α-cyclodextrins. Tetrahedron Lett. 1992, 33, 5385–5388. [Google Scholar] [CrossRef]
- Tian, S.; Forgo, P.; D’Souza, V.T. Selective modification at the 3-position of β-cyclodextrin. Tetrahedron Lett. 1996, 37, 8309–8312. [Google Scholar] [CrossRef]
- Fujita, K.; Nagamura, S.; Imoto, T.; Tahara, T.; Koga, T. Regiospecific sulfonation of secondary hydroxyl groups of alpha-cyclodextrin. Its application to preparation of 2A2B-, 2A2C-, and 2A2D-disulfonates. J. Am. Chem. Soc. 1985, 107, 3233–3235. [Google Scholar] [CrossRef]
- Fujita, K.; Tahara, T.; Yamamura, H.; Imoto, T.; Koga, T.; Mihashi, K. Specific preparation and structure determination of 3A,3C,3E-tri-O-sulfonyl-beta-cyclodextrin. J. Org. Chem. 1990, 55, 877–880. [Google Scholar] [CrossRef]
- Guitet, M.; Adam de Beaumais, S.; Blériot, Y.; Vauzeilles, B.; Zhang, Y.; Ménand, M.; Sollogoub, M. Cyclodextrins selectively modified on both rims using an O-3-debenzylative post-functionalisation, a consequence of the Sorrento meeting. Carbohydr. Res. 2012, 356, 278–281. [Google Scholar] [CrossRef]
- Szejtli, J.; Lipták, A.; Jodál, I.; Fügedi, P.; Nánási, P.; Neszmélyi, A. Synthesis and 13C-NMR spectroscopy of methylated beta-cyclodextrins. Starch-Starke 1980, 32, 165–169. [Google Scholar] [CrossRef]
- Yao, X.; Huanga, P.; Nie, Z. Cyclodextrin-based polymer materials: From controlled synthesis to applications. Progr. Polym. Sci. 2019, 93, 1–35. [Google Scholar] [CrossRef]
- Przybyla, M.A.; Yilmaz, G.; Becer, C.R. Natural cyclodextrins and their derivatives for polymer synthesis. Polym. Chem. 2020, 11, 7582–7602. [Google Scholar] [CrossRef]
- Seidi, F.; Jin, Y.; Xiao, H. Polycyclodextrins: Synthesis, functionalization, and applications. Carbohydr. Polym. 2020, 242, 116277. [Google Scholar] [CrossRef] [PubMed]
- Ohno, K.; Wong, B.; Haddleton, D.M. Synthesis of well-defined cyclodextrin-core star polymers. J. Polym. Sci. A Pol. Chem. 2001, 39, 2206–2214. [Google Scholar] [CrossRef]
- Abdouni, Y.; Yilmaz, G.; Becer, C.R. Sequence controlled polymers with a β-cyclodextrin core. Macromol. Rapid Commun. 2017, 38, 1700501. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; Xiong, Q.; Shen, W.; Zhang, Q. Facile synthesis of well-defined cyclodextrin-pendant polymer via ATRP for nanostructure fabrication. RSC Adv. 2014, 4, 30566–30572. [Google Scholar] [CrossRef]
- Diget, J.S.; Städe, L.W.; Nielsen, T.T. Direct synthesis of well-defined zwitterionic cyclodextrin polymers via atom transfer radical polymerization. Eur. Polym. J. 2019, 116, 84–90. [Google Scholar] [CrossRef]
- Perrier, S. 50th Anniversary Perspective: RAFT polymerization—A user guide. Macromolecules 2017, 50, 7433–7447. [Google Scholar] [CrossRef]
- Zhang, L.; Stenzel, M.H. Spherical glycopolymer architectures using RAFT: From stars with a β-cyclodextrin core to thermoresponsive core-shell particles. Aust. J. Chem. 2009, 62, 813–822. [Google Scholar] [CrossRef]
- Koyanagi, K.; Takashima, Y.; Nakamura, T.; Yamaguchi, H.; Harada, A. Radical polymerization by a supramolecular catalyst: Cyclodextrin with a RAFT reagent. Beilstein J. Org. Chem. 2016, 12, 2495–2502. [Google Scholar] [CrossRef]
- Miao, Y.; Zinck, P. Ring-opening polymerization of cyclic esters initiated by cyclodextrins. Polym. Chem. 2012, 3, 1119–1122. [Google Scholar] [CrossRef]
- Gou, P.-F.; Zhu, W.-P.; Xu, N.; Shen, Z.-Q.J. Synthesis and characterization of well-defined cyclodextrin-centered seven-arm star poly(ε-caprolactone)s and amphiphilic star poly(ε-caprolactone-b-ethylene glycol)s. J. Polym. Sci. A Pol. Chem. 2008, 46, 6455–6465. [Google Scholar] [CrossRef]
- Galia, A.; Scialdone, O.; Spanò, T.; Valenti, M.G.; Grignard, B.; Lecomte, P.; Monflier, E.; Tilloy, S.; Rousseau, C. Ring opening polymerization of ε-caprolactone in the presence of wet β-cyclodextrin: Effect of the operative pressure and of water molecules in the β-cyclodextrin cavity. RSC Adv. 2016, 6, 90290–90299. [Google Scholar] [CrossRef]
- Meimoun, J.; Phuphuak, Y.; Miyamachi, R.; Miao, Y.; Bria, M.; Rousseau, C.; Nogueira, G.; Valente, A.; Favrelle-Huret, A.; Zinck, P. Cyclodextrins initiated ring-opening polymerization of lactide using 4-dimethylaminopyridine (DMAP) as catalyst: Study of DMAP/β-CD inclusion complex and access to new structures. Molecules 2022, 27, 1083. [Google Scholar] [CrossRef]
- Faugeras, P.A.; Boëns, B.; Elchinger, P.H.; Brouillette, F.; Montplaisir, D.; Zerrouki, R.; Lucas, R. When cyclodextrins meet click chemistry. Eur. J. Org. Chem. 2012, 2012, 4087–4105. [Google Scholar] [CrossRef]
- Ge, Z.; Xu, J.; Hu, J.; Zhang, Y.; Liu, S. Synthesis and supramolecular self-assembly of stimuli-responsive water-soluble Janus-type heteroarm star copolymers. Soft Matter 2009, 5, 3932–3939. [Google Scholar] [CrossRef]
- Arslan, M.; Sanyal, R.; Sanyal, A. Cyclodextrin embedded covalently crosslinked networks: Synthesis and applications of hydrogels with nano-containers. Polym. Chem. 2020, 11, 615–629. [Google Scholar] [CrossRef]
- Roy, A.; Manna, K.; Dey, S.; Pal, S. Chemical modification of β-cyclodextrin towards hydrogel formation. Carbohydr. Polym. 2023, 306, 120576. [Google Scholar] [CrossRef]
- Iravani, S.; Varma, R.S. Nanosponges for drug delivery and cancer therapy: Recent advances. Nanomaterials 2022, 12, 2440. [Google Scholar] [CrossRef]
- Pawara, S.; Shendea, P.; Trotta, F. Diversity of β-cyclodextrin-based nanosponges for transformation of actives. Int. J. Pharmaceut. 2019, 565, 333–350. [Google Scholar] [CrossRef]
- Krabicová, I.; Appleton, S.L.; Tannous, M.; Hoti, G.; Caldera, F.; Pedrazzo, A.R.; Cecone, C.; Cavalli, R.; Trotta, F. History of cyclodextrin nanosponges. Polymers 2020, 12, 1122. [Google Scholar] [CrossRef] [PubMed]
- Utzeri, G.; Matias, P.M.C.; Murtinho, D.; Valente, A.J. Cyclodextrin-based nanosponges: Overview and opportunities. Front. Chem. 2022, 10, 859406. [Google Scholar] [CrossRef]
- Asela, I.; Donoso-González, O.; Yutronic, N.; Sierpe, R. β- cyclodextrin-based nanosponges functionalized with drugs and gold nanoparticles. Pharmaceutics 2021, 13, 513. [Google Scholar] [CrossRef] [PubMed]
- Guineo-Alvarado, J.; Quilaqueo, M.; Hermosilla, J.; González, S.; Medina, C.; Rolleri, A.; Lim, L.T.; Rubilar, M. Degree of crosslinking in β-cyclodextrin-based nanosponges and their effect on piperine encapsulation. Food Chem. 2021, 340, 128132. [Google Scholar] [CrossRef] [PubMed]
- Sadjadi, S.; Malmir, M.; Heravi, M.M.; Raja, M. Magnetic hybrid of cyclodextrin nanosponge and polyhedral oligomeric silsesquioxane: Efficient catalytic support for immobilization of Pd nanoparticles. Int. J. Biol. Macromol. 2019, 128, 638–647. [Google Scholar] [CrossRef]
- Wadhwa, P.; Vij, M.; Dand, N. Wave-assisted techniques, a greener and quicker alternative to synthesis of cyclodextrin-based nanosponges: A review. Recent Pat. Nanotechnol. 2022. [Google Scholar] [CrossRef] [PubMed]
- Ciesielska, A.; Ciesielski, W.; Girek, B.; Girek, T.; Koziel, K.; Kulawik, D.; Lagiewka, J. Biomedical application of cyclodextrin polymers cross-linked via dianhydrides of carboxylic acids. Appl. Sci. 2020, 10, 8463. [Google Scholar] [CrossRef]
- Rubin Pedrazzo, A.; Caldera, F.; Zanetti, M.; Appleton, S.L.; Dhakar, N.K.; Trotta, F. Mechanochemical green synthesis of hyper-crosslinked cyclodextrin polymers. Beilstein J. Org. Chem. 2020, 16, 1554–1563. [Google Scholar] [CrossRef]
- Suvarna, V.; Bore, B.; Bhawar, C.; Mallya, R. Complexation of phytochemicals with cyclodextrins and their derivatives- an update. Biomed. Pharmacother. 2022, 149, 112862. [Google Scholar] [CrossRef]
- Szente, L.; Szemán, J.; Sohajda, T. Analytical characterization of cyclodextrins: History, official methods and recommended new techniques. J. Pharm. Biomed. Anal. 2016, 130, 347–365. [Google Scholar] [CrossRef]
- Cid-Samamed, A.; Rakmai, J.; Mejuto, J.C.; Simal-Gandara, J.; Astray, G. Cyclodextrins inclusion complex: Preparation methods, analytical techniques and food industry applications. Food Chem. 2022, 384, 132467. [Google Scholar] [CrossRef] [PubMed]
- Lodagekar, A.; Borkar, R.M.; Thatikonda, S.; Chavan, R.B.; Naidu, V.G.M.; Shastri, N.R.; Srinivas, R.; Chella, N. Formulation and evaluation of cyclodextrin complexes for improved anticancer activity of repurposed drug: Niclosamide. Carbohydr. Polym. 2019, 212, 252–259. [Google Scholar] [CrossRef] [PubMed]
- Araújo-Filho, H.G.; Pereira, E.W.M.; Rezende, M.M.; Menezes, P.P.; Araújo, A.A.S.; Barreto, R.S.S.; Martins, A.O.B.P.B.; Albuquerque, T.R.; Silva, B.A.F.; Alcantara, I.S.; et al. D-limonene exhibits superior antihyperalgesic effects in a β-cyclodextrin-complexed form in chronic musculoskeletal pain reducing Fos protein expression on spinal cord in mice. Neuroscience 2017, 358, 158–169. [Google Scholar] [CrossRef]
- Heimfarth, L.; Santos dos Anjos, K.; Barbosa Gomes de Carvalho, Y.M.; Lucena dos Santos, B.; Russo Serafini, M.; de Carvalho Neto, A.G.; Santos Nunes, P.; Araújo Beserra Filho, J.I.; Pereira da Silva, S.; Mussi Ribeiro, A.; et al. Characterization of β-cyclodextrin/myrtenol complex and its protective effect against nociceptive behavior and cognitive impairment in a chronic musculoskeletal pain model. Carbohydr. Polym. 2020, 244, 116448. [Google Scholar] [CrossRef] [PubMed]
- Shende, P.K.; Gaud, R.S.; Bakal, R.; Patil, D. Effect of inclusion complexation of meloxicam with β-cyclodextrin- and β-cyclodextrin-based nanosponges on solubility, in vitro release and stability studies. Colloid. Surf. B-Biointerfaces 2015, 136, 105–110. [Google Scholar] [CrossRef]
- Mura, P. Analytical techniques for characterization of cyclodextrin complexes in the solid state: A review. J. Pharm. Biomed. Anal. 2015, 113, 226–238. [Google Scholar] [CrossRef] [PubMed]
- Lima, P.S.S.; Lucchese, A.M.; Araújo-Filho, H.G.; Menezes, P.P.; Araújo, A.A.S.; Quintans-Júnior, L.J.; Quintans, J.S.S. Inclusion of terpenes in cyclodextrins: Preparation, characterization and pharmacological approaches. Carbohydr. Polym. 2016, 151, 965–987. [Google Scholar] [CrossRef]
- Narayanan, G.; Boy, R.; Gupta, B.S.; Tonelli, A.E. Analytical techniques for characterizing cyclodextrins and their inclusion complexes with large and small molecular weight guest molecules. Polym. Test. 2017, 62, 402–439. [Google Scholar] [CrossRef]
- Szente, L. Analytical methods for cyclodextrins, cyclodextrin derivatives and cyclodextrin complexes. In Comprehensive Supramolecular Chemistry; Atwood, J.L., Davies, J.E.D., Macnicol, D.D., Vötgtle, F., Eds.; Pergamon: Exeter, UK, 1996; Volume 3, pp. 253–278. [Google Scholar]
- Trollope, L.; Cruickshank, D.L.; Noonan, T.; Bourne, S.A.; Sorrenti, M.; Catenacci, L.; Caira, M.R. Inclusion of trans-resveratrol in methylated cyclodextrins: Synthesis and solid-state structures. Beilstein J. Org. Chem. 2014, 10, 136–3151. [Google Scholar] [CrossRef]
- Santana, D.V.S.; Trindade, I.A.S.; Carvalho, Y.M.B.G.; Carvalho-Neto, A.G.; Silva, E.C.D.; Silva-Júnior, E.F.; Leite, R.F.S.; Quintans-Júnior, L.J.; Aquino, T.M.; Serafini, M.R.; et al. Analytical techniques to recognize inclusion complexes formation involving monoterpenes and cyclodextrins: A study case with (–) borneol, a food ingredient. Food Chem. 2021, 339, 127791. [Google Scholar] [CrossRef]
- Hădărugă, N.G.; Bandur, G.N.; David, I.; Hădărugă, D.I. A review on thermal analyses of cyclodextrins and cyclodextrin complexes. Environ. Chem. Lett. 2019, 17, 349–373. [Google Scholar] [CrossRef]
- Dos Santos, P.H.; Mesquita, T.; Miguel-dos-Santos, R.; de Almeida, G.K.M.; de Sá, L.A.; Menezes, P.P.; Araujo, A.A.S.; Lauton-Santos, S. Inclusion complex with β-cyclodextrin is a key determining factor for the cardioprotection induced by usnic acid. Chem-Biol. Interact. 2020, 332, 109297. [Google Scholar] [CrossRef] [PubMed]
- Grebogi, I.H.; Tibola, A.P.O.V.; Barison, A.; Grandizoli, C.W.P.S.; Ferraz, H.G.; Rodriguez, L.N.C. Binary and ternary inclusion complexes of dapsone in cyclodextrins and polymers: Preparation, characterization and evaluation. J. Incl. Phenom. Macrocycl. Chem. 2012, 73, 467–474. [Google Scholar] [CrossRef]
- Dalmolin, M.C.; da Silva, C.E.; Lunelli, C.E.; Zaioncz, S.; Farago, P.V.; Zawadzki, S.F. Modified β-cyclodextrin/amlodipine inclusion complexes: Preparation and application in aqueous systems. J. Mol. Liq. 2019, 276, 531–540. [Google Scholar] [CrossRef]
- Trindade, G.G.G.; Thrivikraman, G.; Menezes, P.P.; França, C.M.; Lima, B.S.; Carvalho, Y.M.B.G.; Souza, E.P.B.S.S.; Duarte, M.C.; Shanmugam, S.; Quintans-Júnior, L.J.; et al. Carvacrol/β-cyclodextrin inclusion complex inhibits cell proliferation and migration of prostate cancer cells. Food Chem. Toxicol. 2019, 125, 198–209. [Google Scholar]
- Kumar, S.; Rao, R. Analytical tools for cyclodextrin nanosponges in pharmaceutical field: A review. J. Incl. Phenom. Macrocycl. Chem. 2019, 94, 11–30. [Google Scholar] [CrossRef]
- Muñoz-Botella, S.; Martín, M.A.; del Castillo, B.; Vázquez, L. Differentiating inclusion complexes from host molecules by tapping- mode atomic force microscopy. Biophys. J. 1996, 71, 86–90. [Google Scholar] [CrossRef]
- Desai, C.; Prabhakar, B. Nano-amorphous composites of cilostazol–HP-β−CD inclusion complexes: Physicochemical characterization, structure elucidation, thermodynamic studies and in vitro evaluation. J. Incl. Phenom. Macrocycl. Chem. 2015, 81, 175–191. [Google Scholar] [CrossRef]
- Varghese, B.; Suliman, F.E.O.; Al-Hajri, A.; Al Bishri, N.S.S.; Al-Rwashda, N. Spectral and theoretical study on complexation of sulfamethoxazole with β- and HPβ-cyclodextrins in binary and ternary systems. Spectroc. Acta A Molec. Biomolec. Spectr. 2018, 190, 392–401. [Google Scholar] [CrossRef]
- Venuti, V.; Crupi, V.; Fazio, B.; Majolino, D.; Acri, G.; Testagrossa, B.; Stancanelli, R.; De Gaetano, F.; Gagliardi, A.; Paolino, D.; et al. Physicochemical characterization and antioxidant activity evaluation of idebenone/hydroxypropyl-β-cyclodextrin inclusion complex. Biomolecules 2019, 9, 531. [Google Scholar] [CrossRef]
- Prados, J.L.; León, A.G.; Olives, A.I.; Martín, M.A.; del Castillo, B. Cyclodextrins modify the proton transfer photoreactions of norharmane. J. Photochem. Photobiol. A Chem. 2005, 173, 287–295. [Google Scholar] [CrossRef]
- Mura, P. Analytical techniques for characterization of cyclodextrin complexes in aqueous solution: A review. J. Pharm. Biomed. Anal. 2014, 101, 238–250. [Google Scholar] [CrossRef] [PubMed]
- Kfoury, M.; Auezova, L.; Greige-Gerges, H.; Fourmentin, S. Encapsulation in cyclodextrins to widen the applications of essential oils. Environ. Chem. Lett. 2019, 17, 129–143. [Google Scholar] [CrossRef]
- Loftsson, T.; Saokham, P.; Rodrigues Sá Couto, A. Self-association of cyclodextrins and cyclodextrin complexes in aqueous solutions. Int. J. Pharmaceut. 2019, 560, 228–234. [Google Scholar] [CrossRef]
- Couto, A.R.S.; Ryzhakov, A.; Loftsson, L. Self-assembly of α-cyclodextrin and β-cyclodextrin: Identification and development of analytical techniques. J. Pharm. Sci. 2018, 107, 2208–2215. [Google Scholar] [CrossRef] [PubMed]
- Kumar, M.; Bhatia, R.; Rawal, R.K. Applications of various analytical techniques in quality control of pharmaceutical excipients. J. Pharm. Biomed. Anal. 2018, 157, 122–136. [Google Scholar] [CrossRef] [PubMed]
- Raymond, B.Y.; Quirino, J.P. Cyclodextrins as mobile phase additives in open-tubular admicellar electrochromatography for achiral and chiral separations. Microchem. J. 2021, 161, 105763. [Google Scholar]
- Djajić, N.; Krmar, J.; Rmandić, M.; Rašević, M.; Otašević, B.; Zečević, M.; Malenović, A.; Protić, A. Modified aqueous mobile phases: A way to improve retention behavior of active pharmaceutical compounds and their impurities in liquid chromatography. J. Chromatogr. Open 2022, 2, 100023. [Google Scholar] [CrossRef]
- Olives, A.I.; González-Ruiz, V.; Martín, M.A. Sustainable and eco-friendly alternatives for liquid chromatographic analysis. ACS Sustain. Chem. Eng. 2017, 5, 5618–5634. [Google Scholar] [CrossRef]
- León, A.G.; Olives, A.I.; del Castillo, B.; Martín, M.A. Influence of the presence of methyl cyclodextrins in high-performance liquid chromatography mobile phases on the separation of β−carboline alkaloids. J. Chromatogr. A 2008, 1192, 254–258. [Google Scholar] [CrossRef]
- González-Ruiz, V.; Olives, A.I.; Martín, M.A. SPE/RP-HPLC using C1 columns: An environmentally friendly alternative to conventional reverse-phase separations for quantitation of beta-carboline alkaloids in human serum samples. Anal. Bioanal. Chem. 2011, 400, 395–401. [Google Scholar] [CrossRef] [PubMed]
- Zhou, J.; Tang, J.; Tang, W. Recent development of cationic cyclodextrins for chiral separation. Trac-Trends Anal. Chem. 2015, 65, 22–29. [Google Scholar] [CrossRef]
- Guo, C.; Xiao, Y. Negatively charged cyclodextrins: Synthesis and applications in chiral analysis—A review. Carbohydr. Polym. 2021, 256, 117517. [Google Scholar] [CrossRef] [PubMed]
- Chankvetadze, B.; Scriba, G.K. Cyclodextrins as chiral selectors in capillary electrophoresis: Recent trends in mechanistic studies. Trac-Trends Anal. Chem. 2023, 160, 116987. [Google Scholar] [CrossRef]
- Pereira Lourenço, L.; Armani Aguiar, F.; Moraes de Oliveira, A.R.; Masetto de Gaitani, C. Quantitative determination of lercanidipine enantiomers in commercial formulations by capillary electrophoresis. J. Anal. Methods Chem. 2015, 2015, 294270. [Google Scholar]
- Szabó, Z.-I.; Tóth, G.; Völgyi, G.; Komjáti, B.; Hancu, G.; Szente, L.; Sohajda, T.; Béni, S.; Muntean, D.-L.; Noszál, B. Chiral separation of asenapine enantiomers by capillary electrophoresis and characterization of cyclodextrin complexes by NMR spectroscopy, mass spectrometry and molecular modeling. J. Pharm. Biomed. Anal. 2016, 117, 398–404. [Google Scholar] [CrossRef]
- Vargas-Martínez, M.G.; Ramírez-Galicia, G. Molecular modeling and chiral separation of benzodiazepines by capillary electrophoresis using highly sulfated cyclodextrins. J. Mex. Chem. Soc. 2018, 62, 358–370. [Google Scholar] [CrossRef]
- Kiss, E.; Szabó, V.A.; Horváth, P. Simple circular dichroism method for selection of the optimal cyclodextrin for drug complexation. J. Incl. Phenom. Macrocycl. Chem. 2019, 95, 223–233. [Google Scholar] [CrossRef]
- Kasliwal, N.; Negi, J.S. Development, characterization and performance evaluation of oro-dispersible tablet containing aceclofenac hydroxypropyl-β-cyclodextrin binary system. J. Incl. Phenom. Macrocycl. Chem. 2011, 71, 215–224. [Google Scholar] [CrossRef]
- Alizadeh, B.; Zarghi, A.; Aboofazeli, R.; Mahboubi, A. Application of supramolecular technology for the delivery of erythropoietin: Synthesis of β-cyclodextrin-erythropoietin inclusion complex via a host-guest interaction. J. Drug Deliv. Sci. Technol. 2023, 84, 104460. [Google Scholar] [CrossRef]
- Miskolczy, Z.; Megyesi, M.; Biczók, L. Entropy-driven inclusion of natural protoberberine alkaloids in sulfobutylether-β-cyclodextrin. Molecules 2022, 27, 7514. [Google Scholar] [CrossRef] [PubMed]
- Sursyakova, V.V.; Levdansky, V.A.; Rubaylo, A.I. Determining binding constants for 1:1 and 1:2 inclusion complexes of ester botulin derivatives with (2-hydroxypropyl)-β-cyclodextrin by affinity capillary electrophoresis. Electrophoresis 2021, 42, 700–707. [Google Scholar] [CrossRef] [PubMed]
- Sursyakova, V.V.; Levdansky, V.A.; Rubaylo, A.I. Evaluation of the effect of background electrolyte composition and independence of parameters in determining binding constants of betulin derivatives to beta- and dimethyl-beta-cyclodextrins by affinity capillary electrophoresis. J. Sep. Sci. 2022, 45, 3745–3753. [Google Scholar] [CrossRef] [PubMed]
- Sursyakova, V.V.; Levdansky, V.A.; Rubaylo, A.I. Determination of binding constants for strong complexation by affinity capillary electrophoresis: The example of complexes of ester betulin derivatives with (2-hydroxypropyl)-γ-cyclodextrin. Anal. Bioanal. Chem. 2020, 412, 5615–5625. [Google Scholar] [CrossRef] [PubMed]
- Castro-Puyana, M.; Crego, A.L.; Marina, M.L.; García-Ruiz, C. Enantioselective separation of azole compounds by EKC. Reversal of migration order of enantiomers with CD concentration. Electrophoresis 2007, 28, 2667–2674. [Google Scholar] [CrossRef]
- Sapte, S.; Pore, Y. Inclusion complexes of cefuroxime axetil with β-cyclodextrin: Physicochemical characterization, molecular modeling and effect of L-arginine on complexation. J. Pharm. Biomed. Anal. 2016, 6, 300–306. [Google Scholar] [CrossRef]
- Rescifina, A.; Surdob, E.; Cardile, V.; Avola, R.; Graziano, A.C.E.; Stancanelli, R.; Tommasini, S.; Pistarà, S.; Ventura, C.A. Gemcitabine anticancer activity enhancement by water soluble celecoxib/sulfobutyl ether-β-cyclodextrin inclusion complex. Carbohydr. Polym. 2019, 206, 792–800. [Google Scholar] [CrossRef]
- Lavania, K.; Garg, A. Inclusion complex of chrysin with hydroxypropyl-β-cyclodextrin (HP-β-CD) preparation, characterization, and dissolution study. BioNanoScience 2023, 13, 616–624. [Google Scholar] [CrossRef]
- Mashaqbeh, H.; Obaidat, R.; Al-Shar’I, N. Evaluation and characterization of curcumin-β-cyclodextrin and cyclodextrin-based nanosponge inclusion complexation. Polymers 2021, 13, 4073. [Google Scholar] [CrossRef]
- Petralito, S.; Zanardi, I.; Spera, R.; Memoli, A.; Travagli, V. Spectroscopic characterization of both aqueous and solid-state diacerhein/hydroxypropyl-β-cyclodextrin inclusion complexes. Spectroc. Acta A Molec. Biomolec. Spectr. 2014, 127, 355–360. [Google Scholar] [CrossRef]
- Ahad, A.; Bin Jardan, Y.A.; Hassan, M.Z.; Raish, M.; Ahmad, A.; Al-Mohizea, A.M.; Al-Jenoobi, F.I. Formulation and characterization of eprosartan mesylate and β-cyclodextrin inclusion complex prepared by microwave technology. Drug Deliv. 2022, 29, 1512–1522. [Google Scholar] [CrossRef] [PubMed]
- Lin, Z.Y.; Wang, X.X.; Kou, S.B.; Shi, J.H. Exploring the inclusion interaction of estradiol with β-CD and HP-β-CD with the help of molecular dynamics simulation as well as multi-spectroscopic approaches. Spectroc. Acta A Molec. Biomolec. Spectr. 2022, 269, 120764. [Google Scholar] [CrossRef] [PubMed]
- Tóth, G.; Jánoska, A.; Szabó, Z.-I.; Völgyi, G.; Orgován, G.; Szente, L.; Noszál, B. Physicochemical characterisation and cyclodetrin complexation of erlotinib. Supramol. Chem. 2016, 28, 656–664. [Google Scholar] [CrossRef]
- Sherje, A.P.; Kulkarni, V.; Murahari, M.; Nayak, U.Y.; Bhat, P.; Suvarna, V.; Dravyakar, B. Inclusion complexation of etodolac with hydroxypropyl-beta-cyclodextrin and auxiliary agents: Formulation, characterization and molecular modeling studies. Mol. Pharm. 2017, 14, 1231–1242. [Google Scholar] [CrossRef] [PubMed]
- Pirnau, A.; Floare, C.G.; Bogdan, M. The complexation of flurbiprofen with β-cyclodextrin: A NMR study in aqueous solution. J. Incl. Phenom. Macrocycl. Chem. 2014, 78, 113–120. [Google Scholar] [CrossRef]
- Dai, Y.; Sun, C.; Yang, Y.; Wang, S.; Li, H.; Chen, D.D.Y. β-Cyclodextrin and folic acid host–guest interaction binding parameters determined by Taylor dispersion analysis and affinity capillary electrophoresis. Electrophoresis 2023, 44, 1027–1036. [Google Scholar] [CrossRef]
- Stancanelli, R.; Venuti, V.; Arigó, A.; Calabró, M.L.; Cannavá, C.; Crupi, V.; Majolino, D.; Tommasini, S.; Ventura, C.A. Isoflavone aglycons-sulfobutyl ether-β-cyclodextrin inclusion complexes: In solution and solid-state studies. J. Incl. Phenom. Macrocycl. Chem. 2015, 83, 27–36. [Google Scholar] [CrossRef]
- Zornoza, A.; Vélaz, I.; González-Gaitano, G.; Martínez-Ohárriz, M.C. A comprehensive study of gemfibrozil complexation with β-cyclodextrins in aqueous solution using different analytical techniques. Int. J. Mol. Sci. 2022, 23, 16119. [Google Scholar] [CrossRef]
- Prabu, S.; Samad, N.A.; Ahmad, N.A.; Jumbri, K.; Raoov, M.; Rahim, N.Y.; Samikannu, K.; Mohamad, S. Studies on the supramolecular complex of a guanosine with beta-cyclodextrin and evaluation of its anti-proliferative activity. Carbohydr. Res. 2020, 497, 108138. [Google Scholar] [CrossRef]
- Mohandoss, M.; Atchudan, R.; Edison, T.R.J.I.; Mandal, T.P.; Palanisamy, S.; Youb, S.G.; Napoleon, A.A.; Shima, J.J.; Lee, Y.R. Enhanced solubility of guanosine by inclusion complexes with cyclodextrin derivatives: Preparation, characterization, and evaluation. Carbohydr. Polym. 2019, 224, 115166. [Google Scholar] [CrossRef]
- Suzuki, R.; Inoue, Y.; Limmatvapirat, S.; Murata, I.; Kanamoto, I. Molecular interactions of the inclusion complexes of hinokitiol and various cyclodextrins. AAPS PharmSciTech. 2017, 18, 2717–2726. [Google Scholar] [CrossRef] [PubMed]
- Prabu, S.; Sivakumar, K.; Swaminathan, M.; Rajamohan, R. Preparation and characterization of host–guest system between inosine and β-cyclodextrin through inclusion mode. Spectroc. Acta A Molec. Biomolec. Spectr. 2015, 147, 151–157. [Google Scholar] [CrossRef] [PubMed]
- Obaid, A.; Jamil, A.K.M.; Prabu, S.; Saharin, S.M.; Mohamad, S. Spectroscopic studies for the inclusion complexation of ketoprofen enantiomers with β-cyclodextrin. Spectroc. Acta A Molec. Biomolec. Spectr. 2020, 241, 118674. [Google Scholar] [CrossRef]
- Bedogni, G.; Arrúa, E.; Seremeta, K.; Okulik, N.; Salomon, C. Elucidating the complexation of nifurtimox with cyclodextrins. J. Mol. Liq. 2023, 382, 121852. [Google Scholar] [CrossRef]
- Yousef, F.O.; Ghanemb, R.; El-Barghouthi, M.-I.; Abu-Shattal, E.D.; Al-Sa’doni, H.H.; Bodoor, K. Heptakis(2,6-di-O-methyl)-β-CD as a host of olanzapine: Experimental and computational study. J. Mol. Struct. 2023, 1276, 134812. [Google Scholar] [CrossRef]
- Commey, K.; Nakatake, A.; Enaka, A.; Nishi, K.; Tsukigawa, K.; Yamaguchi, K.; Ikeda, H.; Iohara, D.; Hirayama, F.; Otagiri, M.; et al. Study of the inclusion complexes formed between 4-phenylbutyrate and α-, β- and γ-cyclodextrin in solution and evaluation on their taste-masking properties. J. Pharm. Pharmacol. 2023, 75, 236–244. [Google Scholar] [CrossRef] [PubMed]
- Fisli, H.; Hennig, A.; Chelaghmi, M.L.; Abdaoui, M. The relationship between solvatochromic properties and in silico ADME parameters of new chloroethylnitrosourea derivatives with potential anticancer activity and their β-cyclodextrin complexes. Spectroc. Acta A Molec. Biomolec. Spectr. 2021, 253, 119579. [Google Scholar] [CrossRef]
- Yan, J.; Li, Z.; Zhu, F.; Chi, S.; Wang, Q.; Rong, M.; Xie, W.; Zhao, Y. Inclusion complex of 20(S)-protopanaxatriol with modified β-cyclodextrin: Characterization, solubility, and interaction with bovine serum albumin. Anal. Biochem. 2022, 53, 114753. [Google Scholar] [CrossRef]
- Ghosh, B.; Roy, N.; Roy, D.; Mandal, S.; Mondal, M.; Dakua, V.K.; Dutta, A.; Sen, S.; Kumar, A.; Chakraborty, R.; et al. Exploring inclusion complex of an antithyroid drug (PTU) with α-cyclodextrin for innovative applications by physicochemical approach optimized by molecular docking. J. Mol. Liq. 2023, 380, 121708. [Google Scholar] [CrossRef]
- Alizadeh, N.; Poorbagher, N. Host-guest inclusion complexes of sulfabenzamide with β- and methyl-β-cyclodextrins: Characterization, antioxidant activity and DFT calculation. J. Mol. Struct. 2022, 1260, 32809. [Google Scholar] [CrossRef]
- Bani-Yaseen, A.D.; Mo’ala, A. Spectral, thermal, and molecular modeling studies on the encapsulation of selected sulfonamide drugs in β-cyclodextrin nano-cavity. Spectroc. Acta A Molec. Biomolec. Spectr. 2014, 131, 424–431. [Google Scholar] [CrossRef] [PubMed]
- Yakavets, I.; Guereschi, C.; Lamy, L.; Kravchenko, I.; Lassalle, H.P.; Zorin, V.; Bezdetnaya, L. Cyclodextrin nanosponge as a temoporfin nanocarrier: Balancing between accumulation and penetration in 3D tumor spheroids. Eur. J. Pharm. Biopharm 2020, 154, 33–42. [Google Scholar] [CrossRef] [PubMed]
- Mohandoss, S.; Velu, K.S.; Stalin, T.; Ahmad, N.; Alomar, S.Y.; Lee, Y.R. Tenofovir antiviral drug solubility enhancement with β-cyclodextrin inclusion complex and in silico study of potential inhibitor against SARS-CoV-2 main protease (Mpro). J. Mol. Liq. 2023, 377, 121544. [Google Scholar] [CrossRef] [PubMed]
- Ravi, A.; Pathigoolla, A.; Balan, H.; Gupta, R.; Raj, G.; Varghese, R.; Sureshan, K.M. Adamantoid scaffolds for multiple cargo loading and cellular delivery as β-cyclodextrin inclusion complexes. Angew. Chem. Int. Ed. 2023, 62, e202307324. [Google Scholar] [CrossRef] [PubMed]
- Yaşayan, G.; Şatıroğlu Sert, B.; Tatar, E.; Küçükgüzel, İ. Fabrication and characterisation studies of cyclodextrin-based nanosponges for sulfamethoxazole delivery. J. Incl. Phenom. Macrocycl. Chem. 2020, 97, 175–186. [Google Scholar] [CrossRef]
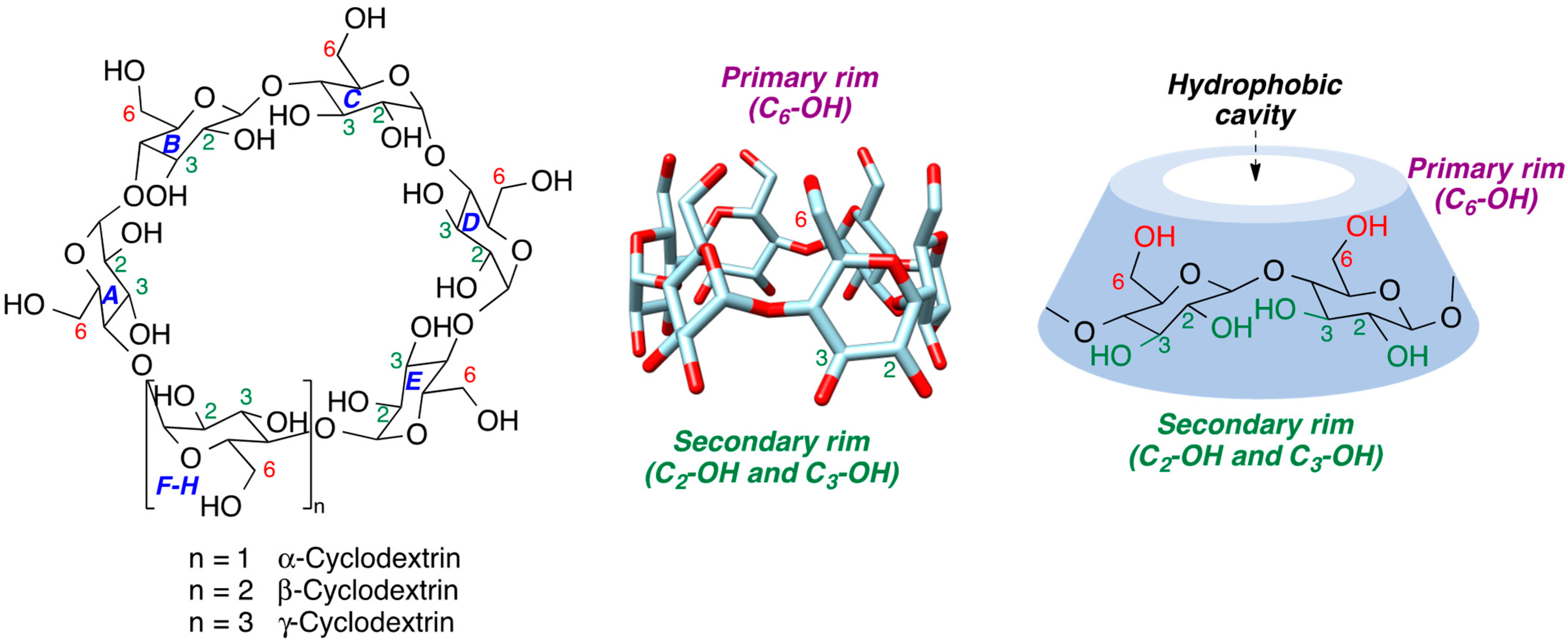
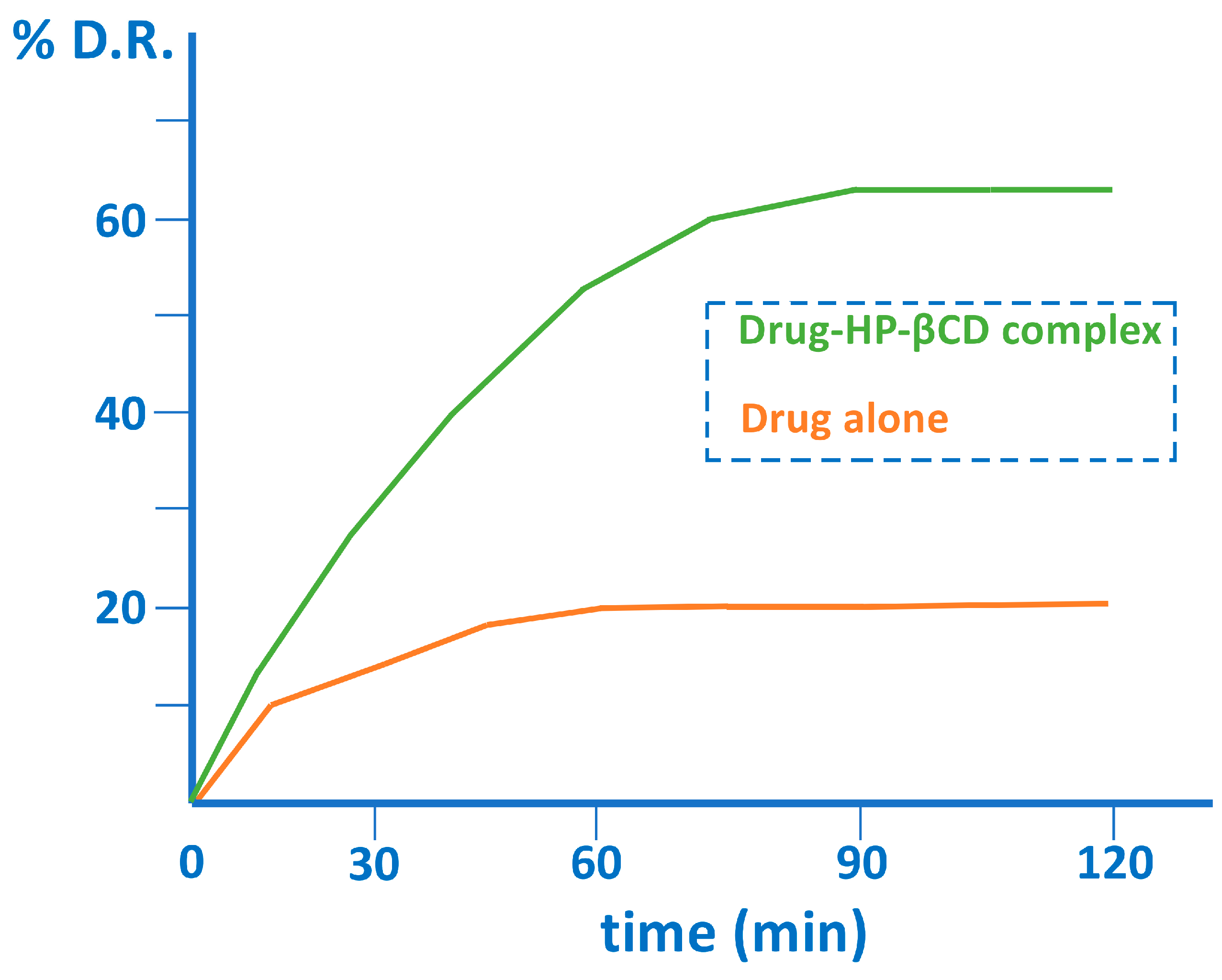
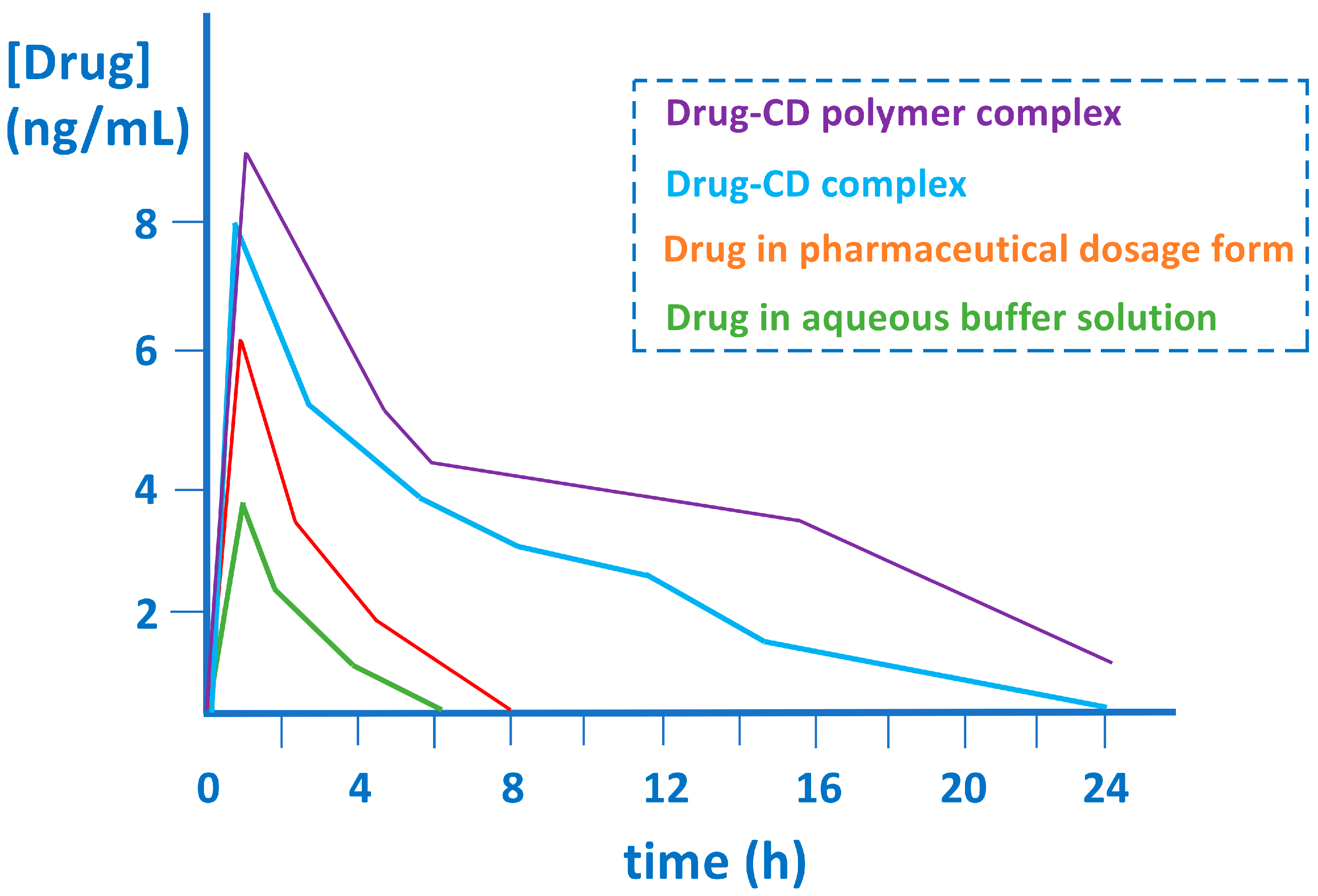
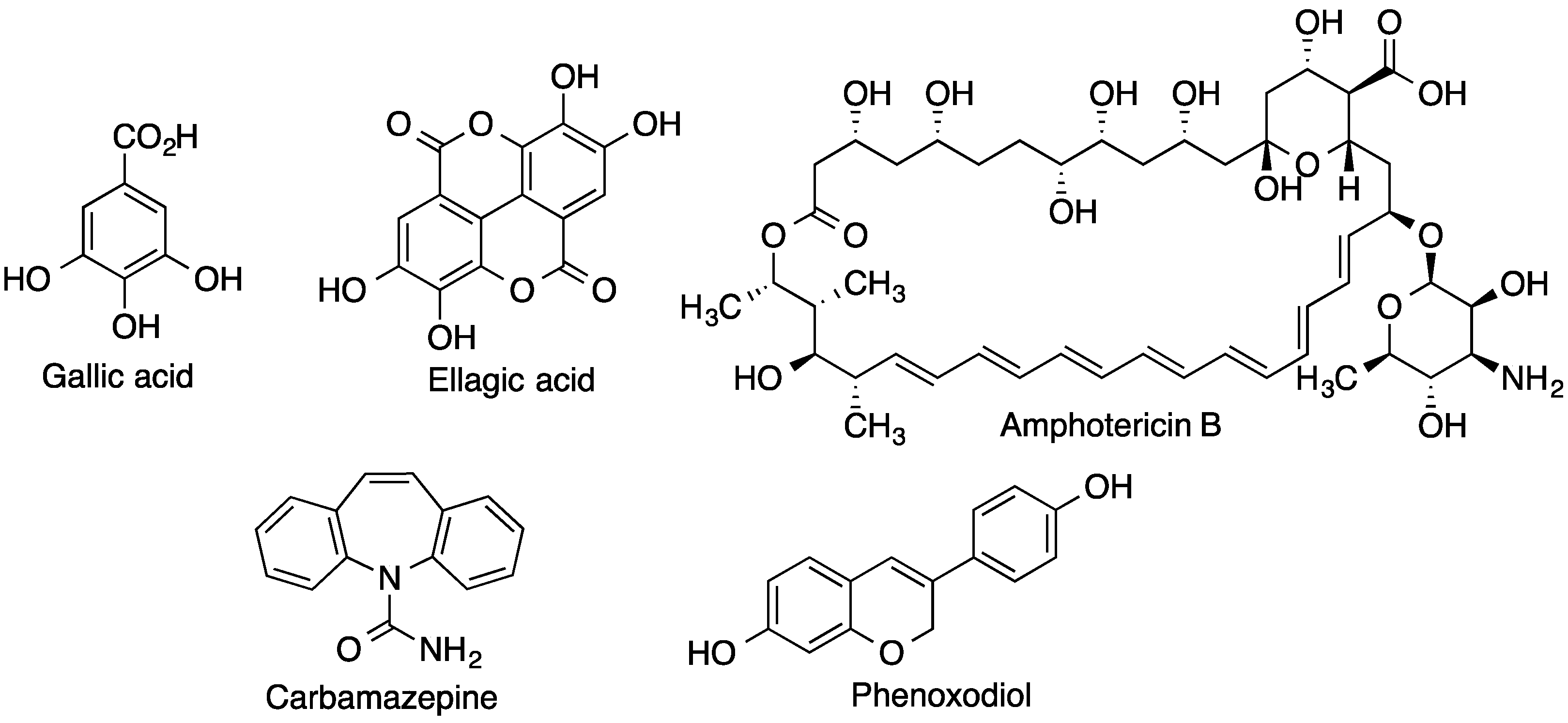
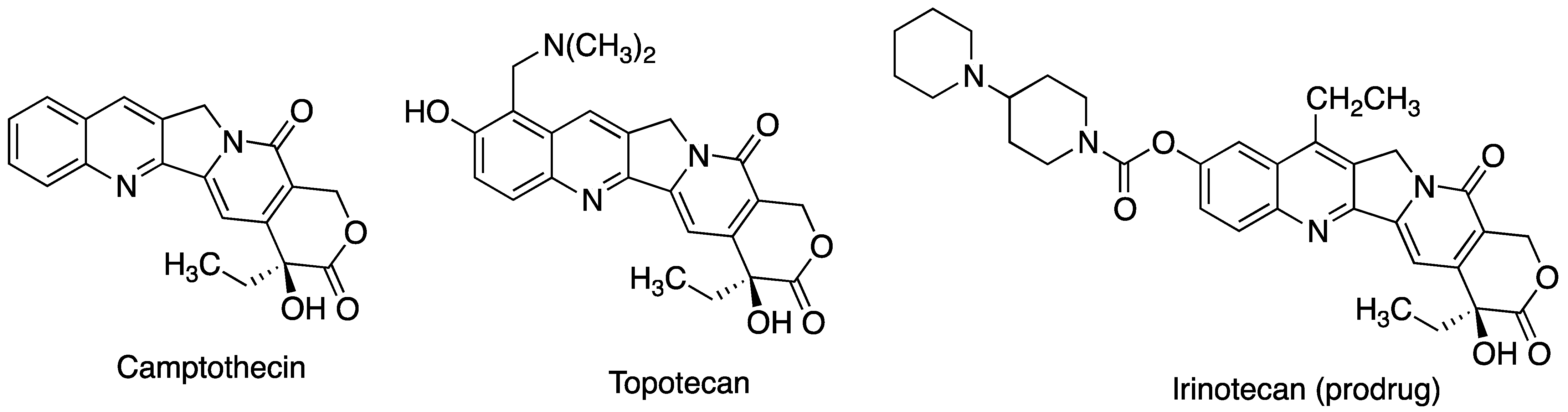


| CD | Cavity Diameter (Å) | Outer Diameter (Å) | Cavity Volume (Å3) | Height (Å) | Molecular Weight (g/mol) | Aqueous Solubility (mg/mL) |
|---|---|---|---|---|---|---|
| α | 4.7–5.3 | 14.6 | 174 | 7.9 | 973 | 145 |
| β | 6.0–6.5 | 15.4 | 262 | 7.9 | 1135 | 18.5 |
| γ | 7.5–8.3 | 17.5 | 427 | 7.9 | 1297 | 232 |
| Drug | Bioactivity | Cyclodextrin/Polymer | Improvement/Enhancement | Ref. |
|---|---|---|---|---|
| Atorvastatin | Anti-hyperlipidemic drug that interferes in cholesterol biosynthesis | β-CD-based nanosponges cross-linked with carbonyl diimidazole | Improved solubilization and dissolution rate; enhanced oral bioavailability | [68] |
| Boswellic acids | Triterpenic acids with anti-inflammatory activity | Complexes with β-CD and HPβ-CD | Drug release and oral bioavailability enhancement due to the increase in the solubility | [30] |
| Camptothecin | Alkaloid with anticancer activity | HPβ-CD; 6-O-capro-β-CD; and polymeric nanoparticles of these CDs with poly-ε-caprolactone and poly (lactide-co-glycolide) | Enhanced anti-tumor activity due to the stabilization of the active form (lactone) | [52] |
| Camptothecin, Luotonin A | Alkaloids with anticancer activity | Complexes with β-CD and HPβ-CD | Enhanced solubility and stability. Improvement in anti-tumor activity with luotonin A reaching camptothecin-like activity | [50] |
| Carbamazepine | Treatment of epilepsy and neuropathic pain | Inclusion complexes with β-CD, HPβ-CD, and γ-CD | Improved equilibrium solubility, in vitro dissolution rates, and oral bioavailability | [69] |
| Curcumin | Natural hydrophobic polyphenolic compound with a variety of pharmacological activities | γ-CD complex | Increase in plasma levels of curcumin; significant increase in oral bioavailability | [70,71] |
| Cyclosporin | Peptide isolated from fungus acting as immunosuppressant and in ophthalmic preparations | Polymers based on HPβ-CD and poly(ethyleneglycol) diglycidyl ether | The polymer incorporating HPβ-CD allows good penetration of cyclosporine through sclera and ocular tissues | [72] |
| Doxorubicin | Anti-cancer drug | β-CD grafted onto graphene oxide with L-phenylalanine as a linker | Enhancement of 10–40% in cytotoxicity activity with regard to free drugs | [73] |
| Doxorubicin | Anti-cancer drug | Nanogels based on β-cyclodextrin-modified chitosan | Enhancement in the sustained release profile into the tumor cells; increased anti-tumor activity | [74] |
| Eugenol | Methoxyphenol derivative with analgesic, anti-inflammatory properties employed as cement component in clinical dentistry | Complexes with α-CD and β-CD | Suggested increased bioavailability | [75] |
| Flavonoids | Natural polyphenolic compounds with antioxidant and anti-inflammatory activity | Several CDs | Enhanced oral bioavailability with an increase in the biological activity | [76] |
| Flurbiprofen | Nonsteroidal anti-inflammatory drug employed in the treatment of intraocular inflammation | γ-CD-based polypseudorotaxane hydrogels | Improved the precorneal drug retention ability, corneal permeability, and intraocular bioavailability | [77] |
| Genistein | Isoflavone with anti-cancer activity | Amino-appended β-CDs | Complex solubility increased 1000-fold compared with free genistein; cytotoxic activity also increases | [78] |
| Ginsenoside Re | A triterpene present in ginseng root with effects on ischemic damage of the cardiovascular system | Complexes with α-CD, β-CD, and γ-CD | Dissolution rate is increased ca. 10% and bioavailability is enhanced by 1.71% for the complex with γ-CD with regard to the free compound | [79] |
| Irinotecan, Topotecan, Doxorubicin | Anticancer drugs and topoisomerase inhibitors | Heptakis-[6-deoxy-6-(3-sulfanyl HX acid)]-β-cyclodextrin; HX = acetic or propionic acid | pH-controlled release of anticancer drugs due to negative charge of polyanionic CDs | [80] |
| Melatonin/sulforaphane hybrid | Treatment of ischemic stroke | HPβ-CD complexes | A 34-fold increase in solubility; stabilization at several pH and temperature values | [81] |
| 17-α-Methyltestosterone | Androgen | β-CD complex | A 6-fold hydrosolubility enhancement, with sustained release of the hormone | [82] |
| Methotrexate 5-Fluorouracil | Antimetabolites employed for cancer treatment | β-CD/glycerol organogel based on self-aggregation of β-CD | Sustained drug release providing an improved therapeutic effect | [83] |
| Morin | Flavonoid present in tea, wine, fruits, and vegetables acting as an antioxidant, anti-inflammatory | Complexes with HPβ-CD | Increased solubility and dissolution rate; oral bioavailability increased 4.2-fold compared with the free natural product | [84] |
| Nifedipine | Calcium-channel blocking agent for hypertension treatment | Multicomponent complexes with β-CD and aspartic acid | Enhancement of solubility and increase in dissolution rate | [85] |
| Nintedanib | Kinase inhibitor for the treatment of pulmonary fibrosis | Complexes with sulfobutyl ether-β-cyclodextrin | Improved stability at physiological pH and enhanced membrane transport | [86] |
| Norfloxacin | Fluoroquinolone antibiotic employed for urinary tract infections | β-Cyclodextrin-based nanosponges cross-linked with diphenyl carbonate | Controlled release of norfloxacin with enhanced membrane permeability and antibiotic efficiency | [87] |
| Oleuropein Hydroxytyrosol Tyrosol | Polyphenols present in olives and olive oil (functional foods) with antioxidant activity | Complexes with native β-CD | A 12–14% enhancement in the anti-oxidant activity (DPPH method) | [88] |
| Sulforaphane | Natural product isolated from broccoli with anticancer properties | Complexes with α-CD | Chemical stabilization | [89] |
| Tamoxifen | Antiestrogen—employed in hormone treatment of breast cancers | M-β-CD; HP-β-CD; and Sulfobutyl ether-β-cyclodextrin | Enhanced solubility and dissolution rate of tamoxifen inclusion complexes. Significant improvement in oral bioavailability | [90] |
| Umbelliferon | Coumarin derivative with lipid-lowering capability, and anticarcinogenic and HIV-inhibition activities | Complexes with α-CD | Potential sunscreen agent for protection from UV radiation | [91] |
| Guest | Host a | Solution State | Solid State | Ref. | |||
|---|---|---|---|---|---|---|---|
| Stoich. | Kass | Technique a | Preparation | Technique | |||
| Aceclofenac | HPβ-CD | 1:1 | 222.11 | HPLC b | Kneading Coprecipitation | FTIR, DSC, PXRD | [235] |
| Adamantane– Erythropoietin | mPEG β-CD | 1:1 | NP | 1H-NMR | Lyophilization | FTIR, DSC, TGA, PXRD, MALDI-TOF | [236] |
| Berberine Coptisine Palmatine Epiberberine Dehydrocorydaline | SBE-β-CD | 1:1 1:1 1:1 1:1 1:1 | 19,200 ± 103 19,100 ± 1300 4700 ± 200 3000 ± 300 2500 ± 200 | Fluorescence b, UV/Vis, ITC | [237] | ||
| Betulin 3,28- diphthalate Betulin 3,28- disuccinate Betulin 3,28-disulfate | HPβ-CD | 1:1 1:2 1:1 1:2 1:1 1:2 | 7.1 × 104 3.6 × 108 8.3 × 104 3.5 × 108 4.0 × 104 1.3 × 107 | ACE b | [238] | ||
| Betulin 3,28- diphthalate Betulin 3,28- disuccinate Betulin 3,28-disulfate | DMβ-CD | 1:1 1:2 1:1 1:2 1:1 1:2 | 9.5 × 104 3.3 × 107 1.8 × 105 --- 9.3 × 104 1.7 × 108 | ACE b | [239] | ||
| Betulin 3,28- diphthalate Betulin 3,28- disuccinate | HPγ-CD | 1:1 1:1 | 1.7 × 107 1.3 × 107 | ACE b | [240] | ||
| Bifonazole Clotrimazole Miconazole Tioconazole | β-CD SBEβ-CD β-CD SBβ-CD β-CD SBEβ-CD β-CD SBEβ-CD | 1:1 1:1 1:1 1:1 1:1 1:1 1:1 1:1 | 2.5 × 103 5.2 × 104 4.5 × 102 2.9 × 103 5.0 × 102 1.5 × 103 1.5 × 103 9.3 × 103 | CD b, UV/Vis | [235] | ||
| Bifonazole Miconazole | β-CD HPβ-CD β-CD HPβ-CD | 1:1 1:1 1:1 1:1 | 2767 2487 932 1012 | CE b | [241] | ||
| Cefuroxime Axetil | β-CD β-CD-L-Arg | 1:1 1:1 | 339.74 ± 1.5 498.98 ± 2.7 | UV/Vis b | Spray drying | DSC, PXRD, SEM | [242] |
| Celecoxib | SBEβ-CD | 1:1 | 8131 | UV/Vis b, HPLC, 1H-NMR, H-H ROESY | Freeze drying | [243] | |
| Chysin | HPβ-CD | 1:1 | 1090 | UV/Vis b 1H-NMR | Kneading Coprecipitation | FTIR, TGA, SEM | [244] |
| Curcumin | β-CD β-CD NS4 β-CD NS6 β-CD NS8 | 1:1 1:1 1:1 1:1 | 487.34 4972.90 4167.50 3567.87 | HPLC b | Freeze drying | FTIR, DSC, PXRD | [245] |
| Diacerhein | HPβ-CD | 1:1 1:1 | 45 * 22 # | UV/Vis b,* HPLC b,# | Kneading Coevaporation Freeze drying | FTIR, DSC | [246] |
| Eprosartan mesylate | β-CD | 1:1 | 280.78 | UV/Vis b 1H-NMR | Microwave irradiation | FTIR, DSC, PXRD, SEM | [247] |
| Estradiol | β-CD HPβ-CD | 1:1 1:1 | 3.14 × 104 3.22 × 104 | Fluorescence b UV/Vis, CD | [248] | ||
| Erlotinib | α-CD β-CD γ-CD HPβ-CD RAMEB CMβ-CD SBβ-CD | 1:1 1:1 1:1 1:1 1:1 1:1 1:1 | 142 ± 9 * 268 ± 11 * 324 # 54 ± 4 * 235 ± 10 * 434 ± 13 * 1396 ± 66 * 2380 ± 62 * | ACE b,*, LC-MS b,#, 1H NMR, H-H ROESY, UV, ESI-MS | Co-evaporation, kneading, vacuum desiccation, coprecipitation | XRD | [249] |
| Etodolac | HPβ-CD HPβ-CD-L-Arg | 1:1 1:1 | 162.11 ± 6.24 2573.31 ± 11.31 | UV/Vis b, 1H NMR | Coevaporation Spray drying | FTIR, DSC, PXRD | [250] |
| Flurbiprofen | β-CD | 1:1 | 2483.8 | 1H NMR *, H-H ROESY | [251] | ||
| Folic Acid | β-CD | 1:1 1:2 | 944 ± 79 * 159 ± 26 # | TDA-CE * ACE# | [252] | ||
| Genistein | Ab-CD1 Aβ-CD2 Aβ-CD3 Aβ-CD4 | 1:1 1:1 1:1 1:1 | 31,300 15,692 35,386 20,745 | UV/Vis b, 1H-NMR, H-H ROESY | Lyophilization | FTIR, PXRD, SEM | [79] |
| Genistein Daidzein | SBEβ-CD | 1:1 1:1 | 34,926 ± 4500 13,131 ± 980 | UV/Vis b | Kneading | ATR-FTIR | [253] |
| Gemfibrozil | β-CD HPβ-CD Meβ-CD | 1:1 1:1 1:1 1:2 | 760 ± 30 * 530 ± 20 * 440 ± 20 * | Fluorescence b,* 1H NMR b, UV b | Co-evaporation, kneading, vacuum desiccation, coprecipitation | [254] | |
| Ginsenoside Re | α-CD β-CD γ-CD | 1:1 1:1 1:1 | 22 612 1.4 × 104 | HPLC b | Lyophilization | FTIR, DSC, PXRD | [80] |
| Guanosine | β-CD | 1:1 1:1 | 59.79 * 151.97# | UV/Vis b,* Fluorescence b.# 1H-NMR, 13C-NMR | Coprecipitation | FTIR, DSC, TGA, PXRD, FESEM | [255] |
| Guanosine | β-CD HPβ-CD SBEβ-CD | 1:1 1:1 1:1 | 87.53 *, 70.79 # 91.61 *, 98.97 # 103.29 *, 190.76 # | UV/Vis b,* Fluorescence b,# | Coprecipitation | FTIR, DSC, TGA, PXRD, FESEM | [256] |
| Harmane Harmine | β-CD DMβ-CD TMβ-CD HPβ-CD β-CD DMβ-CD TMβ-CD HPβ-CD | 1:1 + 1:2 1:1 + 1:2 | 345 ± 42 207 ± 50 121 ± 29 602 ± 46 201 ± 14 148 ± 27 195 ± 14 353 ± 46 | Fluorescence b UV/Vis | 14 | ||
| Hinokitiol | α-CD β-CD | 1:2 1:1 | 175 187 | UV/Vis b, H-H ROESY | Grinding | FTIR, DSC, PXRD, Fluorescence | [257] |
| Inosine | β-CD | 1:1 1:1 | 33.59 * 104.53 # | UV/Vis b,* Fluorescence b,# | Kneading Coevaporation | FTIR, DSC; XRD, SEM | [258] |
| (R)-Ketoprofen (S)-Ketoprofen | β-CD | 1:1 1:1 | 2750 *, 4088 # 1299 *, 2547 # | UV/Vis b,* Fluorescence b,# | Coevaporation | RAMAN | [259] |
| Myrtenol | β-CD | 1:1 | -- | 1H-NMR, H-H ROESY | Kneading Slurry | DSC, TGA, PXRD, SEM | [200] |
| Nifedipine | β-CD β-CD:Asp | 1:1 1:1 | 99 ± 2 117 ± 4 | HPLC b, 1H-NMR | Kneading | FTIR, DCS, TGA, PXRD, SEM | [86] |
| Nifurtimox | β-CD SBEβ-CD | 1:1 1:1 | 236.79 359.57 | UV/Vis b, 1H-NMR, 13C-NMR | Coevaporation | FTIR, DSC, TGA, PXRD, SEM | [260] |
| Olanzapine | β-CD DMβ-CD | 1:1 1:1 | 100 ± 3 278 ± 5 | UV/Vis b, 1H-NMR | Kneading | FTIR | [261] |
| 4-Phenylbutyrate | α-CD β-CD γ-CD | 1:1 1:1 2:1 | 481 ± 26 178 ± 23 119 ± 9 | HPLC b, CD, 1H-NMR, H-H COSY, H-H ROESY | [262] | ||
| PNU 2FPNU 2MPNU 2MOPNU | β-CD | 1:1 1:1 1:1 1:1 | 1316 600 2200 5071 | Fluorescence b | [263] | ||
| 20S-Protopanaxatriol | EDBA-bis-β-CD | 1:1 | 995.94 ± 0.07 | UV/Vis b, 1H NMR, H-H ROESY | Coprecipitation | FTIR, PXRD, SEM | [264] |
| 6-Propyl-2-thiouracil | α-CD | 1:1 | 3297.57 ± 0.15 | UV/Vis b, 1H-NMR | Coevaporation | FTIR, DSC, TGA, PXRD, SEM | [265] |
| Sulfabenzamide | β-CD Mβ-CD | 1:1 1:1 | 2.4 × 105 2.2 × 104 | UV/Vis b, 1H-NMR | Lyophilization | FTIR, PXRD, SEM | [266] |
| Sulfisoxazole Sulfamethizole Sulfamethazine | β-CD | 1:1 1:1 1:1 | 650 1532 714 | UV/Vis b 1H-NMR | Coevaporation | DSC | [267] |
| Temoporfin | β-CD NS cmβ-CD NS | 1:1 1:1 | 6.3 × 106 1.2 × 106 | Fluorescence b | [268] | ||
| Tenofovir | β-CD | 1:1 | 863 ± 32 | UV/Vis b | Coprecipitation | FTIR, DSC, TGA, PXRD, SEM | [269] |
| Trioxaadamantane Adamantoid scaffolds: G2 G3 G4 | β-CD | 1:1 1:1 1:1 1:1 | 969 ± 62 173 ± 11 2990 ± 65 4140 ± 193 | ITC b, 1H-NMR | Cocrystallization | PXRD | [270] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sarabia-Vallejo, Á.; Caja, M.d.M.; Olives, A.I.; Martín, M.A.; Menéndez, J.C. Cyclodextrin Inclusion Complexes for Improved Drug Bioavailability and Activity: Synthetic and Analytical Aspects. Pharmaceutics 2023, 15, 2345. https://doi.org/10.3390/pharmaceutics15092345
Sarabia-Vallejo Á, Caja MdM, Olives AI, Martín MA, Menéndez JC. Cyclodextrin Inclusion Complexes for Improved Drug Bioavailability and Activity: Synthetic and Analytical Aspects. Pharmaceutics. 2023; 15(9):2345. https://doi.org/10.3390/pharmaceutics15092345
Chicago/Turabian StyleSarabia-Vallejo, Álvaro, María del Mar Caja, Ana I. Olives, M. Antonia Martín, and J. Carlos Menéndez. 2023. "Cyclodextrin Inclusion Complexes for Improved Drug Bioavailability and Activity: Synthetic and Analytical Aspects" Pharmaceutics 15, no. 9: 2345. https://doi.org/10.3390/pharmaceutics15092345
APA StyleSarabia-Vallejo, Á., Caja, M. d. M., Olives, A. I., Martín, M. A., & Menéndez, J. C. (2023). Cyclodextrin Inclusion Complexes for Improved Drug Bioavailability and Activity: Synthetic and Analytical Aspects. Pharmaceutics, 15(9), 2345. https://doi.org/10.3390/pharmaceutics15092345