Mechanistic Insight in Permeability through Different Membranes in the Presence of Pharmaceutical Excipients: A Case of Model Hydrophobic Carbamazepine
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Determination of CBZ Equillibrium Solubility in F127 and SBE-β-CD Solutions
2.3. HPLC Analysis
2.4. Determination of CMC of F127 and CAC of SBE-β-CD in Buffer Solution pH 6.8
2.5. Estimation of the CBZ/F127 Association Constant, and the Stability Constant of CBZ/SBE-β-CD Complex
2.6. Free CBZ Fraction Estimation
2.7. Solubilizing Capacity Calculation
2.8. Light Scattering Measurements
2.9. Aggregation Number Determination
2.10. CBZ Partition Coefficients Determinations
2.11. Viscosity Measurements
2.12. Measurements of the CBZ In Vitro Permeability
3. Results and Discussion
3.1. CMC and CAC Measurements
3.2. Solubility Determinations
3.3. Solubilizing Capacity and Aggregation Behavior of F127 in the Presence of CBZ
3.4. Determination of the CBZ Partition Coefficients F127 Micelles/Buffer and SBE-β-CD/Buffer
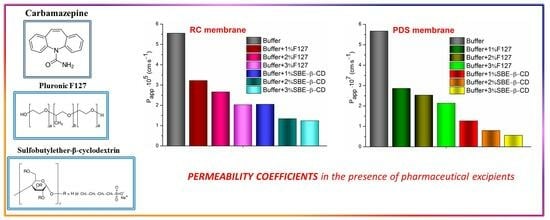
3.5. Determination of the Membrane Permeability Coefficients
3.6. Pratio Evaluation
R = 0.9999; F = 6336.62; n = 3
3.7. Disclosing the Permeability Variations Using a Quasi-Equilibrium Transport Model
3.8. Correction of Permeability Coefficients for the Free Drug Concentration
4. Conclusions
5. Future Perspectives
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| CMC | Critical micelle concentration |
| CAC | Critical aggregation concentration |
| Ka | Association constant |
| Kc | Stability constant |
| Ctotal | Total CBZ concentration |
| Cfree | Free drug concentration |
| Cmicellar | Micellar (F127) drug concentration |
| Ccomplex | Drug concentration in the CBZ/SBE-β-CD complex. |
| CF127 | Concentration of F127 |
| CSBE-β-CD | Concentration of SBE-β-CD |
| ffree | fraction of free CBZ molecules |
| Equilibrium CBZ solubility in the saturated solution of pure buffer | |
| CBZ equilibrium solubility in the presence of F127 or SBE-β-CD | |
| χ | Solubilizing capacity |
| Nagg | Aggregation number |
| Mw (micelle) | Molecular weight of F127 micelle |
| Mw (monomer) | Molecular weight of F127 monomer |
| Km/buf = KF127/buf | Micelle (F127)/buffer partition coefficient |
| KSBE-β-CD/buf | SBE-β-CD/buffer partition coefficient |
| RC | Regenerated cellulose membrane |
| PDS | Polydimethylsiloxane-polycarbonate membrane |
| J | Steady state flux through the membrane (µmol∙cm−2∙s−1) |
| Papp | Permeability coefficient (cm∙s−1) |
| Pratio | A ratio of Papp with the excipient to Papp with pure buffer solutions |
| Permeability coefficient calculated within a quasi-equilibrium mathematical mass transport model | |
| Absolute permeability coefficient for the free drug concentration | |
| Difference between the permeability coefficients in pure buffer and the corrected values |
References
- Fine-Shamir, N.; Beig, A.; Miller, J.M.; Dahan, A. The solubility, permeability and the dose as key factors in formulation development for oral lipophilic drugs: Maximizing the bioavailability of carbamazepine with a cosolvent-based formulation. Int. J. Pharm. 2020, 582, 119307. [Google Scholar] [CrossRef] [PubMed]
- Fasinu, P.; Pillay, V.; Ndesendo, V.M.K.; du Toit, L.C.; Choonara, Y.E. Diverse approaches for the enhancement of oral drug bioavailability. Biopharm. Drug Dispos. 2011, 32, 185–209. [Google Scholar] [CrossRef] [PubMed]
- Alqahtani, M.S.; Kazi, M.; Alsenaidy, M.A.; Ahmad, M.Z. Advances in oral drug delivery. Front. Pharmacol. 2021, 12, 618411. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Zhao, L.; Wu, B.; Chen, F. Improving solubility of poorly water-soluble drugs by protein-based strategy: A review. Int. J. Pharm. 2023, 634, 122704. [Google Scholar] [CrossRef] [PubMed]
- Pandey, N.; Ghosh, A. An outlook on permeability escalation through cocrystallization for developing pharmaceuticals with improved biopharmaceutical properties. J. Drug Deliv. Sci. Tech. 2022, 76, 103757. [Google Scholar] [CrossRef]
- Gopi, S.; Amalraj, A. Effective drug delivery system of biopolymers based on nanomaterials and hydrogels—A review. Drug Des. Open Access 2016, 5, 129. [Google Scholar] [CrossRef]
- Lu, Y.; Park, K. Polymeric micelles and alternative nanonized delivery vehicles for poorly soluble drugs. Int. J. Pharm. 2013, 453, 198–214. [Google Scholar] [CrossRef]
- Semalty, A. Cyclodextrin and phospholipid complexation in solubility and dissolution enhancement: A critical and meta-analysis. Expert Opin. Drug Deliv. 2014, 11, 1255–1272. [Google Scholar] [CrossRef]
- Elder, D.P.; Holm, R.; de Diego, H.L. Use of pharmaceutical salts and cocrystals to address the issue of poor solubility. Int. J. Pharm. 2013, 453, 88–100. [Google Scholar] [CrossRef]
- Hussain, A.; Singh, S.; Sharma, D.; Webster, T.J.; Shafaat, K.; Faruk, A. Elastic liposomes as novel carriers: Recent advances in drug delivery. Int. J. Nanomed. 2017, 12, 5087–5108. [Google Scholar] [CrossRef]
- Miller, J.M.; Dahan, A. Predicting the solubility–permeability interplay when using cyclodextrins in solubility-enabling formulations: Model validation. Int. J. Pharm. 2012, 430, 388–391. [Google Scholar] [CrossRef]
- Miller, J.M.; Beig, A.; Carr, R.A.; Webster, G.K.; Dahan, A. The solubility–permeability interplay when using cosolvents for solubilization: Revising the way we use solubility-enabling formulations. Mol. Pharm. 2012, 9, 581–590. [Google Scholar] [CrossRef]
- Volkova, T.V.; Drozd, K.V.; Surov, A.O. Effect of polymers and cyclodextrins on solubility, permeability and distribution of enzalutamide and apalutamide antiandrogens. J. Mol. Liq. 2020, 322, 114937. [Google Scholar] [CrossRef]
- Desai, D.; Wong, B.; Huang, Y.; Ye, Q.; Tang, D.; Guo, H.; Huang, M.; Timmins, P. Surfactant-mediated dissolution of metformin hydrochloride tablets: Wetting effects versus ion pairs diffusivity. J. Pharm. Sci. 2014, 103, 920–926. [Google Scholar] [CrossRef] [PubMed]
- Loftsson, T.; Vogensen, S.B.; Brewster, M.E.; Konráðsdóttir, F. Effects of cyclodextrins on drug delivery through biological membranes. J. Pharm Sci. 2007, 96, 2532–2546. [Google Scholar] [CrossRef] [PubMed]
- Katneni, K.; Charman, S.A.; Porter, C.J. Permeability assessment of poorly water-soluble compounds under solubilizing conditions: The reciprocal permeability approach. J. Pharm Sci. 2006, 95, 2170–2185. [Google Scholar] [CrossRef] [PubMed]
- di Cagno, M.; Luppi, B. Drug “supersaturation” states induced by polymeric micelles and liposomes: A mechanistic investigation into permeability enhancements. Eur. J. Pharm. Sci. 2013, 48, 775–780. [Google Scholar] [CrossRef] [PubMed]
- Tzanova, M.M.; Nguyen, L.; Moretti, F.; Grassi, M.; Magnano, G.C.; Voinovich, D.; Stein, P.C.; Hiorth, M.; di Cagno, M.P. Interpreting permeability as a function of free drug fraction: The case studies of cyclodextrins and liposomes. Eur. J. Pharm. Sci. 2023, 189, 106559. [Google Scholar] [CrossRef] [PubMed]
- Tzanova, M.M.; Moretti, F.; Grassi, G.; Stein, P.C.; Hiorth, M.; Abrami, M.; Grassi, M.; di Cagno, M.P. Modelling drug diffusion through unstirred water layers allows real-time quantification of free/loaded drug fractions and release kinetics from colloidal-based formulations. Eur. J. Pharm. Biopharm. 2022, 178, 168–178. [Google Scholar] [CrossRef] [PubMed]
- Eriksen, J.B.; Christensen, S.B.; Bauer-Brandl, A.; Brandl, M. Dissolution/permeation of albendazole in the presence of cyclodextrin and bile salts: A mechanistic in-vitro study into factors governing oral bioavailability. J. Pharm. Sci. 2022, 111, 1667–1673. [Google Scholar] [CrossRef]
- Buckley, S.T.; Frank, K.J.; Fricker, G.; Brandl, M. Biopharmaceutical classification of poorly soluble drugs with respect to "enabling formulations". Eur. J. Pharm. Sci. 2013, 50, 8–16. [Google Scholar] [CrossRef] [PubMed]
- Berben, P.; Bauer-Brandl, A.; Brandl, M.; Faller, B.; Flaten, G.E.; Jacobsen, A.-C.; Brouwers, J.; Augustijns, P. Drug permeability profiling using cell-free permeation tools: Overview and applications. Eur. J. Pharm. Sci. 2018, 119, 219–233. [Google Scholar] [CrossRef] [PubMed]
- Feldstein, M.M.; Raigorodskii, I.M.; Iordanskii, A.L.; Hadgraft, J. Modeling of percutaneous drug transport in vitro using skin-imitating Carbosil membrane. J. Control Release 1998, 52, 25–40. [Google Scholar] [CrossRef] [PubMed]
- Wu, I.Y.; Bala, S.; Škalko-Basnet, N.; di Cagno, M.P. Interpreting non-linear drug diffusion data: Utilizing Korsmeyer-Peppas model to study drug release from liposomes. Eur. J. Pharm. Sci. 2019, 138, 105026. [Google Scholar] [CrossRef] [PubMed]
- Liu, W.; Dang, L.; Black, S.; Wei, H. Solubility of carbamazepine (form III) in different solvents from (275 to 343) K. J. Chem. Eng. Data 2008, 53, 2204–2206. [Google Scholar] [CrossRef]
- Rustichelli, C.; Gamberini, G.; Ferioli, V.; Gamberini, M.C.; Ficarra, R.; Tommasini, S. Solid-state study of polymorphic drugs: Carbamazepine. J. Pharm. Biomed. Anal. 2000, 23, 41–54. [Google Scholar] [CrossRef] [PubMed]
- Stella, V.J.; Rajewski, R.A. Sulfobutylether-β-cyclodextrin. Int. J. Pharm. 2020, 583, 119396. [Google Scholar] [CrossRef]
- Brewster, M.E.; Vandecruys, R.; Verreck, G.; Peeters, J. Supersaturating drug delivery systems: Effect of hydrophilic cyclodextrins and other excipients on the formation and stabilization of supersaturated drug solutions. Pharmazie 2008, 63, 217–220. [Google Scholar] [CrossRef]
- Raval, A.; Pillai, S.A.; Bahadur, A.; Bahadur, P. Systematic characterization of Pluronic® micelles and their application for solubilization and in vitro release of some hydrophobic anticancer drugs. J. Mol. Liq. 2017, 230, 473–481. [Google Scholar] [CrossRef]
- Sahu, A.; Kasoju, N.; Goswami, P.; Bora, U. Encapsulation of curcumin in Pluronic block copolymer micelles for drug delivery applications. J. Biomater. Appl. 2011, 25, 619–639. [Google Scholar] [CrossRef]
- Volkova, T.; Kumeev, R.; Kochkina, N.; Terekhova, I. Impact of Pluronics of different structure on pharmacologically relevant properties of sulfasalazine and methotrexate. J. Mol. Liq. 2019, 289, 111076. [Google Scholar] [CrossRef]
- Higuchi, T.; Connors, K. Phase-solubility techniques. Adv. Anal. Chem. Instrum. 1965, 4, 117–123. [Google Scholar]
- Avdeef, A.; Fuguet, E.; Llinàs, A.; Ràfols, C.; Bosch, E.; Völgyi, G.; Verbić, T.; Boldyreva, E.; Takács-Novák, K. Equilibrium solubility measurement of ionizable drugs—Consensus recommendations for improving data quality. ADMET DMPK 2016, 4, 117–178. [Google Scholar] [CrossRef]
- Tan, C.H.; Huang, Z.J.; Huang, X.G. Rapid determination of surfactant critical micelle concentration in aqueous solutions using fiber-optic refractive index sensing. Anal. Biochem. 2010, 401, 144–147. [Google Scholar] [CrossRef] [PubMed]
- Stephenson, B.C.; Rangel-Yagui, C.O.; Junior, A.P.; Tavares, L.C.; Beers, K.; Blankschtein, D. Experimental and theoretical investigation of the micellar-assisted solubilization of ibuprofen in aqueous media. Langmuir 2006, 22, 1514–1525. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Z.; Chu, B. Light-scattering study on the association behavior of triblock polymers of ethylene oxide and propylene oxide in aqueous solution. J. Colloid Interface Sci. 1988, 126, 171–180. [Google Scholar] [CrossRef]
- Nolan, S.L.; Phillips, R.J.; Cotts, P.M.; Dungan, S.R. Light scattering study on the effect of polymer composition on the structural properties of PEO–PPO–PEO micelles. J. Colloid Interface Sci. 1997, 191, 291–302. [Google Scholar] [CrossRef] [PubMed]
- Wan, D.-H.; Zheng, O.; Zhow, Y.; Wu, L.-Y. Solubilization of ibuprofen in pluronic block copolymer F127 micelles. Acta Phys.-Chim. Sin. 2010, 26, 3243–3248. [Google Scholar] [CrossRef]
- Wang, X.; Brusseau, M.L. Solubilization of some low-polarity organic compounds by hydroxypropyl-β-cyclodextrin. Environ. Sci. Technol. 1993, 27, 2821–2825. [Google Scholar] [CrossRef]
- Volkova, T.V.; Simonova, O.R.; Perlovich, G.L. New antifungal compound: Impact of cosolvency, micellization and complexation on solubility and permeability processes. Pharmaceutics 2021, 13, 1865. [Google Scholar] [CrossRef]
- Do, T.T.; Van Hooghten, R.; Van den Mooter, G. A study of the aggregation of cyclodextrins: Determination of the critical aggregation concentration, size of aggregates and thermodynamics using isodesmic and K2–K models. Int. J. Pharm. 2017, 521, 318–326. [Google Scholar] [CrossRef] [PubMed]
- Loftsson, T.; Masson, M.; Sigurdsson, H.H. Cyclodextrins and drug permeability through semi-permeable cellophane membranes. Int. J. Pharm. 2002, 232, 35–43. [Google Scholar] [CrossRef] [PubMed]
- Loftsson, T.; Sigurdsson, H.H.; Jansook, P. Anomalous properties of cyclodextrins and their complexes in aqueous solutions. Materials 2023, 16, 2223. [Google Scholar] [CrossRef] [PubMed]
- Sharma, P.K.; Reilly, M.J.; Jones, D.N.; Robinson, P.M.; Bhatia, S.R. The effect of pharmaceuticals on the nanoscale structure of PEO–PPO–PEO micelles. Colloids Surf. B 2008, 61, 53–60. [Google Scholar] [CrossRef] [PubMed]
- Alexandridis, P.; Holzwarth, J.F.; Hatton, T.A. Micellization of poly(ethy1ene oxide)-poly(propy1ene oxide)-poly(ethy1ene oxide) triblock copolymers in aqueous aolutions: Thermodynamics of copolymer association. Macromolecules 1994, 27, 2414–2425. [Google Scholar] [CrossRef]
- Leonties, A.R.; Stingă, G.; Ilie, C.; Anghel, D.F. Physicochemical studies on ketoprofen encapsulated in pluronic F127 nanomicelles for drug applications. Rev. Roum. Chim. 2017, 62, 657–662. [Google Scholar]
- Kadam, Y.; Yerramilli, U.; Bahadur, A. Solubilization of poorly water-soluble drug carbamezapine in Pluronic® micelles: Effect of molecular characteristics, temperature and added salt on the solubilizing capacity. Colloids Surf. B 2009, 72, 141–147. [Google Scholar] [CrossRef] [PubMed]
- Jain, A.S.; Date, A.A.; Pissurlenkar, R.R.S.; Coutinho, E.C.; Nagarsenker, M.S. Sulfobutyl ether7β-cyclodextrin (SBE7β-CD) carbamazepine complex: Preparation, characterization, molecular modeling, and evaluation of in vivo anti-epileptic activity. AAPS PharmSciTech. 2011, 12, 1163–1175. [Google Scholar] [CrossRef]
- Smith, J.S.; MacRae, R.J.; Snowden, M.J. Effect of SBE7-β-cyclodextrin complexation on carbamazepine release from sustained release beads. Eur. J. Pharm. Biopharm. 2005, 60, 80. [Google Scholar] [CrossRef]
- Attwood, D.; Collett, J.H.; Tait, C.J. The micellar properties of the poly(oxyethylene)–poly(oxypropylene) copolymer pluronic F127 in water and electrolyte solution. Int. J. Pharm. 1985, 26, 25–33. [Google Scholar] [CrossRef]
- Sharma, P.K.; Bhatia, S.R. Effect of anti-inflammatories on Pluronic F127: Micellar assembly, gelation and partitioning. Int. J. Pharm. 2004, 278, 361–377. [Google Scholar] [CrossRef] [PubMed]
- Singla, P.; Singh, O.; Sharma, S.; Betlem, K.; Aswal, V.K.; Peeters, M.; Mahajan, R.K. Temperature-dependent solubilization of the hydrophobic antiepileptic drug lamotrigine in different pluronic micelles—A spectroscopic, heat transfer method, small-angle neutron scattering, dynamic light scattering, and in vitro release study. ACS Omega 2019, 4, 11251–11262. [Google Scholar] [CrossRef] [PubMed]
- Wanka, G.; Hoffmann, H.; Ulbricht, W. Phase diagrams and aggregation behavior of poly(oxyethylene)-poly(oxypropylene)-poly(oxyethylene) triblock copolymers in aqueous solutions. Macromolecules 1994, 27, 4145–4159. [Google Scholar] [CrossRef]
- Shayanfar, A.; Soltani, S.; Jouyban, A. Prediction of blood-brain distribution: Effect of ionization. Biol. Pharm. Bull. 2011, 34, 266–271. [Google Scholar] [CrossRef]
- Dahan, A.; Miller, J.M.; Hoffman, A.; Amidon, G.E.; Amidon, G.L. The solubility-permeability interplay in using cyclodextrins as pharmaceutical solubilizers: Mechanistic modeling and application to progesterone. J. Pharm. Sci. 2010, 99, 2739–2749. [Google Scholar] [CrossRef]
- Beig, A.; Agbaria, R.; Dahan, A. Oral delivery of lipophilic drugs: The tradeoff between solubility increase and permeability decrease when using cyclodextrin-based formulations. PLoS ONE. 2013, 8, e68237. [Google Scholar] [CrossRef]
- Beig, A.; Miller, J.M.; Dahan, A. Accounting for the solubility–permeability interplay in oral formulation development for poor water solubility drugs: The effect of PEG-400 on carbamazepine absorption. Eur. J. Pharm. Biopharm. 2012, 81, 386–391. [Google Scholar] [CrossRef]
- Brewster, M.E.; Noppe, M.; Peeters, J.; Loftsson, T. Effect of the unstirred water layer on permeability enhancement by hydrophilic cyclodextrins. Int. J. Pharm. 2007, 342, 250–253. [Google Scholar] [CrossRef]
- Cho, M.J.; Chen, F.J.; Huczek, D.L. Effects of inclusion complexation on the transepithelial transport of a lipophilic substance in vitro. Pharm. Res. 1995, 12, 560–564. [Google Scholar] [CrossRef]
- Kerns, E.H.; Di, L. Druglike Properties: Concepts, Structure Design and Methods. Academic Press: New York, NY, USA, 2008. [Google Scholar]
- Yano, K.; Masaoka, Y.; Kataoka, M.; Sakuma, S.; Yamashita, S. Mechanisms of membrane transport of poorly soluble drugs: Role of micelles in oral absorption processes. J. Pharm. Sci. 2010, 99, 1336–1345. [Google Scholar] [CrossRef] [PubMed]
- Waters, L.J.; Dennisa, L.; Bibi, A.; Mitchell, J.C. Surfactant and temperature effects on paraben transport through silicone membranes. Colloids Surf. B 2013, 108, 23–28. [Google Scholar] [CrossRef] [PubMed]
- Fischer, S.M.; Brandl, M.; Fricker, G. Effect of the non-ionic surfactant Poloxamer 188 on passive permeability of poorly soluble drugs across Caco-2 cell monolayers. Eur. J. Pharm. Biopharm. 2011, 79, 416–422. [Google Scholar] [CrossRef] [PubMed]
- Poelma, F.G.J.; Breäs, R.; Tukker, J.J.; Crommelin, D.J.A. Intestinal absorption of drugs. The influence of mixed micelles on the disappearance kinetics of drugs from the small intestine of the rat. J. Pharm. Pharmacol. 1991, 43, 317–324. [Google Scholar] [CrossRef] [PubMed]





| Conditions | CMC (F127) (mol·L−1) | CAC (SBE-β-CD) (mol·L−1) |
|---|---|---|
| in the absence of CBZ | 1.640 (±0.066)∙10−4 | 1.716 (±0.069)∙10−2 |
| CCBZ = 4.06∙10−4 mol·L−1 | 1.321 (±0.053)∙10−4 | 1.724 (±0.069)∙10−2 |
| Cexipient (mmol·L−1) | Permeability Results | |||||
|---|---|---|---|---|---|---|
| Papp (cm∙s−1) | 1 | (cm·s−1) 2 | (mol·L−1) 3 | (cm·s−1) 4 | 5 | |
| RC | ||||||
| 0 | (5.55 ± 0.15) × 10−5 | - | - | - | (5.55 ± 0.15) × 10−5 | - |
| F127 | ||||||
| 1.33 | (3.22 ± 0.16) × 10−5 | 0.585 | 3.46 × 10−5 | 4.73 × 10−4 | 4.99 × 10−5 | 0.56 × 10−5 |
| 1.83 | (2.66 ± 0.08) × 10−5 | 0.484 | 2.52 × 10−5 | 3.77 × 10−4 | 5.86 × 10−5 | −0.31 × 10−5 |
| 2.30 | (2.03 ± 0.07) × 10−5 | 0.369 | 2.00 × 10−5 | 4.73 × 10−4 | 5.82 × 10−5 | −0.27 × 10−5 |
| SBE-β-CD | ||||||
| 6.89 | (2.05 ± 0.08) × 10−5 | 0.373 | 1.81 × 10−5 | 4.46 × 10−4 | 7.15 × 10−5 | −1.6 × 10−5 |
| 13.78 | (1.34 ± 0.07) × 10−5 | 0.244 | 1.09 × 10−5 | 3.88 × 10−4 | 7.96 × 10−5 | −2.41 × 10−5 |
| 20.67 | (1.24 ± 0.06) × 10−5 | 0.225 | 7.74 × 10−6 | 5.59 × 10−4 | 1.05 × 10−4 | −4.95 × 10−5 |
| PDS | ||||||
| 0 | (5.67 ± 0.17) × 10−7 | - | - | - | (5.67 ± 0.17) × 10−7 | |
| F127 | ||||||
| 1.33 | (2.86 ± 0.16) × 10−7 | 0.504 | 3.54 × 10−7 | 4.64 × 10−4 | 4.41 × 10−7 | 1.26 × 10−7 |
| 1.83 | (2.53 ± 0.09) × 10−7 | 0.446 | 2.57 × 10−7 | 3.78 × 10−4 | 5.56 × 10−7 | 0.11 × 10−7 |
| 2.30 | (2.14 ±0.07) × 10−7 | 0.377 | 2.05 × 10−7 | 3.12 × 10−4 | 6.12 × 10−7 | −0.45 × 10−7 |
| SBE-β-CD | ||||||
| 6.89 | (1.27 ± 0.08) × 10−7 | 0.224 | 1.85 × 10−7 | 4.40 × 10−4 | 4.66 × 10−7 | 1.01 × 10−7 |
| 13.78 | (7.96 ± 0.20) × 10−8 | 0.140 | 1.11 × 10−7 | 5.14 × 10−4 | 4.72 × 10−7 | 0.95 × 10−7 |
| 20.67 | (5.65 ± 0.15) × 10−8 | 0.100 | 7.91 × 10−8 | 5.00 × 10−4 | 4.76 × 10−7 | 0.91 × 10−7 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Volkova, T.; Simonova, O.; Perlovich, G. Mechanistic Insight in Permeability through Different Membranes in the Presence of Pharmaceutical Excipients: A Case of Model Hydrophobic Carbamazepine. Pharmaceutics 2024, 16, 184. https://doi.org/10.3390/pharmaceutics16020184
Volkova T, Simonova O, Perlovich G. Mechanistic Insight in Permeability through Different Membranes in the Presence of Pharmaceutical Excipients: A Case of Model Hydrophobic Carbamazepine. Pharmaceutics. 2024; 16(2):184. https://doi.org/10.3390/pharmaceutics16020184
Chicago/Turabian StyleVolkova, Tatyana, Olga Simonova, and German Perlovich. 2024. "Mechanistic Insight in Permeability through Different Membranes in the Presence of Pharmaceutical Excipients: A Case of Model Hydrophobic Carbamazepine" Pharmaceutics 16, no. 2: 184. https://doi.org/10.3390/pharmaceutics16020184