Chitosan and Anionic Solubility Enhancer Sulfobutylether-β-Cyclodextrin-Based Nanoparticles as Dexamethasone Ophthalmic Delivery System for Anti-Inflammatory Therapy
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Phase Solubility Studies and Determination of Inclusion Complex Constant
2.3. Job’s Plot
2.4. 1H-NMR Analysis
2.5. CS/SBE-β-CD NPs Preparation
2.6. DEX-Loaded NPs Preparation
2.7. DEX Quantification Using HPLC Method
2.8. NPs Characterization
2.8.1. Particle Size, PDI, and ζ-Potential
2.8.2. Differential Scanning Calorimetry
2.8.3. Fourier-Transform Infrared Spectroscopy (FT-IR)
2.8.4. Encapsulation Efficiency of DEX in NPs
2.8.5. Transmission Electron Microscopy (TEM)
2.9. Preparation of Bovine Eye Cornea
2.10. Ex Vivo Mucoadhesive Studies
2.11. In Vitro and Ex Vivo Permeation Studies
2.12. Bi-Directional Transport Studies on MDCKII-MDR1
2.13. Ocular Irritation Test
3. Results and Discussion
3.1. Characterization of DEX/SBE-β-CD Inclusion Complex
3.2. Nanoparticles Preparation and Characterization
3.3. Characterization of DEX-Loaded NPs
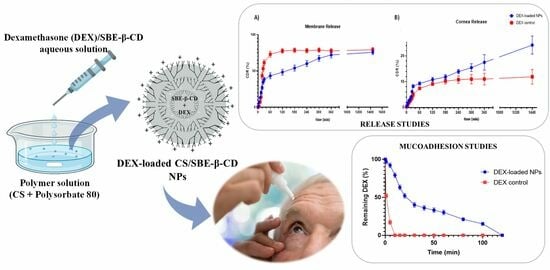
3.4. Ex Vivo Mucoadhesive Studies
3.5. In Vitro and Ex Vivo Permeation Studies
3.6. Bi-Directional Transport Studies on MDCKII-MDR1
3.7. Ocular Irritation Test
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Lam, D.; Rao, S.K.; Ratra, V.; Liu, Y.; Mitchell, P.; King, J.; Tassignon, M.-J.; Jonas, J.; Pang, C.P.; Chang, D.F. Cataract. Nat. Rev. Dis. Primers 2015, 1, 15014. [Google Scholar] [CrossRef]
- Chen, X.; Xu, J.; Chen, X.; Yao, K. Cataract: Advances in Surgery and Whether Surgery Remains the Only Treatment in Future. Adv. Ophthalmol. Pract. Res. 2021, 1, 100008. [Google Scholar] [CrossRef]
- Fang, R.; Yu, Y.-F.; Li, E.-J.; Lv, N.-X.; Liu, Z.-C.; Zhou, H.-G.; Song, X.-D. Global, Regional, National Burden and Gender Disparity of Cataract: Findings from the Global Burden of Disease Study 2019. BMC Public Health 2022, 22, 2068. [Google Scholar] [CrossRef]
- Wang, S.Y.; Stem, M.S.; Oren, G.; Shtein, R.; Lichter, P.R. Patient-Centered and Visual Quality Outcomes of Premium Cataract Surgery: A Systematic Review. Eur. J. Ophthalmol. 2017, 27, 387–401. [Google Scholar] [CrossRef]
- Noh, H.; Yoo, Y.-S.; Shin, K.Y.; Lim, D.H.; Chung, T.-Y. Comparison of Penetrating Femtosecond Laser-Assisted Astigmatic Keratotomy and Toric Intraocular Lens Implantation for Correction of Astigmatism in Cataract Surgery. Sci. Rep. 2021, 11, 7340. [Google Scholar] [CrossRef]
- Zhao, L.; Chen, X.-J.; Zhu, J.; Xi, Y.-B.; Yang, X.; Hu, L.-D.; Ouyang, H.; Patel, S.H.; Jin, X.; Lin, D.; et al. Lanosterol Reverses Protein Aggregation in Cataracts. Nature 2015, 523, 607–611. [Google Scholar] [CrossRef] [PubMed]
- Heruye, S.H.; Nkenyi, L.N.M.; Singh, N.U.; Yalzadeh, D.; Ngele, K.K.; Njie-Mbye, Y.-F.; Ohia, S.E.; Opere, C.A. Current Trends in the Pharmacotherapy of Cataracts. Pharmaceuticals 2020, 13, 15. [Google Scholar] [CrossRef] [PubMed]
- Monson, M.C.; Mamalis, N.; Olson, R.J. Toxic Anterior Segment Inflammation Following Cataract Surgery. J. Cataract. Refract. Surg. 1992, 18, 184–189. [Google Scholar] [CrossRef] [PubMed]
- Cerulli, L.; Missiroli, F. Aging of the Cornea. In Age-Related Changes of the Human Eye; Humana Press: Totowa, NJ, USA, 2008; pp. 45–60. [Google Scholar]
- Grossniklaus, H.E.; Nickerson, J.M.; Edelhauser, H.F.; Bergman, L.A.M.K.; Berglin, L. Anatomic Alterations in Aging and Age-Related Diseases of the Eye. Investig. Ophthalmol. Vis. Sci. 2013, 54, ORSF23–ORSF27. [Google Scholar] [CrossRef] [PubMed]
- Rossi, T.; Romano, M.R.; Iannetta, D.; Romano, V.; Gualdi, L.; D’Agostino, I.; Ripandelli, G. Cataract Surgery Practice Patterns Worldwide: A Survey. BMJ Open Ophthalmol. 2021, 6, e000464. [Google Scholar] [CrossRef] [PubMed]
- Cho, H.; Wolf, K.J.; Wolf, E.J. Management of Ocular Inflammation and Pain Following Cataract Surgery: Focus on Bromfenac Ophthalmic Solution. Clin. Ophthalmol. 2009, 3, 199–210. [Google Scholar] [CrossRef]
- Laurell, C.-G.; Zetterström, C. Effects of Dexamethasone, Diclofenac, or Placebo on the Inflammatory Response after Cataract Surgery. Br. J. Ophthalmol. 2002, 86, 1380–1384. [Google Scholar] [CrossRef]
- Holland, E.J.; Fingeret, M.; Mah, F.S. Use of Topical Steroids in Conjunctivitis: A Review of the Evidence. Cornea 2019, 38, 1062–1067. [Google Scholar] [CrossRef]
- Chennamaneni, S.R.; Mamalis, C.; Archer, B.; Oakey, Z.; Ambati, B.K. Development of a Novel Bioerodible Dexamethasone Implant for Uveitis and Postoperative Cataract Inflammation. J. Control. Release 2013, 167, 53–59. [Google Scholar] [CrossRef]
- Pianini, V.; Passani, A.; Rossi, G.C.M.; Passani, F. Efficacy and Safety of Netilmycin/Dexamethasone Preservative-Free and Tobramycin/Dexamethasone-Preserved Fixed Combination in Patients after Cataract Surgery. J. Ocul. Pharmacol. Ther. 2010, 26, 617–621. [Google Scholar] [CrossRef]
- Patel, A.; Cholkar, K.; Agrahari, V.; Mitra, A.K. Ocular Drug Delivery Systems: An Overview. World J. Pharmacol. 2013, 2, 47–64. [Google Scholar] [CrossRef]
- Missotten, L.; Richard, C.; Trinquand, C. Topical 0.1% Indomethacin Solution versus Topical 0.1% Dexamethasone Solution in the Prevention of Inflammation after Cataract Surgery. The Study Group. Ophthalmologica 2001, 215, 43–50. [Google Scholar] [CrossRef]
- Anselmo, A.C.; Mitragotri, S. An Overview of Clinical and Commercial Impact of Drug Delivery Systems. J. Control. Release 2014, 190, 15–28. [Google Scholar] [CrossRef] [PubMed]
- Rizzo, S.; Gambini, G.; De Vico, U.; Rizzo, C.; Kilian, R. A One-Week Course of Levofloxacin/Dexamethasone Eye Drops: A Review on a New Approach in Managing Patients After Cataract Surgery. Ophthalmol. Ther. 2022, 11, 101–111. [Google Scholar] [CrossRef] [PubMed]
- Gaudana, R.; Ananthula, H.K.; Parenky, A.; Mitra, A.K. Ocular Drug Delivery. AAPS J. 2010, 12, 348–360. [Google Scholar] [CrossRef] [PubMed]
- Barry, P.; Cordovés, L.; Gardner, S. ESCRS Guidelines for Prevention and Treatment of Endophthalmitis following Cataract Surgery: Data, Dilemmas and Conclusions; ESCRS: London, UK, 2013. [Google Scholar]
- Mittal, N.; Kaur, G. Investigations on Polymeric Nanoparticles for Ocular Delivery. Adv. Polym. Technol. 2019, 2019, 1316249. [Google Scholar] [CrossRef]
- Reimondez-Troitiño, S.; Csaba, N.; Alonso, M.J.; de la Fuente, M. Nanotherapies for the Treatment of Ocular Diseases. Eur. J. Pharm. Biopharm. 2015, 95, 279–293. [Google Scholar] [CrossRef]
- Cholkar, K.; Patel, A.; Vadlapudi, A.D.; Mitra, A.K. Novel Nanomicellar Formulation Approaches for Anterior and Posterior Segment Ocular Drug Delivery. Recent Pat. Nanomedicinee 2012, 2, 82–95. [Google Scholar] [CrossRef]
- Başaran, E.; Yenilmez, E.; Berkman, M.S.; Büyükköroğlu, G.; Yazan, Y. Chitosan Nanoparticles for Ocular Delivery of Cyclosporine A. J. Microencapsul. 2014, 31, 49–57. [Google Scholar] [CrossRef]
- Rampino, A.; Borgogna, M.; Blasi, P.; Bellich, B.; Cesàro, A. Chitosan Nanoparticles: Preparation, Size Evolution and Stability. Int. J. Pharm. 2013, 455, 219–228. [Google Scholar] [CrossRef]
- Robinson, J.R.; Longer, M.A.; Veillard, M. Bioadhesive Polymers for Controlled Drug Delivery. Ann. New York Acad. Sci. 1987, 507, 307–314. [Google Scholar] [CrossRef]
- Sogias, I.A.; Williams, A.C.; Khutoryanskiy, V.V. Why Is Chitosan Mucoadhesive? Biomacromolecules 2008, 9, 1837–1842. [Google Scholar] [CrossRef] [PubMed]
- Pawlik, A.; Gawrońska-Szklarz, B.; Baśkiewicz-Masiuk, M.; Machaliński, B.; Safranow, K. Involvement of P-Glycoprotein in the Release of Cytokines from Peripheral Blood Mononuclear Cells Treated with Methotrexate and Dexamethasone. J. Pharm. Pharmacol. 2010, 57, 1421–1425. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Lu, M.; Sun, X.; Li, C.; Kuang, X.; Ruan, X. Expression and Activity of P-Glycoprotein Elevated by Dexamethasone in Cultured Retinal Pigment Epithelium Involve Glucocorticoid Receptor and Pregnane X Receptor. Investig. Opthalmol. Vis. Sci. 2012, 53, 3508–3515. [Google Scholar] [CrossRef] [PubMed]
- Sethi, S.; Malik, M.A.; Goswami, S.; Saxena, P.; Srivastava, A.; Kashyap, S.; Pushker, N.; Bajaj, M.S.; Bakhshi, S.; Kaur, J. Expression of P-Glycoprotein in Human Retinoblastoma and Its Clinical Significance. Tumor Biol. 2014, 35, 11735–11740. [Google Scholar] [CrossRef] [PubMed]
- Loftsson, T.; Stefánsson, E. Topical Dexamethasone Delivery to the Retina: An Aqueous Cyclodextrin-Based Microsuspension. J. Drug Deliv. Sci. Technol. 2023, 81, 104281. [Google Scholar] [CrossRef]
- Haimhoffer, Á.; Rusznyák, Á.; Réti-Nagy, K.; Vasvári, G.; Váradi, J.; Vecsernyés, M.; Bácskay, I.; Fehér, P.; Ujhelyi, Z.; Fenyvesi, F. Cyclodextrins in Drug Delivery Systems and Their Effects on Biological Barriers. Sci. Pharm. 2019, 87, 33. [Google Scholar] [CrossRef]
- Parvathaneni, V.; Elbatanony, R.S.; Shukla, S.K.; Kulkarni, N.S.; Kanabar, D.D.; Chauhan, G.; Ayehunie, S.; Chen, Z.-S.; Muth, A.; Gupta, V. Bypassing P-Glycoprotein Mediated Efflux of Afatinib by Cyclodextrin Complexation—Evaluation of Intestinal Absorption and Anti-Cancer Activity. J. Mol. Liq. 2021, 327, 114866. [Google Scholar] [CrossRef]
- Loftsson, T.; Magnúsdóttir, A.; Másson, M.; Sigurjónsdóttir, J.F. Self-Association and Cyclodextrin Solubilization of Drugs. J. Pharm. Sci. 2002, 91, 2307–2316. [Google Scholar] [CrossRef]
- Ricci, F.; Racaniello, G.F.; Lopedota, A.; Laquintana, V.; Arduino, I.; Lopalco, A.; Cutrignelli, A.; Franco, M.; Sigurdsson, H.H.; Denora, N. Chitosan/Sulfobutylether-β-Cyclodextrin Based Nanoparticles Coated with Thiolated Hyaluronic Acid for Indomethacin Ophthalmic Delivery. Int. J. Pharm. 2022, 622, 121905. [Google Scholar] [CrossRef] [PubMed]
- Tongiani, S.; Ozeki, T.; Stella, V.J. Sulfobutyl Ether-Alkyl Ether Mixed Cyclodextrin Derivatives with Enhanced Inclusion Ability. J. Pharm. Sci. 2009, 98, 4769–4780. [Google Scholar] [CrossRef]
- Zia, V.; Rajewski, R.A.; Stella, V.J. Effect of Cyclodextrin Charge on Complexation of Neutral and Charged Substrates: Comparison of (SBE)7M-β-CD to HP-β-CD. Pharm. Res. 2001, 18, 667–673. [Google Scholar] [CrossRef] [PubMed]
- Connors, K.A. The Stability of Cyclodextrin Complexes in Solution. Chem. Rev. 1997, 97, 1325–1358. [Google Scholar] [CrossRef]
- Piras, A.M.; Zambito, Y.; Burgalassi, S.; Monti, D.; Tampucci, S.; Terreni, E.; Fabiano, A.; Balzano, F.; Uccello-Barretta, G.; Chetoni, P. A Water-Soluble, Mucoadhesive Quaternary Ammonium Chitosan-Methyl-β-Cyclodextrin Conjugate Forming Inclusion Complexes with Dexamethasone. J. Mater. Sci. Mater. Med. 2018, 29, 42. [Google Scholar] [CrossRef]
- Denora, N.; Laquintana, V.; Lopedota, A.; Serra, M.; Dazzi, L.; Biggio, G.; Pal, D.; Mitra, A.K.; Latrofa, A.; Trapani, G.; et al. Novel L-Dopa and Dopamine Prodrugs Containing a 2-Phenyl-Imidazopyridine Moiety. Pharm. Res. 2007, 24, 1309–1324. [Google Scholar] [CrossRef]
- Higuchi, T.; Connors, K.A. Advances in Analytical Chemistry and Instrumentation. Phase Solubility Stud. 1965, 1, 117–212. [Google Scholar]
- Racaniello, G.F.; Laquintana, V.; Summonte, S.; Lopedota, A.; Cutrignelli, A.; Lopalco, A.; Franco, M.; Bernkop-Schnürch, A.; Denora, N. Spray-Dried Mucoadhesive Microparticles Based on S-Protected Thiolated Hydroxypropyl-β-Cyclodextrin for Budesonide Nasal Delivery. Int. J. Pharm. 2021, 603, 120728. [Google Scholar] [CrossRef]
- Ricci, F.; Racaniello, G.F.; Denora, N.; Gentile, L.; Lopalco, A.; Cutrignelli, A.; Franco, M.; Iacobazzi, R.M.; Laquintana, V.; Lopedota, A. Thermoresponsive Mucoadhesive Hydrogel Based on Pluronic F127/Thiolated Glycol Chitosan for Intravesical Administration of Celecoxib/Gemcitabine. J. Drug Deliv. Sci. Technol. 2023, 86, 104687. [Google Scholar] [CrossRef]
- Cutrignelli, A.; Lopedota, A.; Denora, N.; Iacobazzi, R.M.; Fanizza, E.; Laquintana, V.; Perrone, M.; Maggi, V.; Franco, M. A New Complex of Curcumin with Sulfobutylether-β-Cyclodextrin: Characterization Studies and In Vitro Evaluation of Cytotoxic and Antioxidant Activity on HepG-2 Cells. J. Pharm. Sci. 2014, 103, 3932–3940. [Google Scholar] [CrossRef]
- Calvo, P.; Remuñan-López, C.; Vila-Jato, J.L.; Alonso, M.J. Chitosan and Chitosan/Ethylene Oxide-Propylene Oxide Block Copolymer Nanoparticles as Novel Carriers for Proteins and Vaccines. Pharm. Res. 1997, 14, 1431–1436. [Google Scholar] [CrossRef]
- Fülöp, Z.; Saokham, P.; Loftsson, T. Sulfobutylether-β-Cyclodextrin/Chitosan Nano- and Microparticles and Their Physicochemical Characteristics. Int. J. Pharm. 2014, 472, 282–287. [Google Scholar] [CrossRef]
- Serratì, S.; Guida, M.; Di Fonte, R.; De Summa, S.; Strippoli, S.; Iacobazzi, R.M.; Quarta, A.; De Risi, I.; Guida, G.; Paradiso, A.; et al. Circulating Extracellular Vesicles Expressing PD1 and PD-L1 Predict Response and Mediate Resistance to Checkpoint Inhibitors Immunotherapy in Metastatic Melanoma. Mol. Cancer 2022, 21, 20. [Google Scholar] [CrossRef]
- Srividya, B.; Cardoza, R.M.; Amin, P.D. Sustained Ophthalmic Delivery of Ofloxacin from a pH Triggered in Situ Gelling System. J. Control. Release 2001, 73, 205–211. [Google Scholar] [CrossRef] [PubMed]
- Racaniello, G.F.; Pistone, M.; Meazzini, C.; Lopedota, A.; Arduino, I.; Rizzi, R.; Lopalco, A.; Musazzi, U.M.; Cilurzo, F.; Denora, N. 3D Printed Mucoadhesive Orodispersible Films Manufactured by Direct Powder Extrusion for Personalized Clobetasol Propionate Based Paediatric Therapies. Int. J. Pharm. 2023, 643, 123214. [Google Scholar] [CrossRef] [PubMed]
- Jansook, P.; Prajapati, M.; Pruksakorn, P.; Loftsson, T. Antifungal Activity of Econazole Nitrate/Cyclodextrin Complex: Effect of pH and Formation of Complex Aggregates. Int. J. Pharm. 2020, 574, 118896. [Google Scholar] [CrossRef] [PubMed]
- Jain, A.; Jain, S.K. In Vitro Release Kinetics Model Fitting of Liposomes: An Insight. Chem. Phys. Lipids 2016, 201, 28–40. [Google Scholar] [CrossRef] [PubMed]
- Bruschi, M.L. Mathematical Models of Drug Release. In Strategies to Modify the Drug Release from Pharmaceutical Systems; Woodhead Publishing: Cambridge, UK, 2015; pp. 63–86. ISBN 978-0-08-100092-2. [Google Scholar]
- Barzegar-Jalali, M.; Adibkia, K.; Valizadeh, H.; Shadbad, M.R.S.; Nokhodchi, A.; Omidi, Y.; Mohammadi, G.; Nezhadi, S.H.; Hasan, M. Kinetic Analysis of Drug Release from Nanoparticles. J. Pharm. Pharm. Sci. 2008, 11, 167–177. [Google Scholar] [CrossRef]
- Pisani, L.; Iacobazzi, R.M.; Catto, M.; Rullo, M.; Farina, R.; Denora, N.; Cellamare, S.; Altomare, C.D. Investigating Alkyl Nitrates as Nitric Oxide Releasing Precursors of Multitarget Acetylcholinesterase-Monoamine Oxidase B Inhibitors. Eur. J. Med. Chem. 2019, 161, 292–309. [Google Scholar] [CrossRef] [PubMed]
- Gautheron, P.; Dukic, M.; Alix, D.; Sina, J.F. Bovine Corneal Opacity and Permeability Test: An in Vitro Assay of Ocular Irritancy. Fundam. Appl. Toxicol. 1992, 18, 442–449. [Google Scholar] [CrossRef] [PubMed]
- OECD. Draft Revised TG 438 Isolated Chicken Eye Test Method for Identifying (I) Chemicals Inducing Serious Eye Damage and (II) Chemicals Not Requiring Classification for Eye Irritation or Serious Eye Damage. In Streamlined Summary Document Supporting OECD Test Guideline 438 on the Isolated Chicken Eye for Eye Irritation/Corrosion; OECD: Paris, France, 2017. [Google Scholar]
- Dilova, V.; Zlatarova, V.; Spirova, N.; Filcheva, K.; Pavlova, A.; Grigorova, P. Study of Insolubility Problems of Dexamethasone and Digoxin: Cyclodextrin Complexation. Boll. Chim. Farm. 2004, 143, 20–23. [Google Scholar]
- Pistone, M.; Racaniello, G.F.; Arduino, I.; Laquintana, V.; Lopalco, A.; Cutrignelli, A.; Rizzi, R.; Franco, M.; Lopedota, A.; Denora, N. Direct Cyclodextrin-Based Powder Extrusion 3D Printing for One-Step Production of the BCS Class II Model Drug Niclosamide. Drug Deliv. Transl. Res. 2022, 12, 1895–1910. [Google Scholar] [CrossRef]
- Vianna, R.F.L.; Bentley, M.V.L.B.; Ribeiro, G.; Carvalho, F.S.; Neto, A.F.; de Oliveira, D.C.R.; Collett, J.H. Formation of Cyclodextrin Inclusion Complexes with Corticosteroids: Their Characterization and Stability. Int. J. Pharm. 1998, 167, 205–213. [Google Scholar] [CrossRef]
- Doile, M.M.; Fortunato, K.A.; Schmücker, I.C.; Schucko, S.K.; Silva, M.A.S.; Rodrigues, P.O. Physicochemical Properties and Dissolution Studies of Dexamethasone Acetate-β-Cyclodextrin Inclusion Complexes Produced by Different Methods. AAPS PharmSciTech 2008, 9, 314–321. [Google Scholar] [CrossRef]
- Sommonte, F.; Arduino, I.; Racaniello, G.F.; Lopalco, A.; Lopedota, A.A.; Denora, N. The Complexity of the Blood-Brain Barrier and the Concept of Age-Related Brain Targeting: Challenges and Potential of Novel Solid Lipid-Based Formulations. J. Pharm. Sci. 2022, 111, 577–592. [Google Scholar] [CrossRef] [PubMed]
- Bugnicourt, L.; Alcouffe, P.; Ladavière, C. Elaboration of Chitosan Nanoparticles: Favorable Impact of a Mild Thermal Treatment to Obtain Finely Divided, Spherical, and Colloidally Stable Objects. Colloids Surf. A Physicochem. Eng. Asp. 2014, 457, 476–486. [Google Scholar] [CrossRef]
- Zhao, K.; Xie, Y.; Lin, X.; Xu, W. The Mucoadhesive Nanoparticle-Based Delivery System in the Development of Mucosal Vaccines. Int. J. Nanomed. 2022, 17, 4579–4598. [Google Scholar] [CrossRef]
- Vasquez-Martínez, N.; Guillen, D.; Moreno-Mendieta, S.A.; Sanchez, S.; Rodríguez-Sanoja, R. The Role of Mucoadhesion and Mucopenetration in the Immune Response Induced by Polymer-Based Mucosal Adjuvants. Polymers 2023, 15, 1615. [Google Scholar] [CrossRef]
- Rodrigues, L.B.; Leite, H.F.; Yoshida, M.I.; Saliba, J.B.; Junior, A.S.C.; Faraco, A.A.G. In Vitro Release and Characterization of Chitosan Films as Dexamethasone Carrier. Int. J. Pharm. 2009, 368, 1–6. [Google Scholar] [CrossRef]
- Das, S.K.; Kahali, N.; Bose, A.; Khanam, J. Physicochemical Characterization and in Vitro Dissolution Performance of Ibuprofen-Captisol® (Sulfobutylether Sodium Salt of β-CD) Inclusion Complexes. J. Mol. Liq. 2018, 261, 239–249. [Google Scholar] [CrossRef]
- Hombach, J.; Bernkop-Schnürch, A. Mucoadhesive Drug Delivery Systems BT—Drug Delivery; Schäfer-Korting, M., Ed.; Springer: Berlin/Heidelberg, Germany, 2010; pp. 251–266. ISBN 978-3-642-00477-3. [Google Scholar]
- Mantuano, P.; Boccanegra, B.; Conte, E.; De Bellis, M.; Cirmi, S.; Sanarica, F.; Cappellari, O.; Arduino, I.; Cutrignelli, A.; Lopedota, A.A.; et al. β-Dystroglycan Restoration and Pathology Progression in the Dystrophic Mdx Mouse: Outcome and Implication of a Clinically Oriented Study with a Novel Oral Dasatinib Formulation. Biomolecules 2021, 11, 1742. [Google Scholar] [CrossRef]
- Kim, D.-H.; Martin, D.C. Sustained Release of Dexamethasone from Hydrophilic Matrices Using PLGA Nanoparticles for Neural Drug Delivery. Biomaterials 2006, 27, 3031–3037. [Google Scholar] [CrossRef] [PubMed]
- Yu, A.; Shi, H.; Liu, H.; Bao, Z.; Dai, M.; Lin, D.; Lin, D.; Xu, X.; Li, X.; Wang, Y. Mucoadhesive Dexamethasone-Glycol Chitosan Nanoparticles for Ophthalmic Drug Delivery. Int. J. Pharm. 2020, 575, 118943. [Google Scholar] [CrossRef]
- Davidson, H.J.; Kuonen, V.J. The Tear Film and Ocular Mucins. Vet. Ophthalmol. 2004, 7, 71–77. [Google Scholar] [CrossRef]
- Rafie, F.; Javadzadeh, Y.; Javadzadeh, A.R.; Ghavidel, L.A.; Jafari, B.; Moogooee, M.; Davaran, S. In Vivo Evaluation of Novel Nanoparticles Containing Dexamethasone for Ocular Drug Delivery on Rabbit Eye. Curr. Eye Res. 2010, 35, 1081–1089. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, L.; Atif, R.; Eldeen, T.S.; Yahya, I.; Omara, A.; Eltayeb, M. Study the Using of Nanoparticles as Drug Delivery System Based on Mathematical Models for Controlled Release. Int. J. Latest Technol. Eng. Manag. Appl. Sci. 2019, 8, 52–56. [Google Scholar]
- OECD. Test No. 437: Bovine Corneal Opacity and Permeability Test Method for Identifying (i) Chemicals Inducing Serious Eye Damage and (ii) Chemicals Not Requiring Classification for Eye Irritation or Serious Eye Damage; OECD: Paris, France, 2020. [Google Scholar]









| Samples | 1% w/v SBE-β-CD Solution (mL) | 0.5% w/v CS Solution (mL) |
|---|---|---|
| Low Mw CS NPs | 1 | 1.4 |
| Low Mw CS NPs | 2 | 5 |
| Medium Mw CS NPs | 1 | 1.4 |
| Medium Mw CS NPs | 2 | 5 |
| High Mw CS NPs | 1 | 1.4 |
| High Mw CS NPs | 2 | 5 |
| Oligomer CS NPs | 1 | 1.4 |
| Oligomer CS NPs | 2 | 5 |
| Kinetic Model | Equations | |
|---|---|---|
| Zero Order | Dt =amount of drug dissolved in time t; D0 = initial amount of drug in solution; K0 = zero-order release constant; | |
| First Order | C0 = initial drug concentration; K = first-order rate constant, t = time; | |
| Higuchi | KH = Higuchi dissolution constant, t = time; | |
| Hixson–Crowell | fi = fraction of drug dissolved in time t; Kβ = release constant; | |
| Korsmeyer–Peppas | Mt/M∞ = fraction of drug released at time t; K = release rate constant, ɳ = release exponent. |
| Formulation | Diameter (nm) | PDI | ζ-Potential (mV) | EE (%) |
|---|---|---|---|---|
| Low MW CS NPs | 210.3 ± 5.2 | 0.161 ± 0.047 | +29.4 ± 0.7 | / |
| Medium MW CS NPs | 342.7 ± 9.3 | 0.327 ± 0.121 | +17.1 ± 0.5 | / |
| High MW CS NPs | 391.1 ± 12.3 | 0.359 ± 0.174 | +19.6 ± 0.8 | / |
| Oligomer CS NPs | / | / | / | / |
| DEX-loaded Low MW CS NPs | 212.9 ± 5.3 | 0.155 ± 0.048 | +31.7 ± 0.4 | 87.1 |
| Kinetic Model | R2 | n | R2 | n |
|---|---|---|---|---|
| 0–60 min | 60–360 min | |||
| Zero Order | 0.9847 | 0.9967 | ||
| First Order | 0.9963 | 0.9653 | ||
| Higuchi | 0.9377 | 0.9703 | ||
| Hixson–Crowell | 0.9938 | 0.9793 | ||
| Korsmeyer–Peppas | 0.9988 | 1.373 | 0.9612 | 2.502 |
| Formulation | Flux (J) µg h−1 cm−2 | Apparent Permeability Coefficient Papp × 10−6 (cm/s) | Transport Enhancement Ratio R = [Papp(s)/Papp(c)] |
|---|---|---|---|
| DEX-loaded NPs | 6.64 | 2.13 ± 0.08 | 2.04 |
| Control | 3.22 | 1.04 ± 0.02 | / |
| Formulation | Papp AP-BL × 10−7 (cm/s) | Papp BL-AP × 10−7 (cm/s) | ER (PappBL/PappAP) |
|---|---|---|---|
| DEX | 0.66 ± 0.05 | 1.87 ± 0.07 | 2.84 |
| DEX-loaded NPs | 0.92 ± 0.03 | 0.85 ± 0.03 | 0.92 |
| Diazepam | 146 ± 10 | 123 ± 9 | 0.84 |
| FD4 | 10 ± 1 | 2.0 ± 0.2 | 0.2 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Racaniello, G.F.; Balenzano, G.; Arduino, I.; Iacobazzi, R.M.; Lopalco, A.; Lopedota, A.A.; Sigurdsson, H.H.; Denora, N. Chitosan and Anionic Solubility Enhancer Sulfobutylether-β-Cyclodextrin-Based Nanoparticles as Dexamethasone Ophthalmic Delivery System for Anti-Inflammatory Therapy. Pharmaceutics 2024, 16, 277. https://doi.org/10.3390/pharmaceutics16020277
Racaniello GF, Balenzano G, Arduino I, Iacobazzi RM, Lopalco A, Lopedota AA, Sigurdsson HH, Denora N. Chitosan and Anionic Solubility Enhancer Sulfobutylether-β-Cyclodextrin-Based Nanoparticles as Dexamethasone Ophthalmic Delivery System for Anti-Inflammatory Therapy. Pharmaceutics. 2024; 16(2):277. https://doi.org/10.3390/pharmaceutics16020277
Chicago/Turabian StyleRacaniello, Giuseppe Francesco, Gennaro Balenzano, Ilaria Arduino, Rosa Maria Iacobazzi, Antonio Lopalco, Angela Assunta Lopedota, Hakon Hrafn Sigurdsson, and Nunzio Denora. 2024. "Chitosan and Anionic Solubility Enhancer Sulfobutylether-β-Cyclodextrin-Based Nanoparticles as Dexamethasone Ophthalmic Delivery System for Anti-Inflammatory Therapy" Pharmaceutics 16, no. 2: 277. https://doi.org/10.3390/pharmaceutics16020277