Unveiling the Potential of Cannabinoids in Multiple Sclerosis and the Dawn of Nano-Cannabinoid Medicine
Abstract
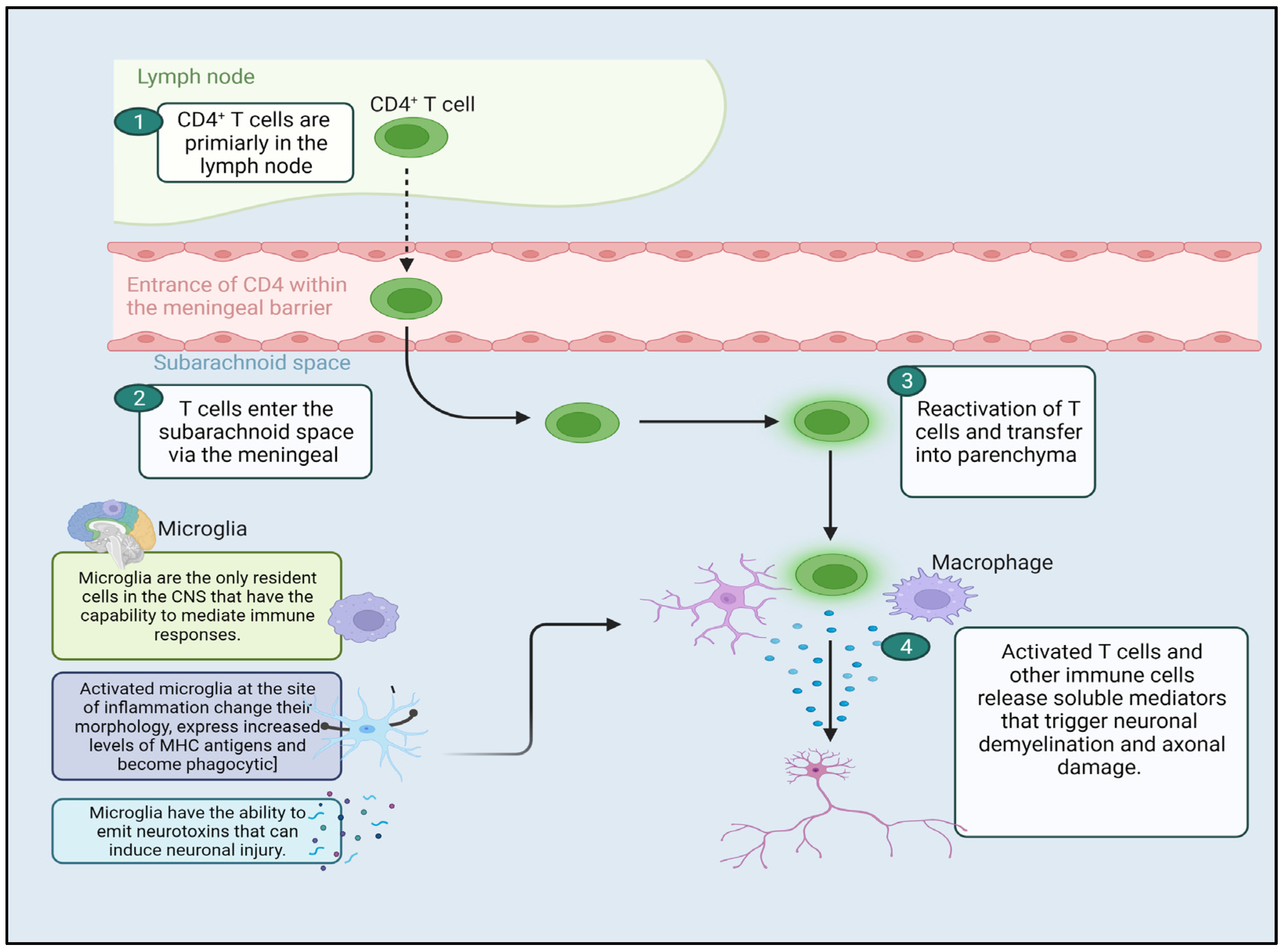
:1. Introduction
2. Treatment Modalities for MS Management
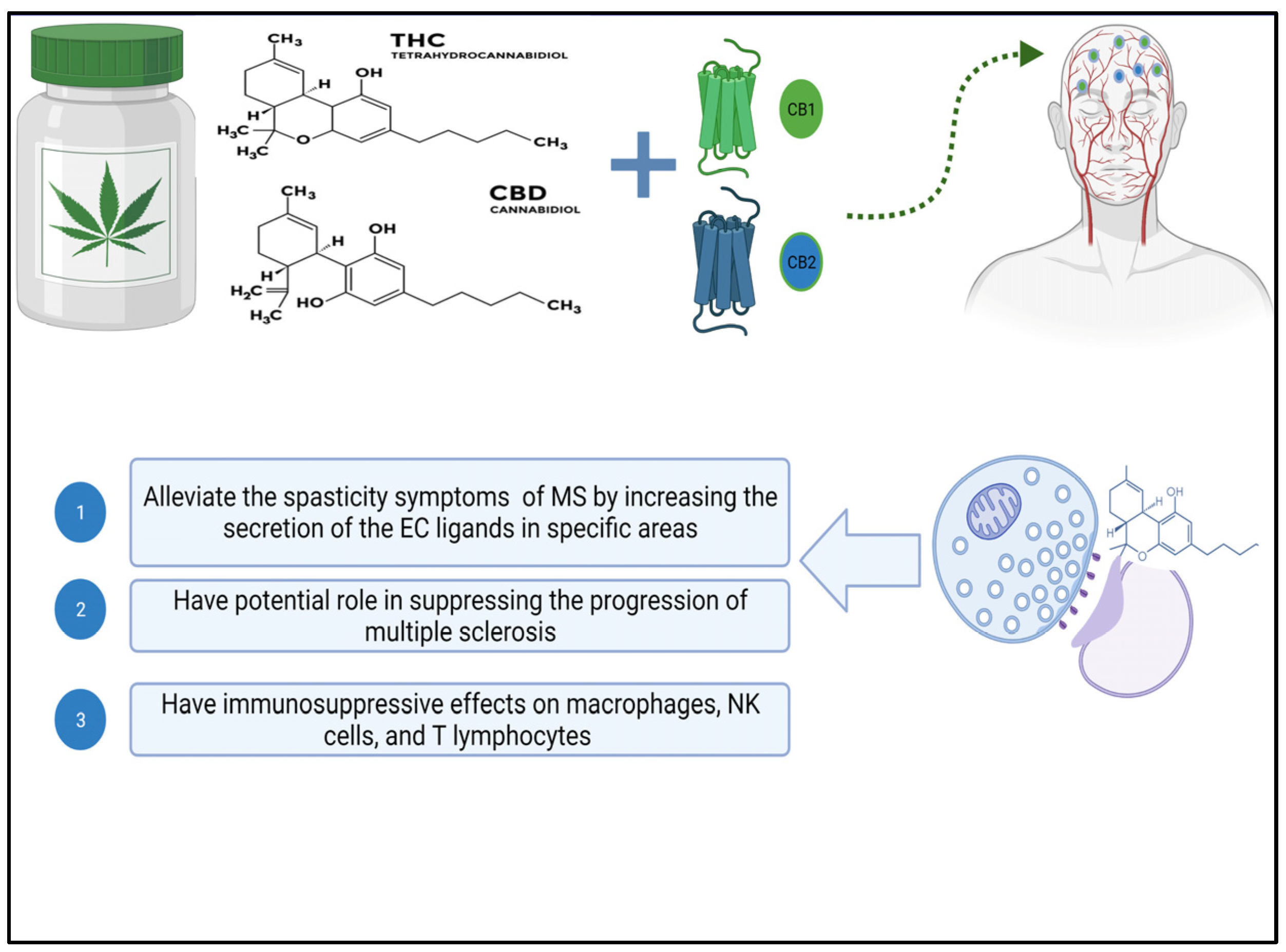
3. Cannabinoids and the Endocannabinoid System (ECS)
3.1. Cannabinoids
3.2. The Endocannabinoid System (ECS)
4. Neuroprotection Effect of Cannabinoids
5. Immunomodulatory Effect of Cannabinoids
6. Therapeutic Potential of Cannabinoids
7. Commercial Therapeutic Cannabinoids in Use
| Drug Name | FDA Approval | Active Constituents | Indication | Ref. |
|---|---|---|---|---|
| Epidiolex | 2018 | Purified CBD formulation. | Treatment of seizures associated with Lennox–Gastaut syndrome (LGS), Dravet syndrome, or tuberous sclerosis complex (TSC). | [87] |
| Nabiximols | No | Combination of CBD (cannabidiol) and THC (delta-9-tetrahydrocannabinol). | Management of spasticity associated with MS. | [88] |
| Ajulemic Acid | No | Synthetic THC-11-oic acid analogue. | Management of chronic neuropathic pain by selectively binding to CB2 receptor. | [89] |
| Sativex | No | 1:1 ratio of Δ-9-tetrahydrocannabinol (THC) and cannabidiol. | Management of spasticity in MS patients. | [90] |
| Cesamet | 1985 | Derivative of Δ-9-tetrahydrocannabinol. | Treatment of chemotherapy-induced nausea with cancer patients. | [91] |
| Marinol | 1985 | Synthetic THC analogue | Management of chemotherapy-induced nausea and vomiting. Treatment of anorexia associated with immune deficiency patients. | [92] |
| Nabiximols | No | Combination of CBD and THC. | Pain management of cancer and MS patients. | [93] |
| Dronabinol | 1985 | Synthetic delta-9-tetrahydrocannabinol. | Management of neuropathic pain. | [94] |
| Nabilone | 1985 | Synthetic THC | Management of Parkinson’s disease and chemotherapy-induced nausea and vomiting. | [95] |
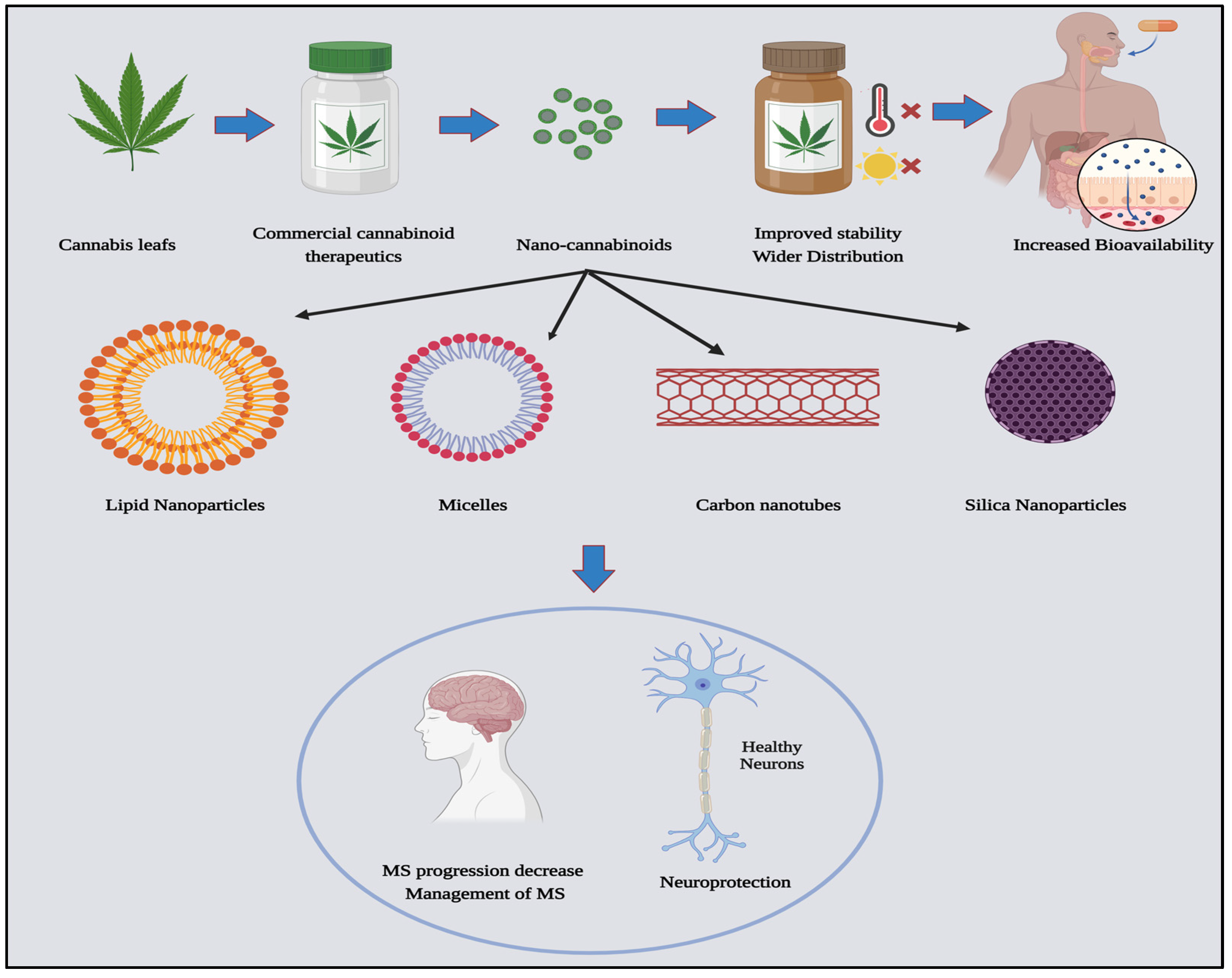
8. Nanomedicine and Cannabinoids for MS Treatment
8.1. Enhancing Drug Stability and Solubility through Nanomedicine
8.2. Mechanism of Nano-Cannabinoids Evading the Blood-Brain Barrier
8.3. Nano-Cannabinoids: Challenges and Potentials
9. Concluding Remarks and Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Rouleau, I.; Dagenais, E.; Tremblay, A.; Demers, M.; Roger, É.; Jobin, C.; Duquette, P. Prospective memory impairment in multiple sclerosis: A review. Prospect. Mem. Clin. Popul. 2017, 32, 922–936. [Google Scholar] [CrossRef]
- Gerhard, L.; Dorstyn, D.S.; Murphy, G.; Roberts, R.M. Neurological, physical and sociodemographic correlates of employment in multiple sclerosis: A meta-analysis. J. Health Psychol. 2020, 25, 92–104. [Google Scholar] [CrossRef]
- Dutta, R.; Trapp, B.D. Pathogenesis of axonal and neuronal damage in multiple sclerosis. Neurology 2007, 68 (Suppl. 3), S22–S31. [Google Scholar] [CrossRef]
- Haddad, F.; Dokmak, G.; Karaman, R. The Efficacy of Cannabis on Multiple Sclerosis-Related Symptoms. Life 2022, 12, 682. [Google Scholar] [CrossRef] [PubMed]
- Popescu, B.F.G.; Pirko, I.; Lucchinetti, C.F. Pathology of Multiple Sclerosis: Where Do We Stand? Contin. Learn. Neurol. 2013, 19, 901. [Google Scholar] [CrossRef] [PubMed]
- Milo, R.; Miller, A. Revised diagnostic criteria of multiple sclerosis. Autoimmun. Rev. 2014, 13, 518–524. [Google Scholar] [CrossRef]
- Coles, A.J.; Compston, D.A.S.; Selma, K.W.; Lake, S.L.; Moran, S.; Margolin, D.H.; Norris, K.; Tandon, P.K. Alemtuzumab vs. interferon beta-1a in early multiple sclerosis. N. Engl. J. Med. 2008, 359, 1786–1801. [Google Scholar] [PubMed]
- Messam, C.A.; Hou, J.; Janabi, N.; Monaco, M.C.; Gravell, M.; Major, E.O. Glial Cell Types; Academic Press: New York, NY, USA, 2002; pp. 369–387. [Google Scholar] [CrossRef]
- Kumar, M.R.P.; Vijayalakshmi, C.; Ramanathan, M. Isoflavones as Nutraceuticals in Stroke: Therapeutic Targets and Signaling Pathways. In Preedy Nutraceuticals, Supplements, and Herbal Medicine in Neurological Disorders; Martin, C.R., Patel, V.B., Preedy, V.R., Eds.; Academic Press: New York, NY, USA, 2023; Chapter 50; pp. 959–978. [Google Scholar] [CrossRef]
- McAteer, M.A.; Choudhury, R.P. Applications of nanotechnology in molecular imaging of the brain. In Nanoneuroscience and Nanoneuropharmacology; Elsevier: Amsterdam, The Netherlands, 2009; pp. 72–96, Chapter 4. [Google Scholar] [CrossRef]
- Broadley, S.A.; Barnett, M.H.; Boggild, M.; Brew, B.J.; Butzkueven, H.; Heard, R.; Hodgkinson, S.; Kermode, A.G.; Lechner-Scott, J.; Macdonell, R.A.; et al. Therapeutic approaches to disease modifying therapy for multiple sclerosis in adults: An Australian and New Zealand perspective Part 3 Treatment practicalities and recommendations. J. Clin. Neurosci. 2014, 21, 1857–1865. [Google Scholar] [CrossRef] [PubMed]
- Hauser, S.L.; Cree, B.A.C. Treatment of Multiple Sclerosis: A Review. Am. J. Med. 2020, 133, 1380–1390.e2. [Google Scholar] [CrossRef]
- Zajicek, J.; Fox, P.; Sanders, H.; Wright, D.; Vickery, J.; Nunn, A.; Thompson, A. Cannabinoids for treatment of spasticity and other symptoms related to multiple sclerosis (CAMS study): Multicentre randomised placebo-controlled trial. Lancet 2003, 362, 1517–1526. [Google Scholar] [CrossRef]
- Chidiac, A.S.; Buckley, N.A.; Noghrehchi, F.; Cairns, R. Paracetamol (acetaminophen) overdose and hepatotoxicity: Mechanism, treatment, prevention measures, and estimates of burden of disease. Expert Opin. Drug Metab. Toxicol. 2023, 19, 297–317. [Google Scholar] [CrossRef]
- IFNB Multiple Sclerosis Study Group. Interferon beta-1b is effective in relapsing-remitting multiple sclerosis. Neurology 1993, 43, 655. [Google Scholar] [CrossRef] [PubMed]
- Reich, D.S.; Lucchinetti, C.F.; Calabresi, P.A. Multiple Sclerosis. N. Engl. J. Med. 2018, 378, 169–180. [Google Scholar] [CrossRef] [PubMed]
- Treating, M.S. Medications. National MS Society. 2023. Available online: https://www.nationalmssociety.org/Treating-MS/Medications (accessed on 8 October 2023).
- Huang, Q.; Xiao, B.; Ma, X.; Qu, M.; Li, Y.; Nagarkatti, P.; Nagarkatti, M.; Zhou, J. MicroRNAs associated with the pathogenesis of multiple sclerosis. J. Neuroimmunol. 2016, 295–296, 148–161. [Google Scholar] [CrossRef] [PubMed]
- Minagar, A. Current and Future Therapies for Multiple Sclerosis. Scientifica 2013, 2013, 249101. [Google Scholar] [CrossRef]
- Kieseier, B.C. The mechanism of action of interferon-β in relapsing multiple sclerosis. CNS Drugs 2011, 25, 491–502. [Google Scholar] [CrossRef] [PubMed]
- Vani, P.B.; Chitra, V. Disease-Modifying Therapies in Multiple Sclerosis. Ann. Rom. Soc. Cell Biol. 2021, 363, 17179–17188. [Google Scholar]
- AbdelRazek, M.A.; Tummala, S.; Khalid, F.; Tauhid, S.; Jalkh, Y.; Khalil, S.; Hurwitz, S.; Zurawski, J.; Bakshi, R. Exploring the effect of glatiramer acetate on cerebral gray matter atrophy in multiple sclerosis. J. Neurol. Sci. 2023, 444, 120501. [Google Scholar] [CrossRef]
- Johnson, K.P.; Brooks, B.R.; Cohen, J.A.; Ford, C.C.; Goldstein, J.; Lisak, R.P.; Myers, L.W.; Panitch, H.S.; Rose, J.W.; Schiffer, R.B.; et al. Copolymer 1 reduces relapse rate and improves disability in relapsing-remitting multiple sclerosis: Results of a phase III multicenter, double-blind, placebo-controlled trial. Neurology 1995, 45, 1268–1276. [Google Scholar] [CrossRef]
- Ruggieri, M.; Avolio, C.; Livrea; Trojano, M. Glatiramer acetate in multiple sclerosis: A review. CNS Drug Rev. 2007, 13, 178–191. [Google Scholar] [CrossRef]
- Neuhaus, O.; Farina, C.; Yassouridis, A.; Wiendl, H.; Then Bergh, F.; Dose, T.; Wekerle, H.; Hohlfeld, R. Multiple sclerosis: Comparison of copolymer-1-reactive T cell lines from treated and untreated subjects reveals cytokine shift from T helper 1 to T helper 2 cells. Proc. Natl. Acad. Sci. USA 2000, 97, 7452–7457. [Google Scholar] [CrossRef] [PubMed]
- Montalban, X.; Hauser, S.L.; Kappos, L.; Arnold, D.L.; Bar-Or, A.; Comi, G.; De Seze, J.; Giovannoni, G.; Hartung, H.P.; Hemmer, B.; et al. Ocrelizumab versus placebo in primary progressive multiple sclerosis. N. Engl. J. Med. 2017, 376, 209–220. [Google Scholar] [CrossRef]
- Hauser, S.L.; Bar-Or, A.; Comi, G.; Giovannoni, G.; Hartung, H.P.; Hemmer, B.; Lublin, F.; Montalban, X.; Rammohan, K.W.; Selmaj, K.; et al. Ocrelizumab versus interferon beta-1a in relapsing multiple sclerosis. N. Engl. J. Med. 2017, 376, 221–234. [Google Scholar] [CrossRef] [PubMed]
- Ineichen, B.V.; Moridi, T.; Granberg, T.; Piehl, F. Rituximab treatment for multiple sclerosis. Mult. Scler. J. 2020, 26, 137–152. [Google Scholar] [CrossRef]
- Yednock, T.A.; Cannon, C.; Fritz, L.C.; Sanchez-Madrid, F.; Steinman, L.; Karin, N. Prevention of experimental autoimmune encephalomyelitis by antibodies against α 4 β l integrin. Nature 1992, 356, 63–66. [Google Scholar] [CrossRef] [PubMed]
- Polman, C.H.; O’Connor, P.W.; Havrdova, E.; Hutchinson, M.; Kappos, L.; Miller, D.H.; Phillips, J.T.; Lublin, F.D.; Giovannoni, G.; Wajgt, A.; et al. A Randomized, Placebo-Controlled Trial of Natalizumab for Relapsing Multiple Sclerosis. N. Engl. J. Med. 2006, 354, 899–910. [Google Scholar] [CrossRef] [PubMed]
- Goodin, D.S.; Arnason, B.G.; Coyle, K.; Frohman, E.; Paty, D.W. The use of mitoxantrone (Novantrone) for the treatment of multiple sclerosis: Report of the Therapeutics and Technology Assessment Subcommittee of the American Academy of Neurology. Neurology 2003, 61, 1332–1338. [Google Scholar] [CrossRef]
- Dąbrowski, G.; Skrajda, M. Cannabinoids from Cannabis sp.: Mechanism of their activity and potential health benefits in human body. J. Educ. Health Sport 2017, 7, 936–945. [Google Scholar]
- Touw, M. The Religious and Medicinal Uses of Cannabis in China, India and Tibet. J. Psychoact. Drugs 2012, 13, 23–34. [Google Scholar] [CrossRef]
- Li, H.L. An Archaeological and Historical Account of Cannabis in China on JSTOR. Econ. Bot. 1974, 28, 437–448. [Google Scholar] [CrossRef]
- Meyer, H.C.; Lee, F.S.; Gee, D.G. The Role of the Endocannabinoid System and Genetic Variation in Adolescent Brain Development. Neuropsychopharmacology 2017, 43, 21–33. [Google Scholar] [CrossRef] [PubMed]
- Ashton, J.C.; Friberg, D.; Darlington, C.L.; Smith, P.F. Expression of the cannabinoid CB2 receptor in the rat cerebellum: An immunohistochemical study. Neurosci. Lett. 2006, 396, 113–116. [Google Scholar] [CrossRef] [PubMed]
- Gong, J.P.; Onaivi, E.S.; Ishiguro, H.; Liu, Q.R.; Tagliaferro, P.A.; Brusco, A.; Uhl, G.R. Cannabinoid CB2 receptors: Immunohistochemical localization in rat brain. Brain Res. 2006, 1071, 10–23. [Google Scholar] [CrossRef]
- Pérez, J. Combined cannabinoid therapy via an oromucosal spray. Drugs Today 2006, 42, 495–503. [Google Scholar] [CrossRef]
- Puighermanal, E.; Busquets-Garcia, A.; Maldonado, R.; Ozaita, A. Cellular and intracellular mechanisms involved in the cognitive impairment of cannabinoids. Philos. Trans. R Soc. B Biol. Sci. 2012, 367, 3254–3263. [Google Scholar] [CrossRef] [PubMed]
- Gutiérrez-Rodríguez, A.; Bonilla-Del Río, I.; Puente, N.; Gómez-Urquijo, S.M.; Fontaine, C.J.; Egaña-Huguet, J.; Elezgarai, I.; Ruehle, S.; Lutz, B.; Robin, L.M.; et al. Localization of the cannabinoid type-1 receptor in subcellular astrocyte compartments of mutant mouse hippocampus. Glia 2018, 66, 1417–1431. [Google Scholar] [CrossRef] [PubMed]
- Jimenez-Blasco, D.; Busquets-Garcia, A.; Hebert-Chatelain, E.; Serrat, R.; Vicente-Gutierrez, C.; Ioannidou, C.; Gomez-Sotres, P.; Lopez-Fabuel, I.; Resch-Beusher, M.; Resel, E.; et al. Glucose metabolism links astroglial mitochondria to cannabinoid effects. Nature 2020, 583, 603–608. [Google Scholar] [CrossRef]
- Tucci, V. (Ed.) Handbook of Neurobehavioral Genetics and Phenotyping; John Wiley & Sons: Hoboken, NJ, USA, 2017; Available online: https://books.google.com.eg/books?hl=en&lr=&id=n7RpDgAAQBAJ&oi=fnd&pg=PA25&ots=P4OtI2xJyg&sig=tfTwPhLxsI_9J7uUHVj3bwiZqvM&redir_esc=y#v=onepage&q&f=false (accessed on 3 July 2022).
- Marsicano, G.; Goodenough, S.; Monory, K.; Hermann, H.; Eder, M.; Cannich, A.; Azad, S.C.; Cascio, M.G.; Gutiérrez, S.O.; Van der Stelt, M.; et al. CB1 cannabinoid receptors and on-demand defense against excitotoxicity. Science 2003, 302, 84–88. [Google Scholar] [CrossRef]
- Kister, I.; Bacon, T.E.; Chamot, E.; Salter, A.R.; Cutter, G.R.; Kalina, J.T.; Herbert, J. Natural History of Multiple Sclerosis Symptoms. Int. J. MS Care 2013, 15, 146–156. [Google Scholar] [CrossRef]
- Wegener, N.; Koch, M. Neurobiology and systems physiology of the endocannabinoid system. Pharmacopsychiatry 2009, 42 (Suppl. S1), S79–S86. [Google Scholar] [CrossRef]
- Maresz, K.; Carrier, E.J.; Ponomarev, E.D.; Hillard, C.J.; Dittel, B.N. Modulation of the cannabinoid CB2 receptor in microglial cells in response to inflammatory stimuli. J. Neurochem. 2005, 95, 437–445. [Google Scholar] [CrossRef]
- Jean-Gilles, L.; Braitch, M.; Latif, M.L.; Aram, J.; Fahey, A.J.; Edwards, L.J.; Robins, R.A.; Tanasescu, R.; Tighe, P.J.; Gran, B.; et al. Effects of pro-inflammatory cytokines on cannabinoid CB 1 and CB 2 receptors in immune cells. Acta Physiol. 2015, 214, 63–74. [Google Scholar] [CrossRef]
- Iuvone, T.; Esposito, G.; Esposito, R.; Santamaria, R.; Di Rosa, M.; Izzo, A.A. Neuroprotective effect of cannabidiol, a non-psychoactive component from Cannabis sativa, on β-amyloid-induced toxicity in PC12 cells. J. Neurochem. 2004, 89, 134–141. [Google Scholar] [CrossRef]
- Zhu, L.X.; Sharma, S.; Stolina, M.; Gardner, B.; Roth, M.D.; Tashkin, D.P.; Dubinett, S.M. Δ-9-Tetrahydrocannabinol Inhibits Antitumor Immunity by a CB2 Receptor-Mediated, Cytokine-Dependent Pathway. J. Immunol. 2000, 165, 373–380. [Google Scholar] [CrossRef]
- Klein, T.W.; Kawakami, Y.; Newton, C.; Friedman, H. Marijuana components suppress induction and cytolytic function of murine cytotoxic T cells in vitro and in vivo. J. Toxicol. Environ. Health Part A Curr. Issues 2009, 32, 465–477. [Google Scholar] [CrossRef]
- Nichols, J.M.; Kaplan, B.L.F. Immune Responses Regulated by Cannabidiol. Cannabis Cannabinoid. Res. 2020, 5, 12–31. [Google Scholar] [CrossRef] [PubMed]
- Berdyshev, E.V.; Boichot, E.; Germain, N.; Allain, N.; Anger, J.P.; Lagente, V. Influence of fatty acid ethanolamides and Δ9-tetrahydrocannabinol on cytokine and arachidonate release by mononuclear cells. Eur. J. Pharmacol. 1997, 330, 231–240. [Google Scholar] [CrossRef] [PubMed]
- Sánchez, C.; Velasco, G.; Guzmán, M. Metabolic stimulation of mouse spleen lymphocytes by low doses of 9-tetrahydrocannabinol. Life Sci. 1997, 60, 1709–1717. [Google Scholar] [CrossRef]
- Furgiuele, A.; Cosentino, M.; Ferrari, M.; Marino, F. Immunomodulatory potential of cannabidiol in multiple sclerosis: A systematic review. J. Neuroimmune Pharmacol. 2021, 16, 251–269. [Google Scholar] [CrossRef]
- Elliott, D.M.; Singh, N.; Nagarkatti, M.; Nagarkatti, P.S. Cannabidiol attenuates experimental autoimmune encephalomyelitis model of multiple sclerosis through induction of myeloid-derived suppressor cells. Front. Immunol. 2018, 9, 1782. [Google Scholar] [CrossRef] [PubMed]
- Kozela, E.; Lev, N.; Kaushansky, N.; Eilam, R.; Rimmerman, N.; Levy, R.; Ben-Nun, A.; Juknat, A.; Vogel, Z. Cannabidiol inhibits pathogenic T cells, decreases spinal microglial activation and ameliorates multiple sclerosis-like disease in C57BL/6 mice. Br. J. Pharmacol. 2011, 163, 1507–1519. [Google Scholar] [CrossRef]
- Peng, J.; Fan, M.; An, C.; Ni, F.; Huang, W.; Luo, J. A narrative review of molecular mechanism and therapeutic effect of cannabidiol (CBD). Basic Clin. Pharmacol. Toxicol. 2022, 130, 439–456. [Google Scholar] [CrossRef] [PubMed]
- Bunman, S.; Muengtaweepongsa, S.; Piyayotai, D.; Charlermroj, R.; Kanjana, K.; Kaew-Amdee, S.; Makornwattana, M.; Kim, S. Analgesic and Anti-Inflammatory Effects of 1% Topical Cannabidiol Gel in Animal Models. Cannabis Cannabinoid Res. 2023; Ahead of Print. [Google Scholar] [CrossRef]
- Maayah, Z.H.; Takahara, S.; Ferdaoussi, M.; Dyck, J.R.B. The anti-inflammatory and analgesic effects of formulated full-spectrum cannabis extract in the treatment of neuropathic pain associated with multiple sclerosis. Inflamm. Res. 2020, 69, 549–558. [Google Scholar] [CrossRef] [PubMed]
- Nichols, J.M.; Kummari, E.; Sherman, J.; Yang, E.J.; Dhital, S.; Gilfeather, C.; Yray, G.; Morgan, T.; Kaplan, B.L. CBD Suppression of EAE Is Correlated with Early Inhibition of Splenic IFN-γ + CD8+ T Cells and Modest Inhibition of Neuroinflammation. J. Neuroimmune Pharmacol. 2021, 16, 346–362. [Google Scholar] [CrossRef] [PubMed]
- Al-Ghezi, Z.Z.; Miranda, K.; Nagarkatti, M.; Nagarkatti, P.S. Combination of cannabinoids, Δ9-tetrahydrocannabinol and cannabidiol, ameliorates experimental multiple sclerosis by suppressing neuroinflammation through regulation of miRNA-mediated signaling pathways. Front. Immunol. 2019, 10, 1921. [Google Scholar] [CrossRef] [PubMed]
- Nahas, G.G.; Morishima, A.; Desoize, B. Effects of cannabinoids on macromolecular synthesis and replication of cultured lymphocytes. Fed. Proc. 1977, 36, 1748–1752. Available online: https://europepmc.org/article/med/844617 (accessed on 13 June 2022). [PubMed]
- Derocq, J.M.; Ségui, M.; Marchand, J.; le Fur, G.; Casellas, P. Cannabinoids enhance human B-cell growth at low nanomolar concentrations. FEBS Lett. 1995, 369, 177–182. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; Bam, M.; Nagarkatti, S.; Nagarkatti, M. Cannabidiol Regulates Gene Expression in Encephalitogenic T cells Using Histone Methylation and noncoding RNA during Experimental Autoimmune Encephalomyelitis. Sci. Rep. 2019, 9, 15780. [Google Scholar] [CrossRef]
- Kozela, E.; Juknat, A.; Kaushansky, N.; Rimmerman, N.; Ben-Nun, A.; Vogel, Z. Cannabinoids decrease the Th17 inflammatory autoimmune phenotype. J. Neuroimmune Pharmacol. 2013, 8, 1265–1276. [Google Scholar] [CrossRef]
- González-García, C.; Torres, I.M.; García-Hernández, R.; Campos-Ruíz, L.; Esparragoza, L.R.; Coronado, M.J.; Grande, A.G.; García-Merino, A.; López, A.J.S. Mechanisms of action of cannabidiol in adoptively transferred experimental autoimmune encephalomyelitis. Exp. Neurol. 2017, 298, 57–67. [Google Scholar] [CrossRef]
- Klein, T.W.; Newton, C.; Friedman, H. Cannabinoid receptors and immunity. Immunol. Today 1998, 19, 373–381. [Google Scholar] [CrossRef]
- Pertwee, R.G. Pharmacology of cannabinoid CB1 and CB2 receptors. Pharmacol. Ther. 1997, 74, 129–180. [Google Scholar] [CrossRef] [PubMed]
- Pertwee, R.G. The diverse CB 1 and CB 2 receptor pharmacology of three plant cannabinoids: Δ 9-tetrahydrocannabinol, cannabidiol and Δ 9-tetrahydrocannabivarin. Br. J. Pharmacol. 2008, 153, 199–215. [Google Scholar] [CrossRef] [PubMed]
- Matsuda, L.A.; Lolait, S.J.; Brownstein, M.J.; Young, A.C.; Bonner, T.I. Structure of a cannabinoid receptor and functional expression of the cloned cDNA. Nature 1990, 346, 561–564. [Google Scholar] [CrossRef] [PubMed]
- Borgelt, L.M.; Franson, K.L.; Nussbaum, A.M.; Wang, G.S. The pharmacologic and clinical effects of medical cannabis. Pharmacotherapy 2013, 33, 195–209. [Google Scholar] [CrossRef]
- National Academies of Sciences, Engineering, and Medicine Health and Medicine Division Board on Population Health and Public Health Practice; Committee on the Health Effects of Marijuana: An Evidence Review and Research Agenda. The Health Effects of Cannabis and Cannabinoids: The Current State of Evidence and Recommendations for Research; National Academies Press: Washington, DC, USA, 2017. Available online: https://books.google.com.eg/books?hl=en&lr=&id=FTW9DgAAQBAJ&oi=fnd&pg=PR1&ots=-jgZ2_8hoS&sig=RfSYnybQwYNAIncjlNhh-5IRCuk&redir_esc=y#v=onepage&q&f=false (accessed on 11 February 2023).
- Jetly, R.; Heber, A.; Fraser, G.; Boisvert, D. The efficacy of nabilone, a synthetic cannabinoid, in the treatment of PTSD-associated nightmares: A preliminary randomized, double-blind, placebo-controlled cross-over design study. Psychoneuroendocrinology 2015, 51, 585–588. [Google Scholar] [CrossRef]
- Volicer, L.; Stelly, M.; Morris, J.; McLaughlin, J.; Volicer, B.J.; Rogers, E.N. Effects of Dronabinol on Anorexia ond Disturbed Behavior in Patients with Alzheimer’s Disease. Ltd. Int. J. Geriat. Psychiatry 1997, 12, 913–919. [Google Scholar] [CrossRef]
- Walther, S.; Mahlberg, R.; Eichmann, U.; Kunz, D. Delta-9-tetrahydrocannabinol for nighttime agitation in severe dementia. Psychopharmacology 2006, 185, 524–528. [Google Scholar] [CrossRef]
- Cohen, L.M.; Ash, E.; Outen, J.D.; Vandrey, R.; Amjad, H.; Agronin, M.; Burhanullah, M.H.; Walsh, P.; Wilkins, J.M.; Leoutsakos, J.M.; et al. Study rationale and baseline data for pilot trial of dronabinol adjunctive treatment of agitation in Alzheimer’s dementia (THC-AD). Int. Psychogeriatr. 2021, 1–6. [Google Scholar] [CrossRef]
- Herrmann, N.; Ruthirakuhan, M.; Gallagher, D.; Verhoeff, N.P.L.; Kiss, A.; Black, S.E.; Lanctôt, K.L. Randomized Placebo-Controlled Trial of Nabilone for Agitation in Alzheimer’s Disease. Am. J. Geriatr. Psychiatry 2019, 27, 1161–1173. [Google Scholar] [CrossRef]
- Squintani, G.; Donato, F.; Turri, M.; Deotto, L.; Teatini, F.; Moretto, G.; Erro, R. Cortical and spinal excitability in patients with multiple sclerosis and spasticity after oromucosal cannabinoid spray. J. Neurol. Sci. 2016, 370, 263–268. [Google Scholar] [CrossRef]
- Ungerleider, J.T.; Andyrsiak, T.; Fairbanks, L.; Ellison, G.W.; Myers, L.W. Delta-9-THC in the treatment of spasticity associated with multiple sclerosis. Adv. Alcohol Subst. Abus. 1988, 7, 39–50. [Google Scholar] [CrossRef]
- Rog, D.J.; Nurmikko, T.J.; Friede, T.; Young, C.A. Randomized, controlled trial of cannabis-based medicine in central pain in multiple sclerosis. Neurology 2005, 65, 812–819. [Google Scholar] [CrossRef] [PubMed]
- Markovà, J.; Essner, U.; Akmaz, B.; Marinelli, M.; Trompke, C.; Lentschat, A.; Vila, C. Sativex® as add-on therapy vs. further optimized first-line ANTispastics (SAVANT) in resistant multiple sclerosis spasticity: A double-blind, placebo-controlled randomised clinical trial. Int. J. Neurosci. 2019, 129, 119–128. [Google Scholar] [CrossRef] [PubMed]
- Überall, M.A. A Review of Scientific Evidence for THC:CBD Oromucosal Spray (Nabiximols) in the Management of Chronic Pain. J. Pain Res. 2020, 399–410. [Google Scholar] [CrossRef] [PubMed]
- Manera, C.; Bertini, S. Cannabinoid-Based Medicines and Multiple Sclerosis. Adv. Exp. Med. Biol. 2021, 1264, 111–129. [Google Scholar] [CrossRef] [PubMed]
- Etges, T.; Karolia, K.; Taylor, T.G.A.; Daka, B.; Wright, S. An observational postmarketing safety registry of patients in the UK, Germany, and Switzerland who have been prescribed Sativex®(THC: CBD, nabiximols) oromucosal spray. Ther. Clin. Risk Manag. 2016, 12, 1667–1675. [Google Scholar] [CrossRef]
- Nahas, G.G.; Frick, H.C.; Lattimer, J.K.; Latour, C.; Harvey, D. Pharmacokinetics of THC in brain and testis, male gametotoxicity and premature apoptosis of spermatozoa. Hum. Psychopharmacol. Clin. Exp. 2002, 17, 103–113. [Google Scholar] [CrossRef]
- De Gregorio, D.; McLaughlin, R.J.; Posa, L.; Ochoa-Sanchez, R.; Enns, J.; Lopez-Canul, M.; Aboud, M.; Maione, S.; Comai, S.; Gobbi, G. Cannabidiol modulates serotonergic transmission and reverses both allodynia and anxiety-like behavior in a model of neuropathic pain. Pain 2019, 160, 136–150. [Google Scholar] [CrossRef]
- Abu-Sawwa, R.; Stehling, C. Epidiolex (cannabidiol) primer: Frequently asked questions for patients and caregivers. J. Pediatr. Pharmacol. Ther. 2020, 25, 75–77. [Google Scholar] [CrossRef]
- Allsop, D.J.; Copeland, J.; Lintzeris, N.; Dunlop, A.J.; Montebello, M.; Sadler, C.; Rivas, G.R.; Holland, R.M.; Muhleisen, P.; Norberg, M.M.; et al. Nabiximols as an Agonist Replacement Therapy During Cannabis Withdrawal: A Randomized Clinical Trial. JAMA Psychiatry 2014, 71, 281–291. [Google Scholar] [CrossRef] [PubMed]
- Karst, M.; Salim, K.; Burstein, S.; Conrad, I.; Hoy, L.; Schneider, U. Analgesic Effect of the Synthetic Cannabinoid CT-3 on Chronic Neuropathic Pain: A Randomized Controlled Trial. JAMA 2003, 290, 1757–1762. [Google Scholar] [CrossRef] [PubMed]
- Sativex® Approved in Germany for the Treatment of Spasticity Due to Multiple Sclerosis. Available online: http://www.gwpharm.com/mechanism-of-action.aspx (accessed on 11 February 2023).
- Harrison, N.J.; Simpson, H. Nabilone (Cesamet). In Cannabinoids and Pain; Springer International Publishing: Cham, Switzerland, 2021; pp. 109–112. [Google Scholar]
- Bagüés, A.; Benítez, D.; Abalo, R. Cannabinoids to Fight Chemotherapy-Induced Adverse Effects. In Handbook of Cancer and Immunology; Springer: Berlin/Heidelberg, Germany, 2023; pp. 1–29. [Google Scholar] [CrossRef]
- Rai, A.; Sargsyan, N.; Prasad, D.N. Nabiximols—An alternative to Gabapentin for Neuropathic Pain. IJCMCR 2023, 24, 3. [Google Scholar] [CrossRef]
- Wilsey, B.; Marcotte, T.; Tsodikov, A.; Millman, J.; Bentley, H.; Gouaux, B.; Fishman, S. A Randomized, Placebo-Controlled, Crossover Trial of Cannabis Cigarettes in Neuropathic Pain. J. Pain 2008, 9, 506–521. [Google Scholar] [CrossRef]
- Sieradzan, K.A.; Fox, S.H.; Hill, M.; Dick, J.P.R.; Crossman, A.R.; Brotchie, J.M. Cannabinoids reduce levodopa-induced dyskinesia in Parkinson’s disease: A pilot study. Neurology 2001, 57, 2108–2111. [Google Scholar] [CrossRef]
- Ojha, S.; Kumar, B. A review on nanotechnology based innovations in diagnosis and treatment of multiple sclerosis. J. Cell Immunother. 2018, 4, 56–64. [Google Scholar] [CrossRef]
- Zeng, Y.; Li, Z.; Zhu, H.; Gu, Z.; Zhang, H.; Luo, K. Recent Advances in Nanomedicines for Multiple Sclerosis Therapy. ACS Appl. Bio. Mater. 2020, 3, 6571–6597. [Google Scholar] [CrossRef]
- Gunasekaran, T.; Haile, T.; Nigusse, T.; Dhanaraju, M.D. Nanotechnology: An effective tool for enhancing bioavailability and bioactivity of phytomedicine. Asian Pac. J. Trop. Biomed. 2014, 4, S1–S7. [Google Scholar] [CrossRef]
- Patel, V.R.; Agrawal, Y.K. Nanosuspension: An approach to enhance solubility of drugs. J. Adv. Pharm. Technol. Res. 2011, 2, 81. [Google Scholar] [CrossRef]
- Patra, J.K.; Das, G.; Fraceto, L.F.; Campos, E.V.R.; Rodriguez-Torres, M.D.P.; Acosta-Torres, L.S.; Diaz-Torres, L.A.; Grillo, R.; Swamy, M.K.; Sharma, S.; et al. Nano based drug delivery systems: Recent developments and future prospects. J. Nanobiotechnol. 2018, 16, 71. [Google Scholar] [CrossRef]
- Soares, S.; Sousa, J.; Pais, A.; Vitorino, C. Nanomedicine: Principles, Properties, and Regulatory Issues. Front. Chem. 2018, 6, 360. [Google Scholar] [CrossRef]
- Wu, L.; Zhang, J.; Watanabe, W. Physical and chemical stability of drug nanoparticles. Adv. Drug Deliv. Rev. 2011, 63, 456–469. [Google Scholar] [CrossRef] [PubMed]
- Rabinow, B.E. Nanosuspensions in drug delivery. Nat. Rev. Drug Discov. 2004, 3, 785–796. [Google Scholar] [CrossRef] [PubMed]
- Patravale, V.B.; Date, A.A.; Kulkarni, R.M. Nanosuspensions: A promising drug delivery strategy. J. Pharm. Pharmacol. 2004, 56, 827–840. [Google Scholar] [CrossRef]
- Goldsmith, M.; Abramovitz, L.; Peer, D. Precision Nanomedicine in Neurodegenerative Diseases. ACS Nano 2014, 8, 1958–1965. [Google Scholar] [CrossRef]
- Griffith, J.I.; Rathi, S.; Zhang, W.; Zhang, W.; Drewes, L.R.; Sarkaria, J.N.; Elmquist, W.F. Addressing BBB Heterogeneity: A New Paradigm for Drug Delivery to Brain Tumors. Pharmaceutics 2020, 12, 1205. [Google Scholar] [CrossRef]
- De Rosa, G.; Salzano, G.; Caraglia, M.; Abbruzzese, A. Nanotechnologies: A strategy to overcome blood-brain barrier. Curr. Drug Metab. 2012, 13, 61–69. [Google Scholar] [CrossRef] [PubMed]
- Ferber, S.; Tiram, G.; Sousa-Herves, A.; Eldar-Boock, A.; Krivitsky, A.; Scomparin, A.; Yeini, E.; Ofek, P.; Ben-Shushan, D.; Vossen, L.I.; et al. Co-targeting the tumor endothelium and P-selectin-expressing glioblastoma cells leads to a remarkable therapeutic outcome. eLife 2017, 6, e25281. [Google Scholar] [CrossRef]
- Zhang, C.; Feng, W.; Vodovozova, E.; Tretiakova, D.; Boldyrevd, I.; Li, Y.; Kürths, J.; Yu, T.; Semyachkina-Glushkovskaya, O.; Zhu, D. Photodynamic opening of the blood-brain barrier to high weight molecules and liposomes through an optical clearing skull window. Biomed. Opt. Express 2018, 9, 4850–4862. [Google Scholar] [CrossRef]
- Aryal, M.; Arvanitis, C.D.; Alexander, M.; McDannold, N. Ultrasound-mediated blood–brain barrier disruption for targeted drug delivery in the central nervous system. Adv. Drug Deliv. Rev. 2014, 72, 94–109. [Google Scholar] [CrossRef]
- Song, K.-H.; Harvey, B.K.; Borden, M.A. State-of-the-art of microbubble-assisted blood-brain barrier disruption. Theranostics 2018, 8, 4393. [Google Scholar] [CrossRef] [PubMed]
- Hwang, D.; Dismuke, T.; Tikunov, A.; Rosen, E.P.; Kagel, J.R.; Ramsey, J.D.; Lim, C.; Zamboni, W.; Kabanov, A.V.; Gershon, T.R. Poly (2-oxazoline) nanoparticle delivery enhances the therapeutic potential of vismodegib for medulloblastoma by improving CNS pharmacokinetics and reducing systemic toxicity. Nanomedicine 2021, 32, 102345. [Google Scholar] [CrossRef] [PubMed]
- Sindhwani, S.; Syed, A.M.; Ngai, J.; Kingston, B.R.; Maiorino, L.; Rothschild, J.; MacMillan, P.; Zhang, Y.; Rajesh, N.U.; Hoang, T.; et al. The entry of nanoparticles into solid tumours. Nat. Mater. 2020, 19, 566–575. [Google Scholar] [CrossRef] [PubMed]
- Tylawsky, D.E.; Kiguchi, H.; Vaynshteyn, J.; Gerwin, J.; Shah, J.; Islam, T.; Boyer, J.A.; Boué, D.R.; Snuderl, M.; Greenblatt, M.B.; et al. P-selectin-targeted nanocarriers induce active crossing of the blood–brain barrier via caveolin-1-dependent transcytosis. Nat. Mater. 2023, 22, 391–399. [Google Scholar] [CrossRef] [PubMed]
- Calapai, F.; Cardia, L.; Sorbara, E.E.; Navarra, M.; Gangemi, S.; Calapai, G.; Mannucci, C. Cannabinoids, blood–brain barrier, and brain disposition. Pharmaceutics 2020, 12, 265. [Google Scholar] [CrossRef] [PubMed]
- Adusumilli, N.C.; Hazuka, E.L.; Friedman, A.J. Nanotechnology to deliver cannabinoids in dermatology. Precis. Nanomed. 2021, 4, 787–794. [Google Scholar] [CrossRef]
- Gajofatto, A.; Benedetti, M.D. Treatment strategies for multiple sclerosis: When to start, when to change, when to stop? World J. Clin. Cases WJCC 2015, 3, 545. [Google Scholar] [CrossRef]
- Lucas, C.J.; Galettis; Schneider, J. The pharmacokinetics and the pharmacodynamics of cannabinoids. Br. J. Clin. Pharmacol. 2018, 84, 2477. [Google Scholar] [CrossRef]
- Millar, S.A.; Maguire, R.F.; Yates, A.S.; O’sullivan, S.E. Towards Better Delivery of Cannabidiol (CBD). Pharmaceuticals 2020, 13, 219. [Google Scholar] [CrossRef]
- Grotenhermen, F. Pharmacokinetics and pharmacodynamics of cannabinoids. Clin. Pharmacokinet. 2003, 42, 327–360. [Google Scholar] [CrossRef]
- Fraguas-Sánchez, A.I.; Fernández-Carballido, A.; Martin-Sabroso, C.; Torres-Suárez, A.I. Stability characteristics of cannabidiol for the design of pharmacological, biochemical and pharmaceutical studies. J. Chromatogr. B 2020, 1150, 122188. [Google Scholar] [CrossRef] [PubMed]
- Onaivi, E.S.; Chauhan, B.P.S.; Sharma, V. Challenges of cannabinoid delivery: How can nanomedicine help? Nanomedicine 2020, 15, 2023–2028. [Google Scholar] [CrossRef] [PubMed]
- Rebelatto, E.R.L.; Rauber, G.S.; Caon, T. An update of nano-based drug delivery systems for cannabinoids: Biopharmaceutical aspects & therapeutic applications. Int. J. Pharm. 2023, 635, 122727. [Google Scholar] [CrossRef]
- Aparicio-Blanco, J.; Sebastián, V.; Benoit, J.P.; Torres-Suárez, A.I. Lipid nanocapsules decorated and loaded with cannabidiol as targeted prolonged release carriers for glioma therapy: In vitro screening of critical parameters. Eur. J. Pharm. Biopharm. 2019, 134, 126–137. [Google Scholar] [CrossRef]
- Durán-Lobato, M.; Álvarez-Fuentes, J.; Fernández-Arévalo, M.; Martín-Banderas, L. Receptor-targeted nanoparticles modulate cannabinoid anticancer activity through delayed cell internalization. Sci. Rep. 2022, 12, 1–17. [Google Scholar] [CrossRef] [PubMed]
- Bruni, N.; della Pepa, C.; Oliaro-Bosso, S.; Pessione, E.; Gastaldi, D.; Dosio, F. Cannabinoid Delivery Systems for Pain and Inflammation Treatment. Molecules 2018, 23, 2478. [Google Scholar] [CrossRef]
- Stella, B.; Baratta, F.; della Pepa, C.; Arpicco, S.; Gastaldi, D.; Dosio, F. Cannabinoid Formulations and Delivery Systems: Current and Future Options to Treat Pain. Drugs 2021, 81, 1513. [Google Scholar] [CrossRef]
| Drug Generic Name | FDA Approval | Chemical Composition | Mechanism of Action | Refs. |
|---|---|---|---|---|
| Betaseron Extavia | 1993, 1995 2009 | Interferon-β1b (IFN-β1b) |
| [17,21] |
| Avonex Rebif Plegridy | 1996 1998 2014 | Interferon-β1a (IFN-β1a) | ||
| Copaxone Glatopa | 1996 2015 | Glatiramer acetate (GA) |
| [17,23,24,25] |
| Kesimpta Briumvi Lemtrada Ocrevus Tysabri Tyruko | 2020 2022 2014 2017 2004 2023 | Ofatumumab (Anti-CD20) Ublituximab (Anti-CD20) Alemtuzumab (Anti-CD52) Ocrelizumab (Anti-CD20) Natalizumab (Anti-α4β1 integrin) Natalizumab (Anti-α4β1 integrin) |
| [17,26,27,28,29,30] |
| Novantrone | 2000 | Mitoxantrone |
| [19,31] |
| Gilenya Tascenso ODT Mayzent Zeposia | 2010 2021 2019 2020 | Fingolimod Fingolimod Siponimod Ozanimod |
| [12,17] |
| Treatment Used | Experimental Design | Results | Ref. |
|---|---|---|---|
| A daily dose of CBD (75 mg/kg) for 5 days | In vivo (mice) | Dimension in the T-cell infiltration and neuroinflammation in the brain and spinal cord’s white matter pathways. | [60] |
| A daily dose of CBD (10 mg/kg/day i.p.) | In vivo (mice) | Decrease the proliferation of T-cells. | [61] |
| A daily dose of CBD + THC (10 mg/kg/day i.p.) | In vivo (mice) | (CBD + 9-THC) Decrease the number of CD3+ T-cells, CD3+ CD4+ T-cells, and demyelination. Furthermore, the combination of THC and CBD improves the clinical symptoms of MS patients. | [61] |
| A daily dose of CBD (20 mg/kg/day i.p.) | In vivo (mice) | Clinical symptoms have a delayed onset and are less severe. | [55] |
| Determination of T-cells in marijuana smokers | In vivo (human subjects) | Reduction in the T-cells proliferation. | [50] |
| A dose of 10−5 to 10−4 of delta 8-THC + delta 9- THC + CBD | In vitro (Animal cell culture) | Decrease the proliferation of T-cells. | [62] |
| Three intraperitoneal (i.p.) injections of CBD (5 mg/kg, one per day) given at the outset of clinical disease | In vitro (cell culture, T-cell line) | Reduction in disease symptoms during the days following the injections, as well as a significant delay in disease development. Additionally, prevention of T-cell proliferation. | [56] |
| A dose (10/100 ng/mL) of Δ-THC | In vitro (B cells) | Increase the proliferation of T-cells. | [63] |
| After MS induction, CBD (10 mg/kg/day i.p.) was given for 7 days | In vitro (CD4+ T lymphocytes) | CD4+ T-cells’ pro-inflammatory phenotype is reversed. | [64] |
| A dose of CBD (0.1–1.5 μM) | In vitro (T-cell line derived from lymph node cells) | CD4+ T-cells and CD19+ B cells succumbed more frequently, whereas CD11b+ monocytes did not. | [65] |
| CBD (5–10 mg/kg/3 times per week or 50 mg/kg/day i.p.) | In vivo (mice) | Clinical symptoms and tissue lesions are reduced dose-dependently. | [66] |
| A dose (5–10 μg/mL) of THC | In vitro (cell culture) | Decrease in the number of Natural killer cell (NK). | [50] |
| Dose of 8 μg/mL or 2.6 × 10−5 M of THC | In vitro (animal cell culture) | Inhibition of T-cell proliferation. | [67] |
| 10−5 to 10−4 M concentrations of delta8, and delta9-tetrahydrocannabinol (THC) | In vitro (human cell culture) | Reduction in the proliferation of T-cells. | [62] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nouh, R.A.; Kamal, A.; Oyewole, O.; Abbas, W.A.; Abib, B.; Omar, A.; Mansour, S.T.; Abdelnaser, A. Unveiling the Potential of Cannabinoids in Multiple Sclerosis and the Dawn of Nano-Cannabinoid Medicine. Pharmaceutics 2024, 16, 241. https://doi.org/10.3390/pharmaceutics16020241
Nouh RA, Kamal A, Oyewole O, Abbas WA, Abib B, Omar A, Mansour ST, Abdelnaser A. Unveiling the Potential of Cannabinoids in Multiple Sclerosis and the Dawn of Nano-Cannabinoid Medicine. Pharmaceutics. 2024; 16(2):241. https://doi.org/10.3390/pharmaceutics16020241
Chicago/Turabian StyleNouh, Roua A., Ahmed Kamal, Oluwaseyi Oyewole, Walaa A. Abbas, Bishoy Abib, Abdelrouf Omar, Somaia T. Mansour, and Anwar Abdelnaser. 2024. "Unveiling the Potential of Cannabinoids in Multiple Sclerosis and the Dawn of Nano-Cannabinoid Medicine" Pharmaceutics 16, no. 2: 241. https://doi.org/10.3390/pharmaceutics16020241





