Effect of Disinfection Method and Testing Methodology on the Performance of a Breath-Enhanced Jet Nebulizer
Abstract
:1. Introduction
2. Methods
2.1. Nebulizers and Sterilization
2.2. Study Design
2.3. Testing Procedure
2.4. Biochemical Determination
2.5. Outcome Variables
2.6. Statistical Analysis
3. Results
3.1. Quality Assurance
3.2. Solution Output
3.2.1. Evaluation without Breathing Simulation
3.2.2. Evaluation with Breathing Simulation
3.2.3. Comparisons
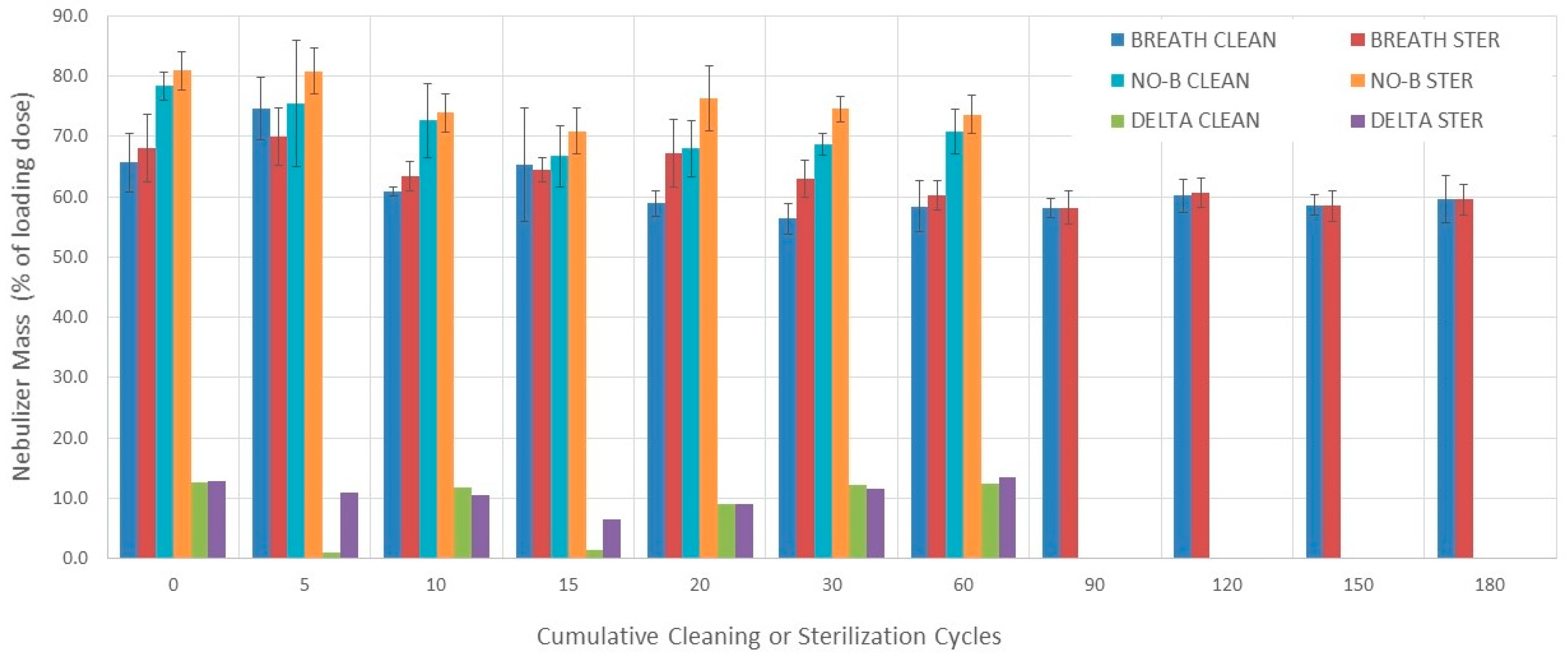
3.3. Nebulizers Mass
3.3.1. Evaluation without Breathing Simulation
3.3.2. Evaluation with Breathing Simulation
3.3.3. Comparisons
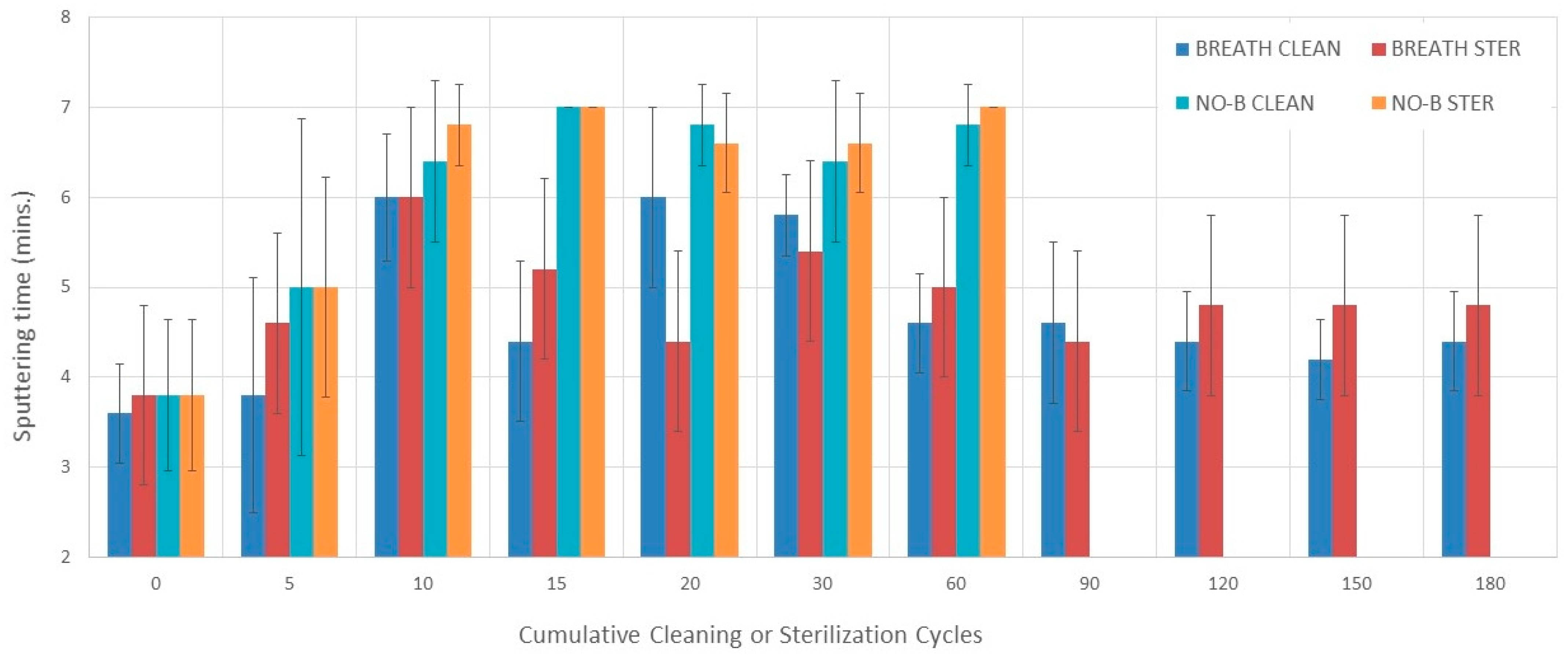
3.4. Sputtering Time
3.4.1. Evaluation without Breathing Simulation
3.4.2. Evaluation with Breathing Simulation
3.4.3. Comparisons
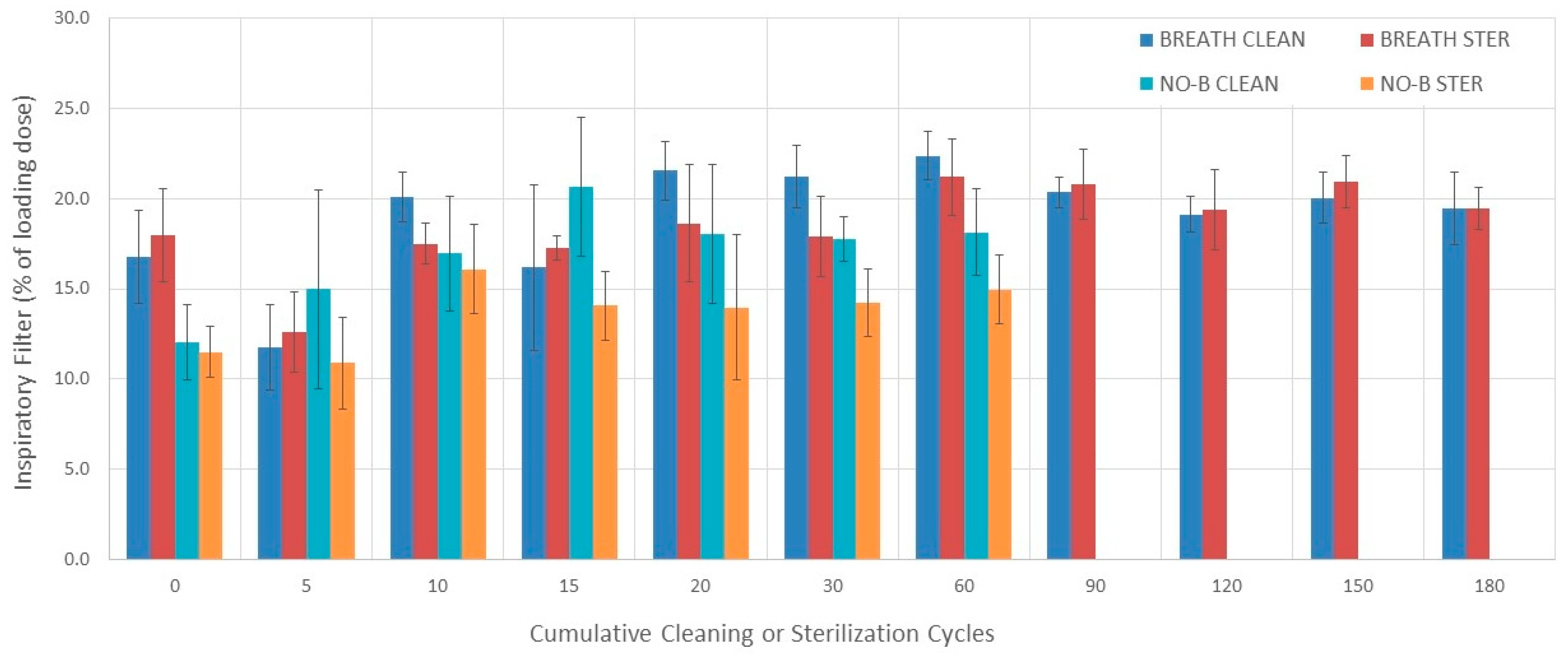
3.5. Inspiratory Filter
3.5.1. Evaluation without Breathing Simulation
3.5.2. Evaluation with Breathing Simulation
3.5.3. Comparisons
3.6. Expiratory Filter
3.6.1. Evaluation without Breathing Simulation
3.6.2. Evaluation with Breathing Simulation
3.6.3. Comparisons
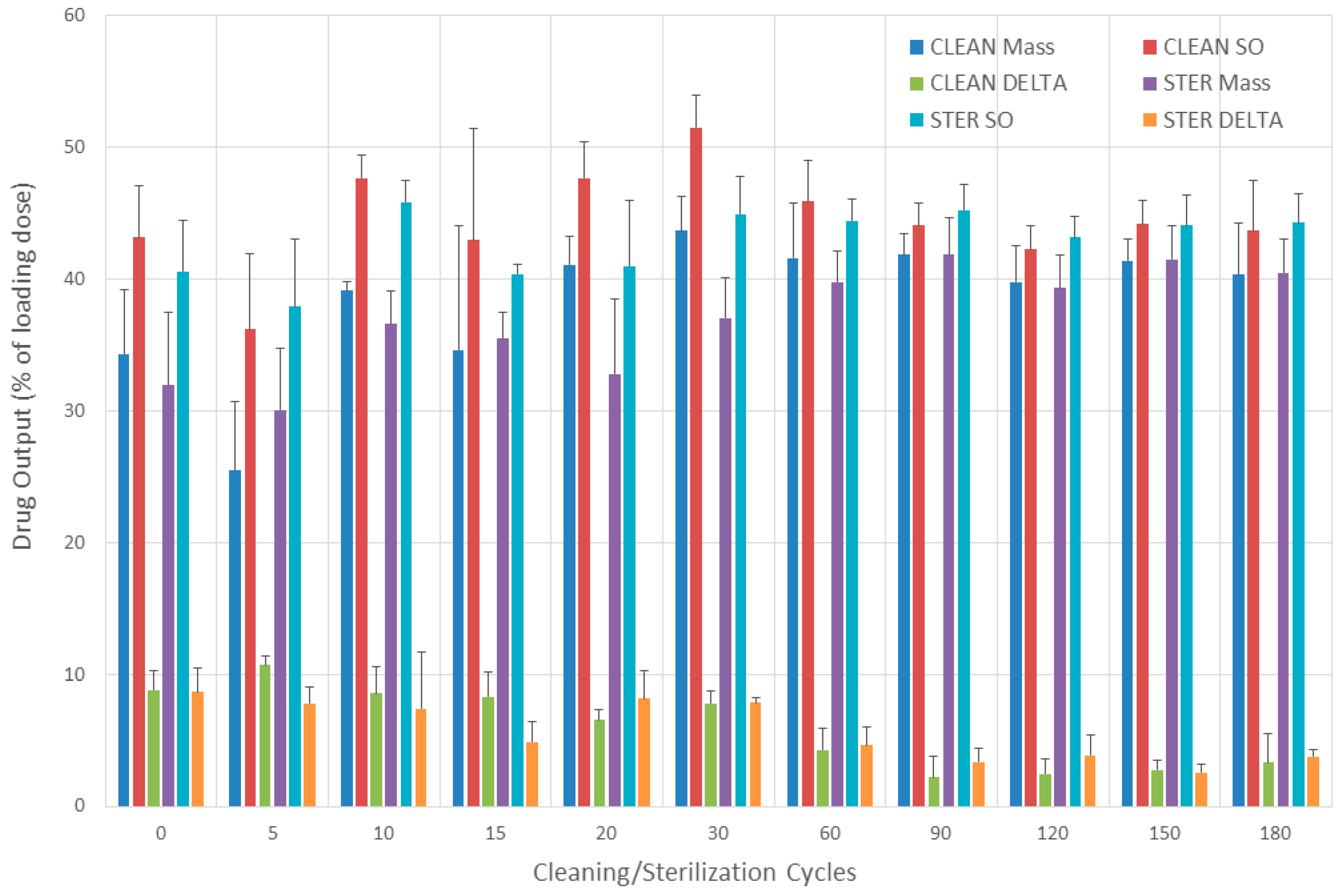
3.7. Drug Output
3.7.1. Evaluation without Breathing Simulation
3.7.2. Evaluation with Breathing Simulation
3.7.3. Comparisons
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Shteinberg, M.; Haq, I.J.; Polineni, D.; Davies, J.C. Cystic fibrosis. Lancet 2021, 397, 2195–2211. [Google Scholar] [CrossRef] [PubMed]
- Sanders, D.B.; Fink, A.K. Background and Epidemiology. Pediatr. Clin. N. Am. 2016, 63, 567–584. [Google Scholar] [CrossRef] [PubMed]
- Mogayzel, P.J.; Naureckas, E.T.; Robinson, K.A.; Mueller, G.; Hadjiliadis, D.; Hoag, J.B.; Lubsch, L.; Hazle, L.; Sabadosa, K.; Marshall, B. Pulmonary Clinical Practice Guidelines Committee. Cystic fibrosis pulmonary guidelines. Am. J. Respir. Crit. Care Med. 2013, 187, 680–689. [Google Scholar] [CrossRef] [PubMed]
- Blau, H.; Mussaffi, H.; Mei Zahav, M.; Prais, D.; Livne, M.; Czitron, B.M.; Cohen, H.A. Microbial contamination of nebulizers in the home treatment of cystic fibrosis. Child. Care Health Dev. 2007, 33, 491–495. [Google Scholar] [CrossRef] [PubMed]
- Vassal, S.; Taamma, R.; Marty, N.; Sardet, A.; D’athis, P.; Brémont, F.; Dalphin, M.L.; Plésiat, P.; Rault, G.; Thubert, J.; et al. Microbiologic contamination study of nebulizers after aerosol therapy in patients with cystic fibrosis. Am. J. Infect. Control 2000, 28, 347–351. [Google Scholar] [CrossRef] [PubMed]
- Riquena, B.; Monte, L.F.V.; Lopes, A.J.; Silva-Filho, L.V.R.F.D.; Damaceno, N.; Aquino, E.D.S.; Marostica, P.J.C.; Ribeiro, J.D. Microbiological contamination of nebulizers used by cystic fibrosis patients: An underestimated problem. J. Bras. Pneumol. 2019, 45, e20170351. [Google Scholar] [CrossRef] [PubMed]
- Weber, D.J.; Gergen, M.F.; Sickbert-Bennett, E.E.; Short, K.A.; Lanza-Kaduce, K.E.; Rutala, W.A. Frequency of contamination of single-patient-use nebulizers over time. Infect. Control Hosp. Epidemiol. 2014, 35, 1543–1546. [Google Scholar] [CrossRef]
- Brzezinski, L.X.; Riedi, C.A.; Kussek, P.; Souza, H.H.; Rosário, N. Nebulizers in cystic fibrosis: A source of bacterial contamination in cystic fibrosis patients? J. Bras. Pneumol. 2011, 37, 341–347. [Google Scholar] [CrossRef]
- Peckham, D.; Williams, K.; Wynne, S.; Denton, M.; Pollard, K.; Barton, R. Fungal contamination of nebuliser devices used by people with cystic fibrosis. J. Cyst. Fibros. 2016, 15, 74–77. [Google Scholar] [CrossRef]
- Denton, M.; Rajgopal, A.; Mooney, L.; Qureshi, A.; Kerr, K.G.; Keer, V.; Pollard, K.; Peckham, D.G.; Conway, S.P. Stenotrophomonas maltophilia contamination of nebulizers used to deliver aerosolized therapy to inpatients with cystic fibrosis. J. Hosp. Infect. 2003, 55, 180–183. [Google Scholar] [CrossRef]
- Pitchford, K.C.; Corey, M.; Highsmith, A.K.; Perlman, R.; Bannatyne, R.; Gold, R.; Levison, H.; Ford-Jones, E.L. Pseudomonas species contamination of cystic fibrosis patients’ home inhalation equipment. J. Pediatr. 1987, 111, 212–216. [Google Scholar] [CrossRef] [PubMed]
- Hutchinson, G.R.; Parker, S.; Pryor, J.A.; Duncan-Skingle, F.; Hoffman, P.N.; Hodson, M.E.; Kaufmann, M.E.; Pitt, T.L. Home-use nebulizers: A potential primary source of Burkholderia cepacia and other colistin-resistant, gram-negative bacteria in patients with cystic fibrosis. J. Clin. Microbiol. 1996, 34, 584–587, Erratum in J. Clin. Microbiol. 1996, 34, 1601. [Google Scholar] [CrossRef] [PubMed]
- Saiman, L.; Siegel, J.D.; LiPuma, J.J.; Brown, R.F.; Bryson, E.A.; Chambers, M.J.; Downer, V.S.; Fliege, J.; Hazle, L.A.; Jain, M.; et al. Infection prevention and control guideline for cystic fibrosis: 2013 update. Infect. Control Hosp. Epidemiol. 2014, 35 (Suppl. S1), S1–S67. [Google Scholar] [CrossRef] [PubMed]
- Available online: https://www.cff.org/managing-cf/nebulizer-care-home (accessed on 21 March 2023).
- Towle, D.; Callan, D.A.; Lamprea, C.; Murray, T.S. Baby bottle steam sterilizers for disinfecting home nebulizers inoculated with non-tuberculous mycobacteria. J. Hosp. Infect. 2016, 92, 222–225. [Google Scholar] [CrossRef] [PubMed]
- Towle, D.; Callan, D.A.; Farrel, P.A.; Egan, M.E.; Murray, T.S. Baby bottle steam sterilizers disinfect home nebulizers inoculated with bacterial respiratory pathogens. J. Cyst. Fibros. 2013, 12, 512–516. [Google Scholar] [CrossRef]
- Hohenwarter, K.; Prammer, W.; Aichinger, W.; Reychler, G. An evaluation of different steam disinfection protocols for cystic fibrosis nebulizers. J. Cyst. Fibros. 2016, 15, 78–84. [Google Scholar] [CrossRef]
- Murray, T.S.; O′Rourke, T.K., Jr.; Feinn, R.; Drapeau, G.; Collins, M.S. Nebulizer cleaning and disinfection practices in families with cystic fibrosis: The relationship between attitudes, practice and microbe colonization. J. Cyst. Fibros. 2019, 18, 823–828. [Google Scholar] [CrossRef]
- Lester, M.; Eidson, D.; Blair, S.; Gray, S.; Sapp, P.; Zupancic, F.J.; Marshall, B.C.; Berlinski, A. Cystic Fibrosis Foundation Nebulizer and Compressor Accessibility Survey. Respir. Care 2021, 66, 1840–1847. [Google Scholar] [CrossRef]
- Yilmaz Yegit, C.; Ergenekon, A.P.; Mursaloglu, H.H.; Cenk, M.; Uzunoglu, B.S.; Tastan, G.; Gokdemir, Y.; Erdem Eralp, E.; Karakoc, F.; Nasr, S.Z.; et al. The effects of nebulizer hygiene training on the practices of cystic fibrosis patients and caregivers. Pediatr. Pulmonol. 2021, 56, 1527–1533. [Google Scholar] [CrossRef]
- Lester, M.K.; Flume, P.A.; Gray, S.L.; Anderson, D.; Bowman, C.M. Nebulizer use and maintenance by cystic fibrosis patients: A survey study. Respir. Care 2004, 49, 1504–1508. [Google Scholar]
- O’Doherty, M.; O’Neill, D.; Rendall, J.C.; Moore, J.E.; Millar, B.C. Nebulizer Cleaning Practices and Adherence to Nebulized Therapies in People With Cystic Fibrosis When Traveling. Respir. Care 2022, 67, 1161–1167. [Google Scholar] [CrossRef] [PubMed]
- Berlinski, A. Aerosol therapy. In Foundations in Neonatal and Respiratory Care, 2nd ed.; Volsko, T.E., Barnhart, S.L., Eds.; Jones & Bartlett LearningL: Boston, MA, USA, 2022; pp. 451–472. [Google Scholar]
- Rau, J.L.; Ari, A.; Restrepo, R.D. Performance comparison of nebulizer designs: Constant-output, breath-enhanced, and dosimetric. Respir. Care 2004, 49, 174–179. [Google Scholar] [PubMed]
- Leung, K.; Louca, E.; Coates, A.L. Comparison of Breath-Enhanced to Breath-Actuated Nebulisers for Rate, Consistency, and Efficiency. Chest 2004, 126, 1619–1627. [Google Scholar] [CrossRef] [PubMed]
- Smith, A.L.; Ramsey, B.W.; Hedges, D.L.; Hack, B.; Williams-Warren, J.; Weber, A.; Gore, E.J.; Redding, G.J. Safety of aerosol tobramycin administration for 3 months to patients with cystic fibrosis. Pediatr. Pulmonol. 1989, 7, 265–271. [Google Scholar] [CrossRef] [PubMed]
- Berlinski, A.; Waldrep, J.C. Effect of aerosol delivery system and formulation on nebulized Budesonide output. J. Aerosol Med. 1997, 10, 307–318. [Google Scholar] [CrossRef]
- Pulmozyme. Highlights of Prescribing Information; Revised 07/2021; Genentech Inc.: San Francisco, CA, USA, 2021. [Google Scholar]
- TOBI. Highlights of Prescribing Information; Revised 12/2018; Novartis Pharmaceutical Corporation: East Hanover, NJ, USA, 2018. [Google Scholar]
- Pulmicort. Highlights of Prescribing Information; Revised 12/2018; AstraZeneca LP: Wilmington, DE, USA, 2018. [Google Scholar]
- Elkins, M.R.; Robinson, M.; Rose, B.R.; Harbour, C.; Moriarty, C.P.; Marks, G.B.; Belousova, E.G.; Xuan, W.; Bye, P.T.P. A controlled trial of long-term inhaled hypertonic saline in patients with cystic fibrosis. N. Engl. J. Med. 2006, 354, 229–240. [Google Scholar] [CrossRef] [PubMed]
- Awad, S.M.; Berlinski, A. Crossover Evaluation of Compressors and Nebulizers Typically Used by Cystic Fibrosis Patients. Respir. Care 2018, 63, 294–300. [Google Scholar] [CrossRef]
- Berlinski, A. In Vitro Comparison of Different Nebulizers Delivering 7% Hypertonic Saline. Respir. Care 2021, 66, 1582–1587. [Google Scholar] [CrossRef]
- Standaert, T.A.; Morlin, G.L.; Williams-Warren, J.; Joy, P.; Pepe, M.S.; Weber, A.; Ramsey, B.W. Effects of repetitive use and cleaning techniques of disposable jet nebulizers on aerosol generation. Chest 1998, 114, 577–586. [Google Scholar] [CrossRef]
- Collins, M.S.; O’Brien, M.; Schramm, C.M.; Murray, T.S. Repeated hot water and steam disinfection of Pari LC Plus® nebulizers alter nebulizer output. J. Cyst. Fibros. 2019, 18, 233–235. [Google Scholar] [CrossRef]
- O′Callaghan, C.; Clarke, A.R.; Milner, A.D. Inaccurate calculation of drug output from nebulisers. Eur. J. Pediatr. 1989, 148, 473–474. [Google Scholar] [CrossRef] [PubMed]
- Tandon, R.; McPeck, M.; Smaldone, G.C. Measuring nebulizer output. Aerosol production vs gravimetric analysis. Chest 1997, 111, 1361–1365. [Google Scholar] [CrossRef] [PubMed]
- Barry, P.W.; O′Callaghan, C. Drug output from nebulizers is dependent on the method of measurement. Eur. Respir. J. 1998, 12, 463–466. [Google Scholar] [CrossRef] [PubMed]
- ISO 27427:2013; Anaesthetic and Respiratory Equipment-Nebulizing Systems and Components, Third Edition. International Organization for Standardization ISO Central Secretariat: Geneva, Switzerland, 2013.
- Barry, P.W.; O′Callaghan, C. An in vitro analysis of the output of salbutamol from different nebulizers. Eur. Respir. J. 1999, 13, 1164–1169. [Google Scholar] [CrossRef] [PubMed]
- Awad, S.; Williams, D.K.; Berlinski, A. Longitudinal evaluation of compressor/nebulizer performance. Respir. Care 2014, 59, 1053–1061. [Google Scholar] [CrossRef]
- Dennis, J.H. Drug nebulizer design and performance: Breath enhanced jet vs constant output jet vs. ultrasonic. J. Aerosol Med. 1995, 8, 227–280. [Google Scholar] [CrossRef]
- Ho, S.L.; Kwong, W.T.; O’Drowsky, L.; Coates, A.L. Evaluation of four breath-enhanced nebulizers for home use. J. Aerosol Med. 2001, 14, 467–475. [Google Scholar] [CrossRef]
- Smaldone, G.C. Drug delivery by nebulization: “Reality testing”. J. Aerosol Med. 1994, 7, 213–216. [Google Scholar] [CrossRef]



| n | Cleaning/Disinfecting Cycles | 0 | 5 | 10 | 15 | 20 | 30 | 60 | 90 | 120 | 150 | 180 |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 5 | Cleaning-Breathing simulation | X | X | X | X | X | X | X | X | X | X | X |
| Disinfecting-Breathing simulation | X | X | X | X | X | X | X | X | X | X | X | |
| 5 | Cleaning-No breathing simulation | X | X | X | X | X | X | X | ||||
| Disinfecting-No breathing simulation | X | X | X | X | X | X | X |
| Variable | Solution Output (mL) | Nebulizer Mass (%) | Sputtering Time (s) | Inspiratory Filter (%) | Expiratory Filter (%) | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| Cycles | CLEAN | STER | CLEAN | STER | CLEAN | STER | CLEAN | STER | CLEAN | STER |
| 0 | 1.29 ± 0.12 | 1.22 ± 0.11 | 65.7 ± 4.9 | 68.1 ± 5.5 | 3.6 ± 0.5 | 3.8 ± 0.8 | 16.8 ± 2.6 | 18.0 ± 2.6 | 5.2 ± 1.1 | 6.5 ± 1.4 |
| 5 | 1.09 ± 0.17 | 1.14 ± 0.15 | 74.5 ± 5.2 | 70.0 ± 4.7 | 3.8 ± 1.3 | 4.6 ± 1.3 | 11.8 ± 2.4 | 12.6 ± 2.2 | 4.5 ± 0.8 | 5.4 ± 1.4 |
| 10 | 1.43 ± 0.05 | 1.37 ± 0.05 | 60.9 ± 0.7 | 63.4 ± 2.5 | 6.0 ± 0.7 | 6.0 ± 0.7 | 20.1 ± 1.4 | 17.5 ± 1.1 | 7.0 ± 1.1 | 7.0 ± 0.7 |
| 15 | 1.29 ± 0.25 | 1.21 ± 0.02 | 65.4 ± 9.4 | 64.5 ± 2.0 | 4.4 ± 0.9 | 5.2 ± 0.4 | 16.2 ± 4.6 | 17.2 ± 0.7 | 6.0 ± 2.1 | 6.0 ± 0.6 |
| 20 | 1.43 ± 0.08 | 1.23 ± 0.15 | 58.9 ± 2.1 | 67.2 ± 5.7 | 6.0 ± 1.0 | 4.4 ± 0.9 | 21.5 ± 1.7 | 18.6 ± 3.3 | 7.8 ± 1.2 | 7.4 ± 1.2 |
| 30 | 1.54 ± 0.08 | 1.35 ± 0.09 | 56.4 ± 2.6 | 63.0 ± 3.1 | 5.8 ± 0.4 | 5.4 ± 0.5 | 21.2 ± 1.7 | 17.9 ± 2.2 | 8.1 ± 1.5 | 6.8 ± 1.3 |
| 60 | 1.38 ± 0.09 | 1.33 ± 0.05 | 58.4 ± 4.2 | 60.3 ± 2.4 | 4.6 ± 0.5 | 5.0 ± 0.0 | 22.4 ± 1.4 | 21.2 ± 2.1 | 7.8 ± 1.6 | 8.2 ± 0.7 |
| 90 | 1.32 ± 0.05 | 1.36 ± 0.06 | 58.2 ± 1.6 | 58.2 ± 2.8 | 4.6 ± 0.9 | 4.4 ± 0.5 | 20.4 ± 0.9 | 20.8 ± 1.9 | 6.8 ± 1.4 | 6.4 ± 1.3 |
| 120 | 1.27 ± 0.05 | 1.30 ±.0.05 | 60.2 ± 2.7 | 60.7 ± 2.5 | 4.4 ± 0.5 | 4.8 ± 0.4 | 19.1 ± 1.0 | 19.4 ± 2.2 | 6.8 ± 1.0 | 6.1 ± 0.5 |
| 150 | 1.33 ± 0.05 | 1.32 ± 0.07 | 58.6 ± 1.7 | 58.5 ± 2.6 | 4.2 ± 0.4 | 4.8 ± 0.4 | 20.0 ± 1.4 | 20.9 ± 1.5 | 7.2 ± 1.1 | 6.7 ± 0.8 |
| 180 | 1.31 ± 0.11 | 1.33 ± 0.07 | 59.7 ± 3.9 | 59.5 ± 2.5 | 4.4 ± 0.5 | 4.8 ± 0.8 | 19.5 ± 2.0 | 19.4 ± 1.2 | 6.3 ± 1.1 | 6.6 ± 1.0 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Agoramurthi, K.; Berlinski, A. Effect of Disinfection Method and Testing Methodology on the Performance of a Breath-Enhanced Jet Nebulizer. Pharmaceutics 2024, 16, 16. https://doi.org/10.3390/pharmaceutics16010016
Agoramurthi K, Berlinski A. Effect of Disinfection Method and Testing Methodology on the Performance of a Breath-Enhanced Jet Nebulizer. Pharmaceutics. 2024; 16(1):16. https://doi.org/10.3390/pharmaceutics16010016
Chicago/Turabian StyleAgoramurthi, Kanjanamala, and Ariel Berlinski. 2024. "Effect of Disinfection Method and Testing Methodology on the Performance of a Breath-Enhanced Jet Nebulizer" Pharmaceutics 16, no. 1: 16. https://doi.org/10.3390/pharmaceutics16010016





