The Necessity to Investigate In Vivo Fate of Nanoparticle-Loaded Dissolving Microneedles
Abstract
:1. Transdermal Drug Delivery System: A Rising Star
2. Challenge of Transdermal Drug Delivery System: Low Transdermal Absorption Rate
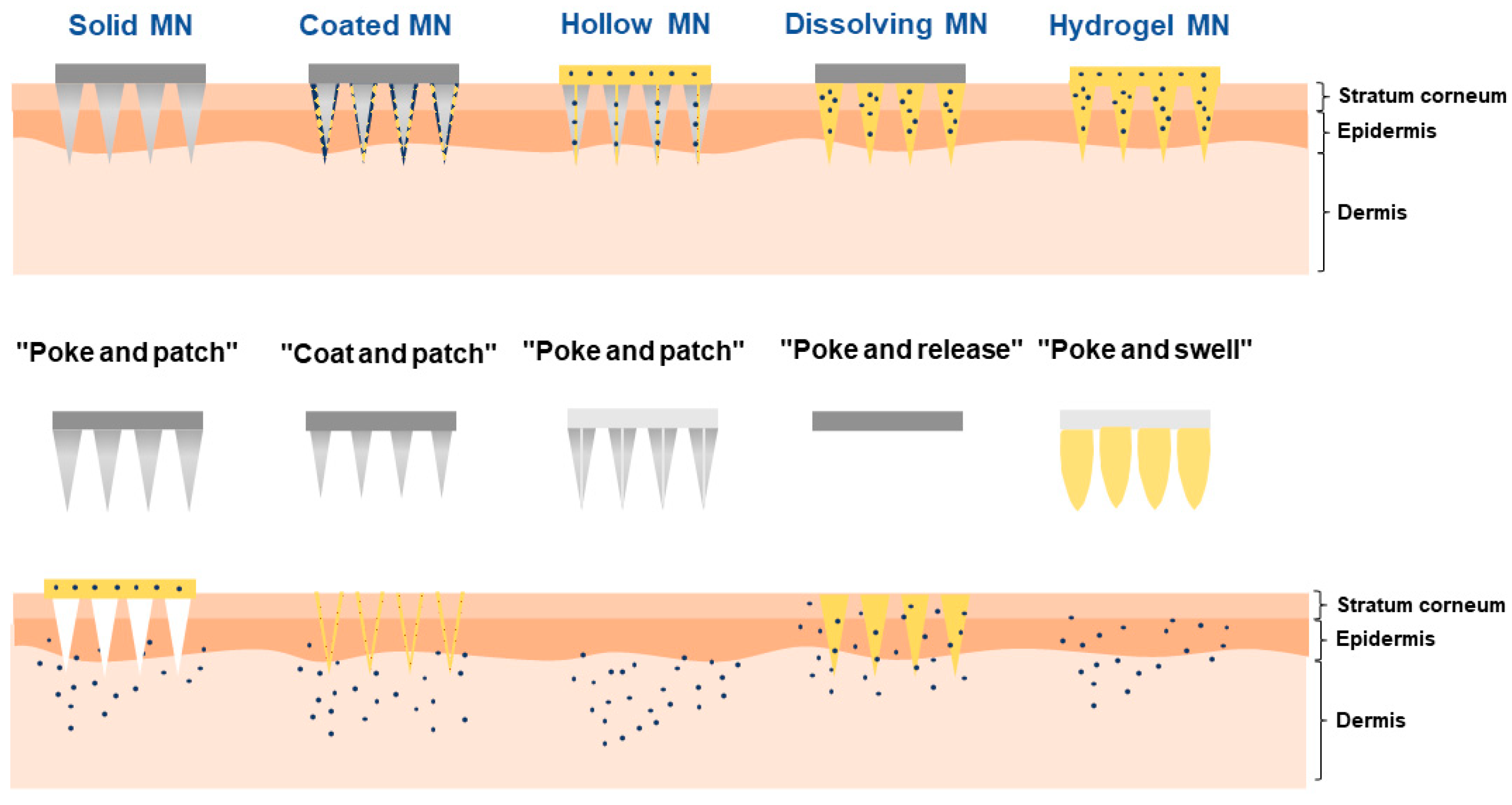
3. Overcoming the Challenge with Dissolving Microneedles
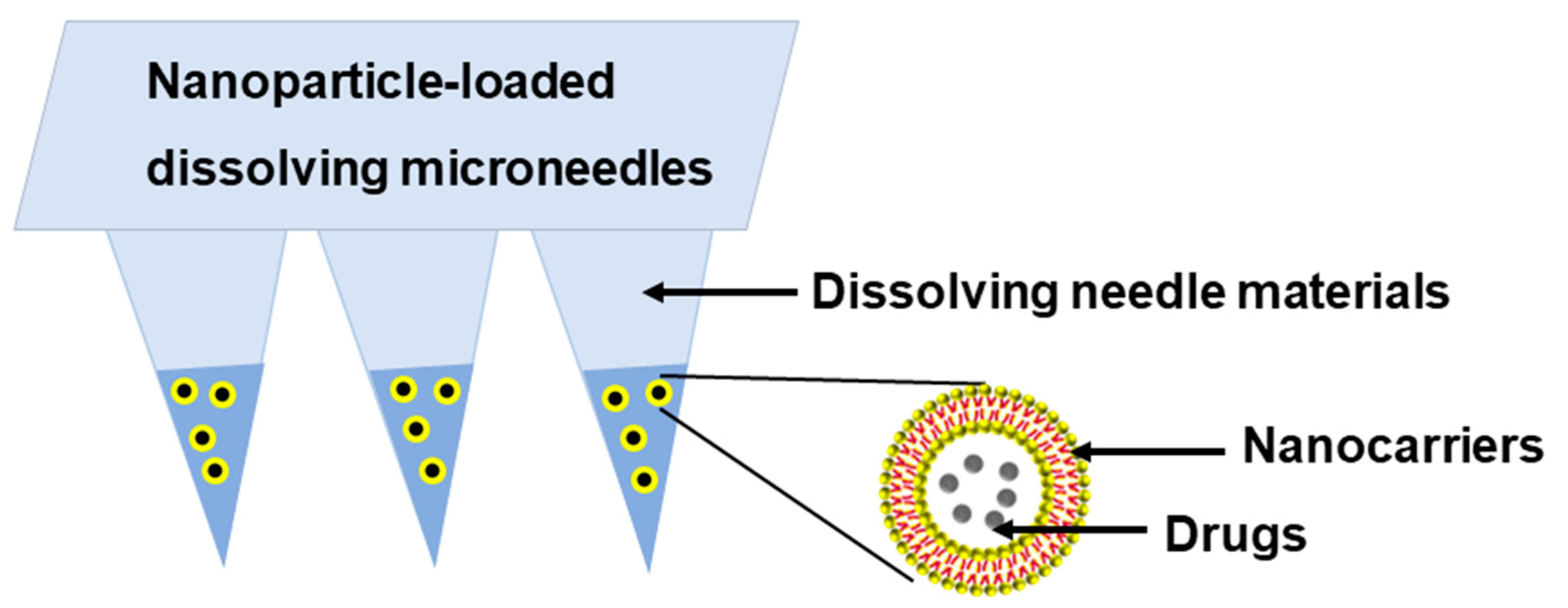
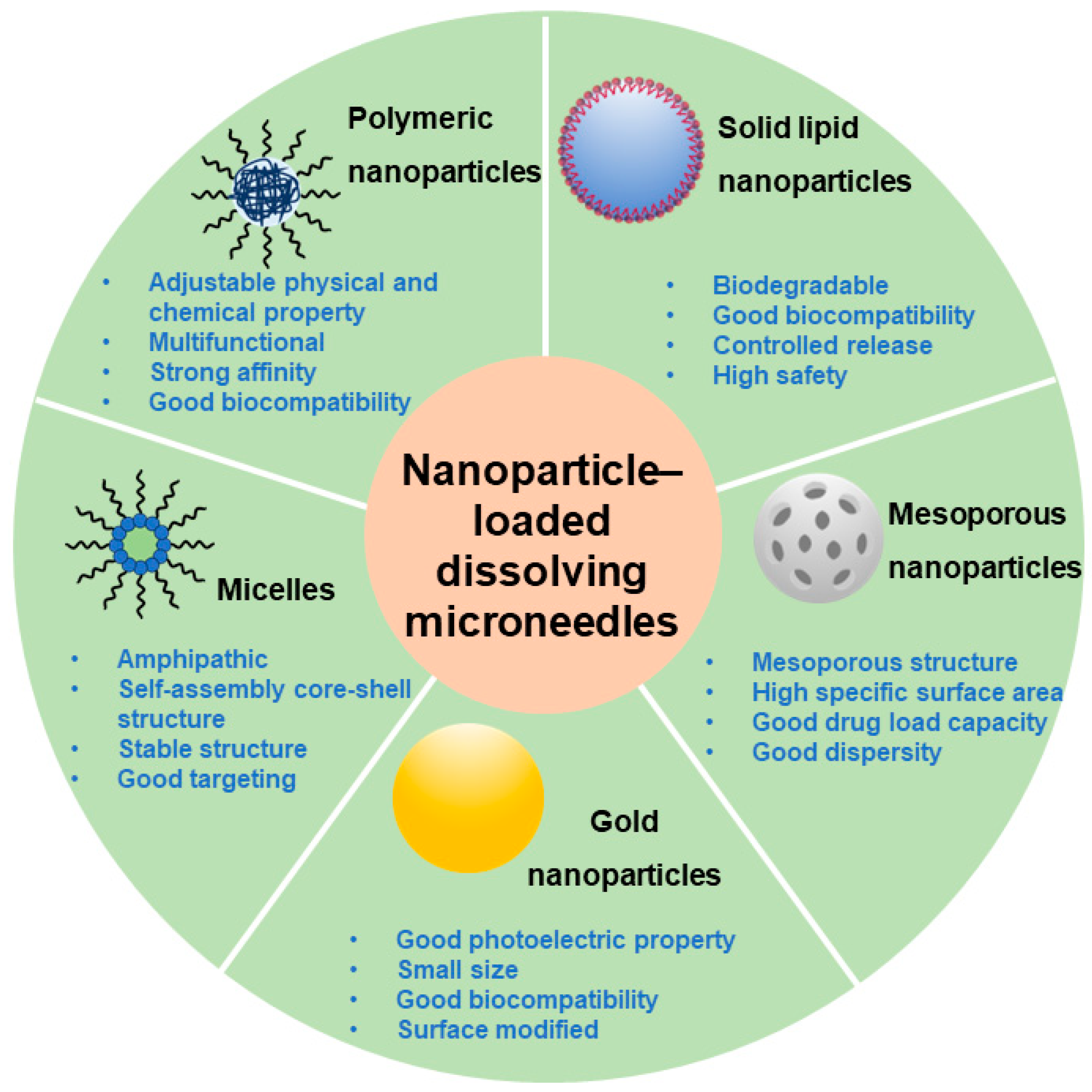
4. Nanoparticle-Loaded DMNs: A Better Choice for Precision Medication
5. Necessity of In Vivo Fate Study
5.1. Necessity Analysis from Clinical Perspective
5.2. Necessity Analysis from the Industrial Perspective
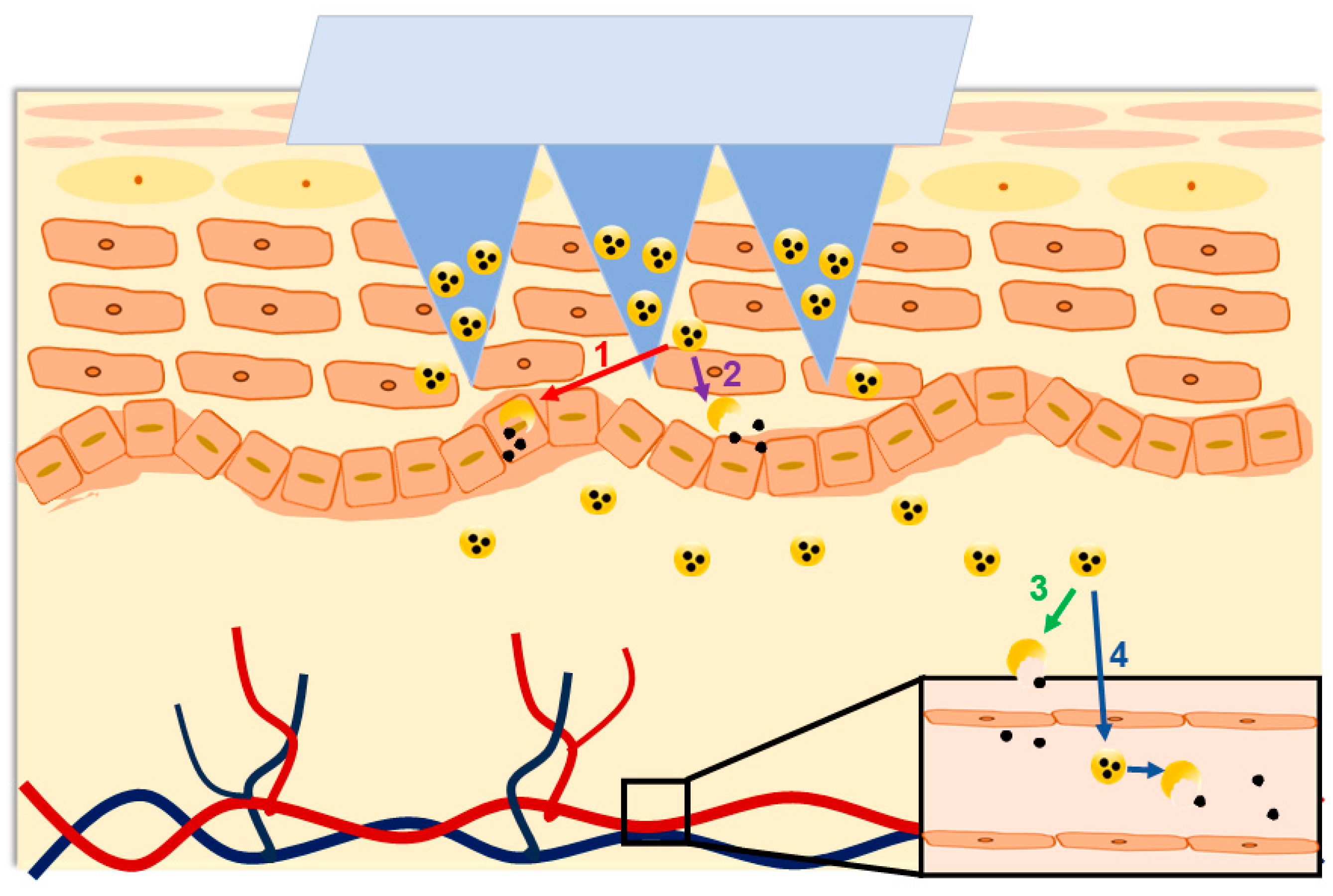
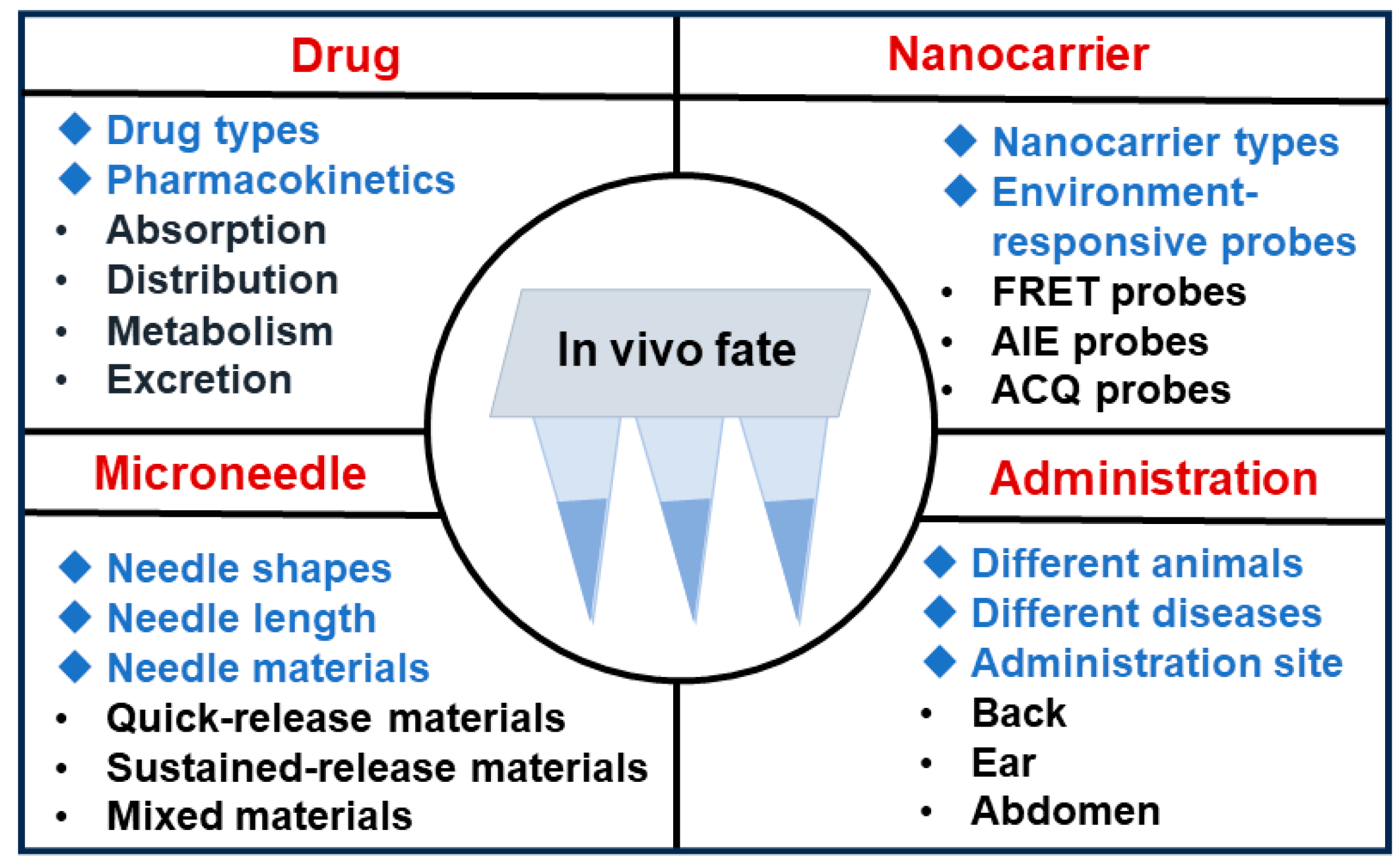
6. Entry Points for In Vivo Fate Studies
7. Outlook
8. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Jain, K.K. An Overview of Drug Delivery Systems. Methods Mol. Biol. 2020, 2059, 1–54. [Google Scholar]
- Park, H.; Otte, A.; Park, K. Evolution of drug delivery systems: From 1950 to 2020 and beyond. J. Control. Release 2022, 342, 53–65. [Google Scholar] [CrossRef] [PubMed]
- Anselmo, A.C.; Mitragotri, S. An overview of clinical and commercial impact of drug delivery systems. J. Control. Release 2014, 190, 15–28. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Yao, W.; Rao, Y.; Lu, X.; Gao, J. pH-Responsive carriers for oral drug delivery: Challenges and opportunities of current platforms. Drug Delivery 2017, 24, 569–581. [Google Scholar] [CrossRef] [PubMed]
- Dai, T.; Jiang, W.; Guo, Z.; Wang, Z.; Huang, M.; Zhong, G.; Liang, C.; Pei, X.; Dai, R. Studies on oral bioavailability and first-pass metabolism of withaferin A in rats using LC-MS/MS and Q-TRAP. Biomed. Chromatogr. 2019, 33, e4573. [Google Scholar] [CrossRef] [PubMed]
- Marwah, H.; Garg, T.; Goyal, A.K.; Rath, G. Permeation enhancer strategies in transdermal drug delivery. Drug Delivery 2016, 23, 564–578. [Google Scholar] [CrossRef]
- Lam, P.L.; Gambari, R. Advanced progress of microencapsulation technologies: In Vivo and In Vitro models for studying oral and transdermal drug deliveries. J. Control. Release 2014, 178, 25–45. [Google Scholar] [CrossRef]
- Ramadon, D.; McCrudden, M.T.C.; Courtenay, A.J.; Donnelly, R.F. Enhancement strategies for transdermal drug delivery systems: Current trends and applications. Drug Delivery Transl. Res. 2022, 12, 758–791. [Google Scholar] [CrossRef]
- Dasht Bozorg, B.; Bhattaccharjee, S.A.; Somayaji, M.R.; Banga, A.K. Topical and transdermal delivery with diseased human skin: Passive and iontophoretic delivery of hydrocortisone into psoriatic and eczematous skin. Drug Delivery Transl. Res. 2022, 12, 197–212. [Google Scholar] [CrossRef]
- Bird, D.; Ravindra, N.M. Transdermal drug delivery and patches-An overview. Med. Devices Sens. 2020, 3, e10069. [Google Scholar] [CrossRef]
- Carter, P.; Narasimhan, B.; Wang, Q. Biocompatible nanoparticles and vesicular systems in transdermal drug delivery for various skin diseases. Int. J. Pharm. 2019, 555, 49–62. [Google Scholar] [CrossRef] [PubMed]
- Jorgensen, A.M.; Gorkun, A.; Mahajan, N.; Willson, K.; Clouse, C.; Jeong, C.G.; Varkey, M.; Wu, M.; Walker, S.J.; Molnar, J.A.; et al. Multicellular bioprinted skin facilitates human-like skin architecture in vivo. Sci. Transl. Med. 2023, 15, eadf7547. [Google Scholar] [CrossRef] [PubMed]
- Manikkath, J.; Sumathy, T.K.; Manikkath, A.; Mutalik, S. Delving Deeper into Dermal and Transdermal Drug Delivery: Factors and Mechanisms Associated with Nanocarrier-mediated Strategies. Curr. Pharm. Des. 2018, 24, 3210–3222. [Google Scholar] [CrossRef] [PubMed]
- Schmitt, T.; Neubert, R.H.H. State of the Art in Stratum Corneum Research. Part II: Hypothetical Stratum Corneum Lipid Matrix Models. Skin Pharmacol. Physiol. 2020, 33, 213–230. [Google Scholar] [CrossRef] [PubMed]
- Gupta, M.; Agrawal, U.; Vyas, S.P. Nanocarrier-based topical drug delivery for the treatment of skin diseases. Expert Opin. Drug Delivery 2012, 9, 783–804. [Google Scholar] [CrossRef]
- Iwai, I.; Han, H.; den Hollander, L.; Svensson, S.; Oefverstedt, L.-G.; Anwar, J.; Brewer, J.; Bloksgaard, M.; Laloeuf, A.; Nosek, D.; et al. The Human Skin Barrier Is Organized as Stacked Bilayers of Fully Extended Ceramides with Cholesterol Molecules Associated with the Ceramide Sphingoid Moiety. J. Investig. Dermatol. 2012, 132, 2215–2225. [Google Scholar] [CrossRef]
- Sa, G.F.F.; Serpa, C.; Arnaut, L.G. Stratum corneum permeabilization with photoacoustic waves generated by piezophotonic materials. J. Control. Release 2013, 167, 290–300. [Google Scholar] [CrossRef]
- Anantrao, J.H.; Nath, P.A.; Nivrutti, P.R. Drug Penetration Enhancement Techniques in Transdermal Drug Delivery System: A Review. J. Pharm. Res. Int. 2021, 33, 46–61. [Google Scholar] [CrossRef]
- Yu, Y.-Q.; Yang, X.; Wu, X.-F.; Fan, Y.-B. Enhancing Permeation of Drug Molecules Across the Skin via Delivery in Nanocarriers: Novel Strategies for Effective Transdermal Applications. Front. Bioeng. Biotechnol. 2021, 9, 646554. [Google Scholar] [CrossRef]
- Alkilani, A.Z.; Nasereddin, J.; Hamed, R.; Nimrawi, S.; Hussein, G.; Abo-Zour, H.; Donnelly, R.F. Beneath the Skin: A Review of Current Trends and Future Prospects of Transdermal Drug Delivery Systems. Pharmaceutics 2022, 14, 1152. [Google Scholar] [CrossRef] [PubMed]
- Jeong, W.Y.; Kwon, M.; Choi, H.E.; Kim, K.S. Recent advances in transdermal drug delivery systems: A review. Biomater. Res. 2021, 25, 24. [Google Scholar] [CrossRef]
- Qindeel, M.; Ullah, M.H.; Fakharud, D.; Ahmed, N.; Rehman, A.U. Recent trends, challenges and future outlook of transdermal drug delivery systems for rheumatoid arthritis therapy. J. Control. Release 2020, 327, 595–615. [Google Scholar] [CrossRef]
- Chakraborty, C.; Bhattacharya, M.; Lee, S.-S. Current Status of Microneedle Array Technology for Therapeutic Delivery: From Bench to Clinic. Mol. Biotechnol. 2023. [Google Scholar] [CrossRef]
- Ma, G.; Wu, C. Microneedle, bio-microneedle and bio-inspired microneedle: A review. J. Control. Release 2017, 251, 11–23. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Jin, Y.; Jiang, M.; Yang, F.; Luo, J.; Wang, Y.; Tao, X. Research Progress on Combination of Microneedle and New Transdermal Drug Delivery Carrier. Chin. J. Mod. Appl. Pharm. 2020, 37, 2170–2176. [Google Scholar]
- Harvey, A.J.; Kaestner, S.A.; Sutter, D.E.; Harvey, N.G.; Mikszta, J.A.; Pettis, R.J. Microneedle-Based Intradermal Delivery Enables Rapid Lymphatic Uptake and Distribution of Protein Drugs. Pharm. Res. 2011, 28, 107–116. [Google Scholar] [CrossRef] [PubMed]
- Donnelly, R.F.; Singh, T.R.R.; Woolfson, A.D. Microneedle-based drug delivery systems: Microfabrication, drug delivery, and safety. Drug Delivery 2010, 17, 187–207. [Google Scholar] [CrossRef] [PubMed]
- Vora, L.K.; Moffatt, K.; Tekko, I.A.; Paredes, A.J.; Volpe-Zanutto, F.; Mishra, D.; Peng, K.; Thakur, R.R.S.; Donnelly, R.F. Microneedle array systems for long-acting drug delivery. Eur. J. Pharm. Biopharm. 2021, 159, 44–76. [Google Scholar] [CrossRef] [PubMed]
- Oh, S.J.; Jung, J.H. Sustainable Drug Release Using Nanoparticle Encapsulated Microneedles. Chem. Asian J. 2022, 17, e202200333. [Google Scholar] [CrossRef] [PubMed]
- McCrudden, M.T.C.; McAlister, E.; Courtenay, A.J.; Gonzalez-Vazquez, P.; Singh, T.R.R.; Donnelly, R.F. Microneedle applications in improving skin appearance. Exp. Dermatol. 2015, 24, 561–566. [Google Scholar] [CrossRef] [PubMed]
- van der Maaden, K.; Jiskoot, W.; Bouwstra, J. Microneedle technologies for (trans)dermal drug and vaccine delivery. J. Control. Release 2012, 161, 645–655. [Google Scholar] [CrossRef]
- Lu, Z.; Du, S.; Li, J.; Zhang, M.; Nie, H.; Zhou, X.; Li, F.; Wei, X.; Wang, J.; Liu, F.; et al. Langmuir-Blodgett-Mediated Formation of Antibacterial Microneedles for Long-Term Transdermal Drug Delivery. Adv. Mater. 2023, 35, e2303388. [Google Scholar] [CrossRef]
- Chen, Y.-H.; Wang, F.-Y.; Chan, Y.-S.; Huang, C.; Huang, Y.-Y. Biofabricating hollow microneedle array with controllable microstructure for cell transplantation. J. Biomed. Mater. Res. Part B 2022, 110, 1997–2005. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.; Chen, M.; Yang, D.; Qin, W.; Quan, G.; Wu, C.; Pan, X. Self-assembly nanomicelle-microneedle patches with enhanced tumor penetration for superior chemo-photothermal therapy. Nano Res. 2022, 15, 2335–2346. [Google Scholar] [CrossRef]
- Liu, D.; Yu, B.; Jiang, G.; Yu, W.; Zhang, Y.; Xu, B. Fabrication of composite microneedles integrated with insulin-loaded CaCO3 microparticles and PVP for transdermal delivery in diabetic rats. Mater. Sci. Eng., C 2018, 90, 180–188. [Google Scholar] [CrossRef]
- Kim, Y.-C.; Park, J.-H.; Prausnitz, M.R. Microneedles for drug and vaccine delivery. Adv. Drug Delivery Rev. 2012, 64, 1547–1568. [Google Scholar] [CrossRef] [PubMed]
- Chen, M.-C.; Lai, K.-Y.; Ling, M.-H.; Lin, C.-W. Enhancing immunogenicity of antigens through sustained intradermal delivery using chitosan microneedles with a patch-dissolvable design. Acta Biomater. 2018, 65, 66–75. [Google Scholar] [CrossRef]
- Shi, Y.; Zhao, J.; Li, H.; Yu, M.; Zhang, W.; Qin, D.; Qiu, K.; Chen, X.; Kong, M. A Drug-Free, Hair Follicle Cycling Regulatable, Separable, Antibacterial Microneedle Patch for Hair Regeneration Therapy. Adv. Healthcare Mater. 2022, 11, e2200908. [Google Scholar] [CrossRef]
- Ping, Y.; Gao, Q.; Li, C.X.; Wang, Y.; Wang, Y.L.; Li, S.; Qiu, M.J.; Zhang, L.Q.; Tu, A.L.; Tian, Y.; et al. Construction of microneedle of Atractylodes macrocephala Rhizoma aqueous extract and effect on mammary gland hyperplasia based on intestinal flora. Front. Endocrinol. 2023, 14, 1158318. [Google Scholar] [CrossRef]
- Fang, Z.; Zhang, B.; Xing, W.; Yu, H.; Xing, C.; Gong, N.; Lu, Y.; Du, G. An Evolving Role of Aqueous Piperazine to Improve the Solubility of Non-Steroidal Anti-Inflammatory Drugs. J. Pharm. Sci. 2022, 111, 2839–2847. [Google Scholar] [CrossRef]
- Du, M.-X.; Yuan, Y.-F.; Zhang, J.-M.; Liu, C.-Y. Hydrogen-Bonding Interactions in Polymer-Organic Solvent Mixtures. Macromolecules 2022, 55, 4578–4588. [Google Scholar] [CrossRef]
- Zaitoon, A.; Luo, X.; Lim, L.-T. Triggered and controlled release of active gaseous/volatile compounds for active packaging applications of agri-food products: A review. Compr. Rev. Food Sci. Food Saf. 2022, 21, 541–579. [Google Scholar] [CrossRef]
- Hueppe, N.; Wurm, F.R.; Landfester, K. Nanocarriers with Multiple Cargo Load—A Comprehensive Preparation Guideline Using Orthogonal Strategies. Macromol. Rapid Commun. 2022, 44, e2200611. [Google Scholar] [CrossRef]
- Ko, C.-N.; Zang, S.; Zhou, Y.; Zhong, Z.; Yang, C. Nanocarriers for effective delivery: Modulation of innate immunity for the management of infections and the associated complications. J. Nanobiotechnol. 2022, 20, 380. [Google Scholar] [CrossRef]
- Chountoulesi, M.; Pispas, S.; Tseti, I.K.; Demetzos, C. Lyotropic Liquid Crystalline Nanostructures as Drug Delivery Systems and Vaccine Platforms. Pharmaceuticals 2022, 15, 429. [Google Scholar] [CrossRef] [PubMed]
- Ran, Q.; Wang, M.; Kuang, W.; Ouyang, J.; Han, D.; Gao, Z.; Gong, J. Advances of Combinative Nanocrystal Preparation Technology for Improving the Insoluble Drug Solubility and Bioavailability. Crystals 2022, 12, 1200. [Google Scholar] [CrossRef]
- Machado, T.O.; Grabow, J.; Sayer, C.; de Araujo, P.H.H.; Ehrenhard, M.L.; Wurm, F.R. Biopolymer-based nanocarriers for sustained release of agrochemicals: A review on materials and social science perspectives for a sustainable future of agri- and horticulture. Adv. Colloid Interface Sci. 2022, 303, 102645. [Google Scholar] [CrossRef]
- De, R.; Mahata, M.K.; Kim, K.-T. Structure-Based Varieties of Polymeric Nanocarriers and Influences of Their Physicochemical Properties on Drug Delivery Profiles. Adv. Sci. 2022, 9, 2105373. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Lv, Q.; Li, X. Recent Advances in Drug Delivery Nanocarriers for Targeting Hepatocellular Carcinoma. J. Nanomater. 2022, 2022, 7798919. [Google Scholar] [CrossRef]
- Moreira, A.F.; Rodrigues, C.F.; Jacinto, T.A.; Miguel, S.P.; Costa, E.C.; Correia, I.J. Microneedle-based delivery devices for cancer therapy: A review. Pharmacol. Res. 2019, 148, 104438. [Google Scholar] [CrossRef]
- Liu, G.-S.; Kong, Y.; Wang, Y.; Luo, Y.; Fan, X.; Xie, X.; Yang, B.-R.; Wu, M.X. Microneedles for transdermal diagnostics: Recent advances and new horizons. Biomaterials 2020, 232, 119740. [Google Scholar] [CrossRef]
- Chen, Z.; Li, H.; Bian, Y.; Wang, Z.; Chen, G.; Zhang, X.; Miao, Y.; Wen, D.; Wang, J.; Wan, G.; et al. Bioorthogonal catalytic patch. Nat. Nanotechnol. 2021, 16, 933. [Google Scholar] [CrossRef] [PubMed]
- Qu, M.; Kim, H.-J.; Zhou, X.; Wang, C.; Jiang, X.; Zhu, J.; Xue, Y.; Tebon, P.; Sarabi, S.A.; Ahadian, S.; et al. Biodegradable microneedle patch for transdermal gene delivery. Nanoscale 2020, 12, 16724–16729. [Google Scholar] [CrossRef]
- Huang, S.; Wen, T.; Wang, J.; Wei, H.; Xiao, Z.; Li, B.; Shuai, X. Nanoparticle-integrated dissolving microneedles for the co-delivery of R848/aPD-1 to synergistically reverse the immunosuppressive microenvironment of triple-negative breast cancer. Acta Biomater. 2024, 176, 344–355. [Google Scholar] [CrossRef]
- Lei, Q.; He, D.; Ding, L.; Kong, F.; He, P.; Huang, J.; Guo, J.; Brinker, C.J.; Luo, G.; Zhu, W.; et al. Microneedle Patches Integrated with Biomineralized Melanin Nanoparticles for Simultaneous Skin Tumor Photothermal Therapy and Wound Healing. Adv. Funct. Mater. 2022, 32, 2113269. [Google Scholar] [CrossRef]
- He, G.; Li, Y.; Younis, M.R.; Fu, L.-H.; He, T.; Lei, S.; Lin, J.; Huang, P. Synthetic biology-instructed transdermal microneedle patch for traceable photodynamic therapy. Nat. Commun. 2022, 13, 6238. [Google Scholar] [CrossRef]
- Bobo, D.; Robinson, K.J.; Islam, J.; Thurecht, K.J.; Corrie, S.R. Nanoparticle-Based Medicines: A Review of FDA-Approved Materials and Clinical Trials to Date. Pharm. Res. 2016, 33, 2373–2387. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Q.; Shao, S.; Wang, J.; Xu, C.; Xiang, J.; Piao, Y.; Zhou, Z.; Yu, Q.; Tang, J.; Liu, X.; et al. Enzyme-activatable polymer-drug conjugate augments tumour penetration and treatment efficacy. Nat. Nanotechnol. 2019, 14, 799–809. [Google Scholar] [CrossRef]
- Blanco, E.; Shen, H.; Ferrari, M. Principles of nanoparticle design for overcoming biological barriers to drug delivery. Nat. Biotechnol. 2015, 33, 941–951. [Google Scholar] [CrossRef]
- Chen, M.; Quan, G.; Sun, Y.; Yang, D.; Pan, X.; Wu, C. Nanoparticles-encapsulated polymeric microneedles for transdermal drug delivery. J. Control. Release 2020, 325, 163–175. [Google Scholar] [CrossRef]
- Prabhu, A.; Jose, J.; Kumar, L.; Salwa, S.; Vijay Kumar, M.; Nabavi, S.M. Transdermal Delivery of Curcumin-Loaded Solid Lipid Nanoparticles as Microneedle Patch: An In Vitro and In Vivo Study. AAPS PharmSciTech 2022, 23, 49. [Google Scholar] [CrossRef] [PubMed]
- Qin, W.; Quan, G.; Sun, Y.; Chen, M.; Yang, P.; Feng, D.; Wen, T.; Hu, X.; Pan, X.; Wu, C. Dissolving Microneedles with Spatiotemporally controlled pulsatile release Nanosystem for Synergistic Chemo-photothermal Therapy of Melanoma. Theranostics 2020, 10, 8179–8196. [Google Scholar] [CrossRef] [PubMed]
- Permana, A.D.; Tekko, I.A.; McCrudden, M.T.C.; Anjani, Q.K.; Ramadon, D.; McCarthy, H.O.; Donnelly, R.F. Solid lipid nanoparticle-based dissolving microneedles: A promising intradermal lymph targeting drug delivery system with potential for enhanced treatment of lymphatic filariasis. J. Control. Release 2019, 316, 34–52. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.W.B.; Li, W.B.; Chang, D.; Wei, Z.Q.; Wang, E.D.; Yu, J.; Xu, Y.Z.; Que, Y.M.; Chen, Y.X.; Fan, C.; et al. A combination therapy for androgenic alopecia based on quercetin and zinc/copper dual-doped mesoporous silica nanocomposite microneedle patch. Bioact. Mater. 2023, 24, 81–95. [Google Scholar] [CrossRef] [PubMed]
- Qu, X.Y.; Guo, X.L.; Zhu, T.T.; Zhang, Z.; Wang, W.C.; Hao, Y.P. Microneedle patches containing mesoporous polydopamine nanoparticles loaded with triamcinolone acetonide for the treatment of oral mucositis. Front. Bioeng. Biotechnol. 2023, 11, 1203709. [Google Scholar] [CrossRef] [PubMed]
- Hao, Y.; Chen, Y.W.; Lei, M.Y.; Zhang, T.Y.; Cao, Y.P.; Peng, J.R.; Chen, L.J.; Qian, Z.Y. Near-Infrared Responsive PEGylated Gold Nanorod and Doxorubicin Loaded Dissolvable Hyaluronic Acid Microneedles for Human Epidermoid Cancer Therapy. Adv. Ther. 2018, 1, 800008. [Google Scholar] [CrossRef]
- Chen, M.L.; Yang, D.; Sun, Y.; Liu, T.; Wang, W.H.; Fu, J.T.; Wang, Q.Q.; Bai, X.Q.; Quan, G.L.; Pan, X.; et al. In Situ Self-Assembly Nanomicelle Microneedles for Enhanced Photoimmunotherapy via Autophagy Regulation Strategy. ACS Nano 2021, 15, 3387–3401. [Google Scholar] [CrossRef]
- Wei, S.H.; Quan, G.L.; Lu, C.; Pan, X.; Wu, C.B. Dissolving microneedles integrated with pH-responsive micelles containing AIEgen with ultra-photostability for enhancing melanoma photothermal therapy. Biomater. Sci. 2020, 8, 5739–5750. [Google Scholar] [CrossRef]
- Hu, H.M.; Ruan, H.; Ruan, S.Y.; Pei, L.X.; Jing, Q.; Wu, T.; Hou, X.L.; Xu, H.; Wang, Y.J.; Feng, N.P.; et al. Acid-responsive PEGylated branching PLGA nanoparticles integrated into dissolving microneedles enhance local treatment of arthritis. Chem. Eng. J. 2022, 431, 134196. [Google Scholar] [CrossRef]
- Permana, A.D.; Mir, M.; Utomo, E.; Donnelly, R.F. Bacterially sensitive nanoparticle-based dissolving microneedles of doxycycline for enhanced treatment of bacterial biofilm skin infection: A proof of concept study. Int. J. Pharm. X 2020, 2, 18. [Google Scholar] [CrossRef]
- Menon, I.; Patil, S.; Bagwe, P.; Vijayanand, S.; Kale, A.; Gomes, K.B.; Kang, S.M.; D’Souza, M. Dissolving Microneedles Loaded with Nanoparticle Formulation of Respiratory Syncytial Virus Fusion Protein Virus-like Particles (F-VLPs) Elicits Cellular and Humoral Immune Responses. Vaccines 2023, 11, 13. [Google Scholar] [CrossRef]
- Gomes, K.B.; D’Souza, B.; Vijayanand, S.; Menon, I.; D’Souza, M.J. A dual-delivery platform for vaccination using antigen-loaded nanoparticles in dissolving microneedles. Int. J. Pharm. 2022, 613, 9. [Google Scholar] [CrossRef]
- Vora, L.K.; Donnelly, R.F.; Larrañeta, E.; González-Vázquez, P.; Thakur, R.R.S.; Vavia, P.R. Novel bilayer dissolving microneedle arrays with concentrated PLGA nanomicroparticles for targeted intradermal delivery: Proof of concept. J. Control. Release 2017, 265, 93–101. [Google Scholar] [CrossRef] [PubMed]
- Cheng, Z.T.; Lin, H.; Wang, Z.; Yang, X.Y.; Zhang, M.; Liu, X.C.; Wang, B.J.; Wu, Z.F.; Chen, D.Q. Preparation and characterization of dissolving hyaluronic acid composite microneedles loaded micelles for delivery of curcumin. Drug Deliv. Transl. Res. 2020, 10, 1520–1530. [Google Scholar] [CrossRef] [PubMed]
- Waghchaure, M.; Govardhane, S.; Shende, P. Enhancement of immunopotentiation using tetanus toxoid-based nanoparticulate dissolvable microneedles. Biomed. Microdevices 2021, 23, 9. [Google Scholar] [CrossRef] [PubMed]
- Dawud, H.; Abu Ammar, A. Rapidly Dissolving Microneedles for the Delivery of Steroid-Loaded Nanoparticles Intended for the Treatment of Inflammatory Skin Diseases. Pharmaceutics 2023, 15, 15. [Google Scholar] [CrossRef] [PubMed]
- Pu, X.Q.; Ju, X.J.; Liu, W.Y.; Liu, Y.Q.; Li, X.J.; Li, Y.; Xie, R.; Wang, W.; Liu, Z.; Chu, L.Y. Stimulus-Responsive Nanoparticle-Integrated Dissolving Microneedles for Synergetic Chemo-Photothermal Therapy of Superficial Skin Tumors. Ind. Eng. Chem. Res. 2022, 61, 7982–7995. [Google Scholar] [CrossRef]
- Mir, M.; Permana, A.D.; Ahmed, N.; Khan, G.M.; Rehman, A.U.; Donnelly, R.F. Enhancement in site-specific delivery of carvacrol for potential treatment of infected wounds using infection responsive nanoparticles loaded into dissolving microneedles: A proof of concept study. Eur. J. Pharm. Biopharm. 2020, 147, 57–68. [Google Scholar] [CrossRef] [PubMed]
- Peng, T.T.; Huang, Y.; Feng, X.Q.; Zhu, C.N.; Yin, S.; Wang, X.Y.; Bai, X.Q.; Pan, X.; Wu, C.B. TPGS/hyaluronic acid dual-functionalized PLGA nanoparticles delivered through dissolving microneedles for markedly improved chemo-photothermal combined therapy of superficial tumor. Acta Pharm. Sin. B 2021, 11, 3297–3309. [Google Scholar] [CrossRef] [PubMed]
- Tripathy, N.; Wang, J.; Tung, M.; Conway, C.; Chung, E.J. Transdermal Delivery of Kidney-Targeting Nanoparticles Using Dissolvable Microneedles. Cell. Mol. Bioeng. 2020, 13, 475–486. [Google Scholar] [CrossRef]
- Hu, X.; Zhang, H.; Wang, Z.; Shiu, C.Y.A.; Gu, Z. Microneedle Array Patches Integrated with Nanoparticles for Therapy and Diagnosis. Small Struct. 2021, 2, 2000097. [Google Scholar] [CrossRef]
- Schupp, T.; Plehiers, P.M. Absorption, distribution, metabolism, and excretion of methylene diphenyl diisocyanate and toluene diisocyanate: Many similarities and few differences. Toxicol. Ind. Health 2022, 38, 500–528. [Google Scholar] [CrossRef]
- Mueck, W.; Stampfuss, J.; Kubitza, D.; Becka, M. Clinical Pharmacokinetic and Pharmacodynamic Profile of Rivaroxaban. Clin. Pharmacokinet. 2014, 53, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Patra, S.; Pradhan, B.; Nayak, R.; Behera, C.; Rout, L.; Jena, M.; Efferth, T.; Bhutia, S.K. Chemotherapeutic efficacy of curcumin and resveratrol against cancer: Chemoprevention, chemoprotection, drug synergism and clinical pharmacokinetics. Semin. Cancer Biol. 2021, 73, 310–320. [Google Scholar] [CrossRef]
- van den Anker, J.; Reed, M.D.; Allegaert, K.; Kearns, G.L. Developmental Changes in Pharmacokinetics and Pharmacodynamics. J. Clin. Pharmacol. 2018, 58, S10–S25. [Google Scholar] [CrossRef]
- Martinez, M.N.; Sinko, B.; Wu, F.; Flanagan, T.; Borbas, E.; Tsakalozou, E.; Giacomini, K.M. A Critical Overview of the Biological Effects of Excipients (Part I): Impact on Gastrointestinal Absorption. AAPS J. 2022, 24, 60. [Google Scholar] [CrossRef] [PubMed]
- Lv, Y.; Wu, W.; Corpstein, C.D.; Li, T.; Lu, Y. Biological and intracellular fates of drug nanocrystals through different delivery routes: Recent development enabled by bioimaging and PK modeling. Adv. Drug Delivery Rev. 2022, 188, 114466. [Google Scholar] [CrossRef] [PubMed]
- Xu, M.; Qi, Y.; Liu, G.; Song, Y.; Jiang, X.; Du, B. Size-Dependent In Vivo Transport of Nanoparticles: Implications for Delivery, Targeting, and Clearance. ACS Nano 2023, 17, 20825–20849. [Google Scholar] [CrossRef]
- Offringa, R.; Kotzner, L.; Huck, B.; Urbahns, K. The expanding role for small molecules in immuno-oncology. Nat. Rev. Drug Discovery 2022, 21, 821–840. [Google Scholar] [CrossRef] [PubMed]
- Shi, Y.; Lu, A.; Wang, X.; Belhadj, Z.; Wang, J.; Zhang, Q. A review of existing strategies for designing long-acting parenteral formulations: Focus on underlying mechanisms, and future perspectives. Acta Pharm. Sin. B 2021, 11, 2396–2415. [Google Scholar] [CrossRef]
- Pang, Z.Y.; Schafroth, M.A.; Ogasawara, D.; Wang, Y.; Nudell, V.; Lal, N.K.; Yang, D.; Wang, K.; Herbst, D.M.; Ha, J.; et al. In Situ identification of cellular in mammalian tissue. Cell 2022, 185, 1793–1805.e17. [Google Scholar] [CrossRef]
- Pires, D.E.V.; Blundell, T.L.; Ascher, D.B. pkCSM: Predicting Small-Molecule Pharmacokinetic and Toxicity Properties Using Graph-Based Signatures. J. Med. Chem. 2015, 58, 4066–4072. [Google Scholar] [CrossRef] [PubMed]
- Anselmo, A.C.; Gokarn, Y.; Mitragotri, S. Non-invasive delivery strategies for biologics. Nat. Rev. Drug Discovery 2019, 18, 19–40. [Google Scholar] [CrossRef] [PubMed]
- Cao, S.-J.; Xu, S.; Wang, H.-M.; Ling, Y.; Dong, J.; Xia, R.-D.; Sun, X.-H. Nanoparticles: Oral Delivery for Protein and Peptide Drugs. AAPS PharmSciTech 2019, 20, 190. [Google Scholar] [CrossRef] [PubMed]
- Kuijper, E.C.; Bergsma, A.J.; Pijnappel, W.W.M.P.; Aartsma-Rus, A. Opportunities and challenges for antisense oligonucleotide therapies. J. Inherit. Metab. Dis. 2021, 44, 72–87. [Google Scholar] [CrossRef] [PubMed]
- Hyjek-Skladanowska, M.; Vickers, T.A.; Napiorkowska, A.; Anderson, B.A.; Tanowitz, M.; Crooke, S.T.; Liang, X.-h.; Seth, P.P.; Nowotny, M. Origins of the Increased Affinity of Phosphorothioate-Modified Therapeutic Nucleic Acids for Proteins. J. Am. Chem. Soc. 2020, 142, 7456–7468. [Google Scholar] [CrossRef]
- Wang, S.; Zhang, J.; Zhou, H.; Lu, Y.C.; Jin, X.; Luo, L.; You, J. The role of protein corona on nanodrugs for organ-targeting and its prospects of application. J. Control. Release 2023, 360, 15–43. [Google Scholar] [CrossRef] [PubMed]
- Du, B.Z. Size-Dependent in vivo Transport and Interactions of Ultrasmall Nanoparticles. ACS Nano 2019, 17, 20825–20849. [Google Scholar]
- Li, X.; Jafari, S.M.; Zhou, F.; Hong, H.; Jia, X.; Mei, X.; Hou, G.; Yuan, Y.; Liu, B.; Chen, S.; et al. The intracellular fate and transport mechanism of shape, size and rigidity varied nanocarriers for understanding their oral delivery efficiency. Biomaterials 2023, 294, 121995. [Google Scholar] [CrossRef]
- Liu, Q.; Guan, J.; Qin, L.; Zhang, X.; Mao, S. Physicochemical properties affecting the fate of nanoparticles in pulmonary drug delivery. Drug Discov. Today 2020, 25, 150–159. [Google Scholar] [CrossRef]
- Lee, S.-H.; Kim, J.-K.; Jee, J.-P.; Jang, D.-J.; Park, Y.-J.; Kim, J.-E. Quality by Design (QbD) application for the pharmaceutical development process. J. Pharm. Investig. 2022, 52, 649–682. [Google Scholar] [CrossRef]
- Camara-Martinez, I.; Blechar, J.A.; Ruiz-Picazo, A.; Garcia-Arieta, A.; Calandria, C.; Merino-Sanjuan, V.; Langguth, P.; Gonzalez-Alvarez, M.; Bermejo, M.; Al-Gousous, J.; et al. Level A IVIVC for immediate release tablets confirms in vivo predictive dissolution testing for ibuprofen. Int. J. Pharm. 2022, 614, 121415. [Google Scholar] [CrossRef]
- Wang, C.; Zeng, Y.; Chen, K.-F.; Lin, J.; Yuan, Q.; Jiang, X.; Wu, G.; Wang, F.; Jia, Y.-G.; Li, W. A self-monitoring microneedle patch for light-controlled synergistic treatment of melanoma. Bioact. Mater. 2023, 27, 58–71. [Google Scholar] [CrossRef]
- Liu, T.; Chen, M.; Fu, J.; Sun, Y.; Lu, C.; Quan, G.; Pan, X.; Wu, C. Recent advances in microneedles-mediated transdermal delivery of protein and peptide drugs. Acta Pharm. Sin. B 2021, 11, 2326–2343. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Wang, M.; Li, L.; Zhang, L.; Ma, B.; Wang, W. A composite peptide-supramolecular microneedle system for melanoma immunotherapy. Nano Res. 2023, 16, 5335–5345. [Google Scholar] [CrossRef]
- Yin, Y.; Su, W.; Zhang, J.; Huang, W.; Li, X.; Ma, H.; Tan, M.; Song, H.; Cao, G.; Yu, S.; et al. Separable Microneedle Patch to Protect and Deliver DNA Nanovaccines Against COVID-19. ACS Nano 2021, 15, 14347–14359. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Wang, Z.; Archibong, E.; Wu, Q.; Chen, G.; Hu, Q.; Ci, T.; Chen, Z.; Wang, J.; Wen, D.; et al. Scattered seeding of CAR T cells in solid tumors augments anticancer efficacy. Natl. Sci. Rev. 2022, 9, nwab172. [Google Scholar] [CrossRef] [PubMed]
- Zou, P. Does Food Affect the Pharmacokinetics of Non-orally Delivered Drugs? A Review of Currently Available Evidence. AAPS J. 2022, 24, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Harish, V.; Tewari, D.; Gaur, M.; Yadav, A.B.; Swaroop, S.; Bechelany, M.; Barhoum, A. Review on Nanoparticles and Nanostructured Materials: Bioimaging, Biosensing, Drug Delivery, Tissue Engineering, Antimicrobial, and Agro-Food Applications. Nanomater 2022, 12, 457. [Google Scholar] [CrossRef] [PubMed]
- Dong, Y.; Li, X.-R.; Chen, Q.; Guo, R.-Y.; Tang, B.-X.; Kan, W.-J.; Zhang, W.; Hu, Y.; Li, J.; Zang, Y.; et al. Microenvironment-Responsive Small-Molecule Probe for Pulmonary Fibrosis Detection. Anal. Chem. 2020, 92, 699–706. [Google Scholar] [CrossRef] [PubMed]
- Sidhu, J.S.; Singh, A.; Garg, N.; Kaur, N.; Singh, N. Gold conjugated carbon dots nano assembly: FRET paired fluorescence probe for cysteine recognition. Sens. Actuators, B 2019, 282, 515–522. [Google Scholar] [CrossRef]
- Wu, H.; Wang, Y.; Wu, W.-N.; Xu, Z.-Q.; Xu, Z.-H.; Zhao, X.-L.; Fan, Y.-C. A novel ‘turn-on’ coumarin-based fluorescence probe with aggregation-induced emission (AIE) for sensitive detection of hydrazine and its imaging in living cells. Spectrochim. Acta Part A 2019, 222, 117272. [Google Scholar] [CrossRef]
- Hu, X.; Zhang, J.; Yu, Z.; Xie, Y.; He, H.; Qi, J.; Dong, X.; Lu, Y.; Zhao, W.; Wu, W. Environment-responsive aza-BODIPY dyes quenching in water as potential probes to visualize the in vivo fate of lipid-based nanocarriers. Nanomedicine 2015, 11, 1939–1948. [Google Scholar] [CrossRef]
- Li, D.; Zhuang, J.; He, H.; Jiang, S.; Banerjee, A.; Lu, Y.; Wu, W.; Mitragotri, S.; Gan, L.; Qi, J. Influence of Particle Geometry on Gastrointestinal Transit and Absorption following Oral Administration. ACS Appl. Mater. Interfaces 2017, 9, 42492–42502. [Google Scholar] [CrossRef]
- Krainer, G.; Hartmann, A.; Schlierf, M. farFRET: Extending the Range in Single-Molecule FRET Experiments beyond 10 nm. Nano Lett. 2015, 15, 5826–5829. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Zhao, E.; Lam, J.W.Y.; Tang, B.Z. AIE luminogens: Emission brightened by aggregation. Mater. Today 2015, 18, 365–377. [Google Scholar] [CrossRef]
- Duo, Y.; Luo, G.; Zhang, W.; Wang, R.; Xiao, G.G.; Li, Z.; Li, X.; Chen, M.; Yoon, J.; Tang, B.Z. Noncancerous disease-targeting AIEgens. Chem. Soc. Rev. 2023, 52, 1024–1067. [Google Scholar] [CrossRef] [PubMed]
- Sartawi, Z.; Blackshields, C.; Faisal, W. Dissolving microneedles: Applications and growing therapeutic potential. J. Control. Release 2022, 348, 186–205. [Google Scholar] [CrossRef] [PubMed]
- Hong, X.; Wu, Z.; Chen, L.; Wu, F.; Wei, L.; Yuan, W. Hydrogel Microneedle Arrays for Transdermal Drug Delivery. Nano-Micro Lett. 2014, 6, 191–199. [Google Scholar] [CrossRef]
- Sunoqrot, S.; Mahmoud, N.N.; Ibrahim, L.H.; Al-Dabash, S.a.; Raschke, H.; Hergenroder, R. Tuning the Surface Chemistry of Melanin-Mimetic Polydopamine Nanoparticles Drastically Enhances Their Accumulation into Excised Human Skin. ACS Biomater. Sci. Eng. 2020, 6, 4424–4432. [Google Scholar] [CrossRef] [PubMed]
- Shi, C.; Yang, D.; Zhao, Y.; Wen, T.; Zhao, W.; Hu, P.; Huang, Z.; Quan, G.; Wu, C.; Pan, X. The spatial-dimensional and temporal-dimensional fate of nanocarrier-loaded dissolving microneedles with different lengths of needles. Med. Drug Discov. 2022, 14, 100124. [Google Scholar] [CrossRef]
- Li, Y.; Hu, X.; Dong, Z.; Chen, Y.; Zhao, W.; Wang, Y.; Zhang, L.; Chen, M.; Wu, C.; Wang, Q. Dissolving Microneedle Arrays with Optimized Needle Geometry for Transcutaneous Immunization. Eur. J. Pharm. Sci. 2020, 151, 105361. [Google Scholar] [CrossRef]
- Aoyagi, S.; Izumi, H.; Fukuda, M. Biodegradable polymer needle with various tip angles and consideration on insertion mechanism of mosquito’s proboscis. Sens. Actuators A 2008, 143, 20–28. [Google Scholar] [CrossRef]
- Zheng, M.; Sheng, T.; Yu, J.; Gu, Z.; Xu, C. Microneedle biomedical devices. Nat. Rev. Bioeng. 2023. [Google Scholar] [CrossRef]
- You, J.; Yang, C.; Han, J.; Wang, H.; Zhang, W.; Zhang, Y.; Lu, Z.; Wang, S.; Cai, R.; Li, H.; et al. Ultrarapid-Acting Microneedles for Immediate Delivery of Biotherapeutics. Adv. Mater. 2023, 35, e2304582. [Google Scholar] [CrossRef] [PubMed]
- Du, G.; Sun, X. Current Advances in Sustained Release Microneedles. Pharm. Front. 2020, 02, e11–e22. [Google Scholar] [CrossRef]
- Bauleth-Ramos, T.; El-Sayed, N.; Fontana, F.; Lobita, M.; Shahbazi, M.A.; Santos, H.A. Recent approaches for enhancing the performance of dissolving microneedles in drug delivery applications. Mater. Today 2023, 63, 239–287. [Google Scholar] [CrossRef]
- Fu, Y.; Shi, C.; Li, X.; Wen, T.; Wu, Q.; Zhang, A.; Hu, P.; Wu, C.; Pan, X.; Huang, Z.; et al. Demonstrating Biological Fate of Nanoparticle-Loaded Dissolving Microneedles with Aggregation-Caused Quenching Probes: Influence of Application Sites. Pharmaceutics 2023, 15, 169. [Google Scholar] [CrossRef]
- Liu, S.; Bai, Q.; Jiang, Y.; Gao, Y.; Chen, Z.; Shang, L.; Zhang, S.; Yu, L.; Yang, D.; Sui, N.; et al. Multienzyme-Like Nanozyme Encapsulated Ocular Microneedles for Keratitis Treatment. SMALL 2023, 2308403. [Google Scholar] [CrossRef]
- Meng, Y.; Li, X.J.; Li, Y.; Zhang, T.Y.; Liu, D.; Wu, Y.Q.; Hou, F.F.; Ye, L.; Wu, C.J.; Feng, X.D.; et al. Novel Double-Layer Dissolving Microneedles for Transmucosal Sequential Delivery of Multiple Drugs in the Treatment of Oral Mucosa Diseases. ACS Appl. Mater. Interfaces 2023, 15, 13892–13906. [Google Scholar] [CrossRef]
- Hu, S.; Zhu, D.; Li, Z.; Cheng, K. Detachable Microneedle Patches Deliver Mesenchymal Stromal Cell Factor-Loaded Nanoparticles for Cardiac Repair. ACS Nano 2022, 16, 15935–15945. [Google Scholar] [CrossRef] [PubMed]
- Gao, X.; Li, J.; Li, J.; Zhang, M.; Xu, J. Pain-free oral delivery of biologic drugs using intestinal peristalsis-actuated microneedle robots. Sci. Adv. 2024, 10, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Panda, A.; Matadh, V.A.; Suresh, S.; Shivakumar, H.N.; Murthy, S.N. Non-dermal applications of microneedle drug delivery systems. Drug Delivery Transl. Res. 2022, 12, 67–78. [Google Scholar] [CrossRef] [PubMed]
- Tao, J.; Wang, B.; Dong, Y.; Chen, X.; Li, S.; Jiang, T.; Zhao, X. Photothermal and Acid-Responsive Fucoidan-CuS Bubble Pump Microneedles for Combined CDT/PTT/CT Treatment of Melanoma. ACS Appl. Mater. Interfaces 2023, 15, 40267–40279. [Google Scholar] [CrossRef]
| Nanoparticle | Needle Material | Needle Length | Indication | Therapeutic Effects | Reference |
|---|---|---|---|---|---|
| Curcumin SLNs | PVA | 416 μm | Parkinson’s disease | Enhancing the lipophilicity of curcumin and the treatment of Parkinson’s disease | [61] |
| Paclitaxel (PTX)/IR-780 SLNs | HA | 800 μm | Melanoma | A curable rate of 100% for primary tumor in 30 days | [62] |
| Doxycycline, diethylcarbamazine, and albendazole sulfone-loaded SLNs | PVA, PVP | 500 μm | Lymphatic filariasis | Effective increase in drug concentration in the lymphatic system for the treatment of lymphatic filariasis | [63] |
| Quercetin and zinc/copper dual-doped mesoporous silica nanoparticles | Sodium hyaluronate, gelatin, sodium alginate | 600 μm | Androgenic alopecia | Efficient enhancement of hair growth | [64] |
| Mesoporous polydopamine nanoparticles loaded with triamcinolone acetonide | HA | 700 μm | Oral mucositis | Excellent anti-inflammatory properties | [65] |
| PEGylated gold nanorod-loaded doxorubicin | HA | 600 μm | Human epidermoid cancer | Remarkable antitumor efficacy | [66] |
| Chloroquine (CQ)/IR780 micelles | HA | 800 μm | Melanoma | Effective elimination of primary and distant tumors | [67] |
| Aggregation-induced emission luminogens (AIEgens) NIR950-loaded polymeric micelles | PVA 103, PVP K30 | 800 μm | Melanoma | Notable elimination of melanoma tumors with a low dose of NIR950 | [68] |
| PEGylated star-shaped PLGA | Peach gum polysaccharide, PVA, HA | 500 μm | Arthritis | Remarkable increase in synovial uptake of Tet and improvement of arthritis | [69] |
| Polymer nanoparticles loaded with doxycycline (DOX) | PVA, PVP | 600 μm | Bacterial biofilm skin infection | Superior antimicrobial and antibiofilm activity | [70] |
| Polymer nanoparticles loaded with RSV fusion protein | HA, trehalose | 520 μm | Respiratory syncytial virus (RSV) vaccine | Induction of robust humoral and cellular immune responses in vivo | [71] |
| PLGA nanoparticles loaded with influenza matrix 2 (M2) protein antigen | HA, trehalose dihydrate | 520 μm | Vaccine | Delivery of antigens into the body with potential for development into vaccines | [72] |
| PLGA nanoparticles loaded with vitamin D3 | PVA, PVP | 600 μm | Micronutrient | Excellent delivery of vitamin D3 into the body | [73] |
| Curcumin (Cur)-loaded micelles | HA, sodium carboxymethyl starch (CMS-Na) | 600 μm | Melanoma | High drug delivery efficiency and promising applications in the treatment of melanoma | [74] |
| Polymer nanoparticles loaded with tetanus toxoid | PVP | 250 μm | Vaccine | Highest production of antibodies | [75] |
| PLGA nanoparticles loaded with dexamethasone (DEX) | Sodium alginate (SA) | 500 μm | Inflammatory skin diseases | Excellent delivery of DEX to inflamed areas of the skin | [76] |
| Polymer nanoparticles loaded with doxorubicin (DOX) | HA | 450 μm | Superficial skin tumors | Significant killing effect on superficial epidermal tumor cells | [77] |
| Poly(caprolactone)(PCL) nanoparticles loaded with Carvacrol (CAR) | PVA, PVP | 850 μm/600 μm | Infected wounds | Antimicrobial potential at lower drug concentrations at the site of infection, with considerable antimicrobial activity against Gram-negative bacteria | [78] |
| PLGA nanoparticles loaded with paclitaxel and indocyanine green | PVA, PVP K30 | 800 μm | Superficial tumor | Favorable anticancer effect and great potential in cancer treatment | [79] |
| Micelle nanoparticles loaded with rhodamine B (RhB) | PVA | 600 μm | Chronic kidney disease | Raised drug concentrations in the kidneys and excellent preservation of kidney health | [80] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chang, Z.; Wu, Y.; Hu, P.; Jiang, J.; Quan, G.; Wu, C.; Pan, X.; Huang, Z. The Necessity to Investigate In Vivo Fate of Nanoparticle-Loaded Dissolving Microneedles. Pharmaceutics 2024, 16, 286. https://doi.org/10.3390/pharmaceutics16020286
Chang Z, Wu Y, Hu P, Jiang J, Quan G, Wu C, Pan X, Huang Z. The Necessity to Investigate In Vivo Fate of Nanoparticle-Loaded Dissolving Microneedles. Pharmaceutics. 2024; 16(2):286. https://doi.org/10.3390/pharmaceutics16020286
Chicago/Turabian StyleChang, Ziyao, Yuhuan Wu, Ping Hu, Junhuang Jiang, Guilan Quan, Chuanbin Wu, Xin Pan, and Zhengwei Huang. 2024. "The Necessity to Investigate In Vivo Fate of Nanoparticle-Loaded Dissolving Microneedles" Pharmaceutics 16, no. 2: 286. https://doi.org/10.3390/pharmaceutics16020286






