NLC-Based Sunscreen Formulations with Optimized Proportion of Encapsulated and Free Filters Exhibit Enhanced UVA and UVB Photoprotection
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Emulsion Preparation
2.3. NLCs Preparation Using Ultrasonication Method
2.3.1. Factorial Design for NLC Formulation
2.3.2. NLCs Preparation Using a High-Pressure Homogenizer
2.4. Factorial Design for NLCs Production Process
2.5. Determination of Particle Size, Polydispersity Index, Zeta Potential, and pH
2.6. Ultraviolet-Visible Spectroscopy
2.7. Atomic Force Microscopy (AFM)
2.8. Rheology
2.9. Analytical Method for Quantification of Uvinul® A Plus, Uvinul® T150, and Tinosorb® S in NLCs
2.9.1. Standard and Sample Preparation
2.9.2. Equipment and Analytical Method
2.9.3. Encapsulation Efficiency and Loading Capacity of the Chemical Filters in the NLCs
2.10. Physicochemical Stability Study
2.11. In Vitro Skin Permeation Study
2.12. Photoprotection Efficacy Studies
2.12.1. In Vitro Photoprotection Test
2.12.2. In Vivo Photoprotection Test
2.13. Statistical Analysis
3. Results
3.1. Formulation and Process Optimization
3.2. SC-NLC Physicochemical Characterization
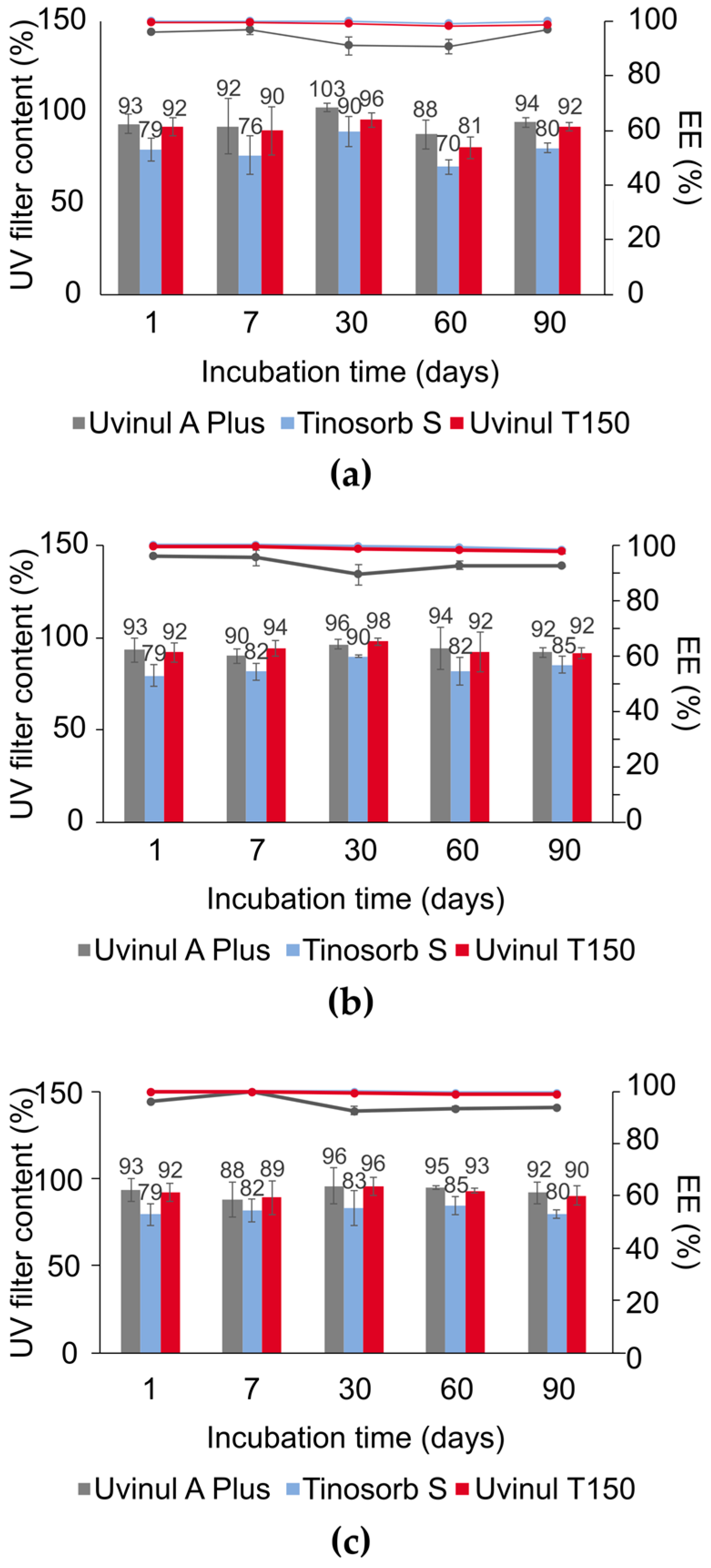
3.3. SC-NLC Physicochemical Stability
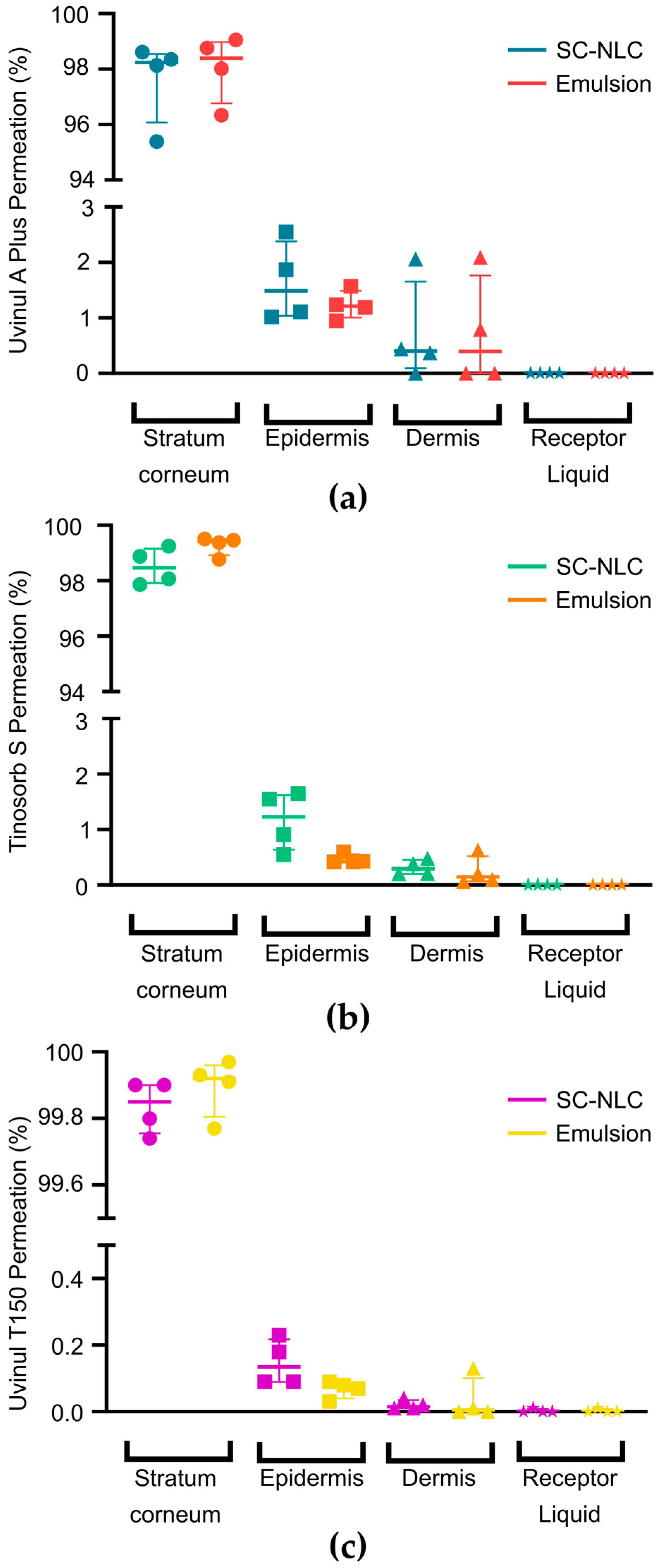
3.4. In Vitro Skin Permeation Test
3.5. In Vitro Photoprotection Test
3.6. In Vivo Photoprotection Evaluation
4. Conclusions
5. Patents
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Santos, A.C.; Marto, J.; Chá-Chá, R.; Martins, A.M.; Pereira-Silva, M.; Ribeiro, H.M.; Veiga, F. Nanotechnology-Based Sunscreens—A Review. Mater. Today Chem. 2022, 23, 100709. [Google Scholar] [CrossRef]
- Summerfield, A.; Meurens, F.; Ricklin, M.E. The Immunology of the Porcine Skin and Its Value as a Model for Human Skin. Mol. Immunol. 2015, 66, 14–21. [Google Scholar] [CrossRef]
- Romanhole, R.C.; Fava, A.L.M.; Tundisi, L.L.; Macedo, L.M.d.; Santos, É.M.d.; Ataide, J.A.; Mazzola, P.G. Unplanned Absorption of Sunscreen Ingredients: Impact of Formulation and Evaluation Methods. Int. J. Pharm. 2020, 591, 120013. [Google Scholar] [CrossRef]
- Puglia, C.; Damiani, E.; Offerta, A.; Rizza, L.; Tirendi, G.G.; Tarico, M.S.; Curreri, S.; Bonina, F.; Perrotta, R.E. Evaluation of Nanostructured Lipid Carriers (NLC) and Nanoemulsions as Carriers for UV-Filters: Characterization, in Vitro Penetration and Photostability Studies. Eur. J. Pharm. Sci. 2014, 51, 211–217. [Google Scholar] [CrossRef]
- Bruce, A.F.; Theeke, L.; Mallow, J. A State of the Science on Influential Factors Related to Sun Protective Behaviors to Prevent Skin Cancer in Adults. Int. J. Nurs. Sci. 2017, 4, 225–235. [Google Scholar] [CrossRef]
- Shi, L.; Shan, J.; Ju, Y.; Aikens, P.; Prud’homme, R.K. Nanoparticles as Delivery Vehicles for Sunscreen Agents. Colloids Surf. A Physicochem. Eng. Asp. 2012, 396, 122–129. [Google Scholar] [CrossRef]
- Pinto, D.; Trink, A.; Giuliani, G.; Rinaldi, F. Protective Effects of Sunscreen (50+) and Octatrienoic Acid 0.1% in Actinic Keratosis and UV Damages. J. Investig. Med. 2022, 70, 92–98. [Google Scholar] [CrossRef]
- Sharma, G.; Khanna, G.; Gupta, S.; Ramzan, M.; Singh, J.; Singh, M.; Mudgill, U.; Gulati, J.S.; Kaur, I.P. Scope of Solid Lipid Nanoparticles per Se as All-Purpose Moisturising Sunscreens. J. Drug Deliv. Sci. Technol. 2022, 75, 103687. [Google Scholar] [CrossRef]
- Asfour, M.H.; Kassem, A.A.; Salama, A. Topical Nanostructured Lipid Carriers/Inorganic Sunscreen Combination for Alleviation of All-Trans Retinoic Acid-Induced Photosensitivity: Box-Behnken Design Optimization, in Vitro and in Vivo Evaluation. Eur. J. Pharm. Sci. 2019, 134, 219–232. [Google Scholar] [CrossRef]
- Cozzi, A.C.; Perugini, P.; Gourion-Arsiquaud, S. Comparative Behavior between Sunscreens Based on Free or Encapsulated UV Filters in Term of Skin Penetration, Retention and Photo-Stability. Eur. J. Pharm. Sci. 2018, 121, 309–318. [Google Scholar] [CrossRef]
- Shetty, P.K.; Venuvanka, V.; Jagani, H.V.; Chethan, G.H.; Ligade, V.S.; Musmade, P.B.; Nayak, U.Y.; Reddy, M.S.; Kalthur, G.; Udupa, N.; et al. Development and Evaluation of Sunscreen Creams Containing Morin-Encapsulated Nanoparticles for Enhanced UV Radiation Protection and Antioxidant Activity. Int. J. Nanomed. 2015, 10, 6477–6491. [Google Scholar] [CrossRef]
- Abdel-Salam, F.S.; Ammar, H.O.; Elkheshen, S.A.; Mahmoud, A.A. Anti-Inflammatory Sunscreen Nanostructured Lipid Carrier Formulations. J. Drug Deliv. Sci. Technol. 2017, 37, 13–19. [Google Scholar] [CrossRef]
- Senzui, M.; Tamura, T.; Miura, K.; Ikarashi, Y.; Watanabe, Y.; Fujii, M. Study on Penetration of Titanium Dioxide (TiO2) Nanoparticles into Intact and Damaged Skin in Vitro. J. Toxicol. Sci. 2009, 35, 107–113. [Google Scholar] [CrossRef]
- Bennett, S.W.; Zhou, D.; Mielke, R.; Keller, A.A. Photoinduced Disaggregation of TiO2 Nanoparticles Enables Transdermal Penetration. PLoS ONE 2012, 7, e48719. [Google Scholar] [CrossRef]
- Shakeel, M.; Jabeen, F.; Shabbir, S.; Asghar, M.S.; Khan, M.S.; Chaudhry, A.S. Toxicity of Nano-Titanium Dioxide (TiO2-NP) through Various Routes of Exposure: A Review. Biol. Trace Elem. Res. 2016, 172, 1–36. [Google Scholar] [CrossRef]
- Wu, J.; Liu, W.; Xue, C.; Zhou, S.; Lan, F.; Bi, L.; Xu, H.; Yang, X.; Zeng, F.D. Toxicity and Penetration of TiO2 Nanoparticles in Hairless Mice and Porcine Skin after Subchronic Dermal Exposure. Toxicol. Lett. 2009, 191, 1–8. [Google Scholar] [CrossRef]
- Sharma, S.; Sharma, R.K.; Gaur, K.; Torres, J.F.C.; Loza-Rosas, S.A.; Torres, A.; Saxena, M.; Julin, M.; Tinoco, A.D. Fueling a Hot Debate on the Application of TiO2 Nanoparticles in Sunscreen. Materials 2019, 12, 2317. [Google Scholar] [CrossRef]
- Dario, M.F.; Oliveira, F.F.; Marins, D.S.S.; Baby, A.R.; Velasco, M.V.R.; Löbenberg, R.; Bou-Chacra, N.A. Synergistic Photoprotective Activity of Nanocarrier Containing Oil of Acrocomia aculeata (Jacq.) Lodd. Ex. Martius—Arecaceae. Ind. Crops Prod. 2018, 112, 305–312. [Google Scholar] [CrossRef]
- Chavda, V.P.; Acharya, D.; Hala, V.; Daware, S.; Vora, L.K. Sunscreens: A Comprehensive Review with the Application of Nanotechnology. J. Drug Deliv. Sci. Technol. 2023, 86, 104720. [Google Scholar] [CrossRef]
- Nikolić, S.; Keck, C.M.; Anselmi, C.; Müller, R.H. Skin Photoprotection Improvement: Synergistic Interaction between Lipid Nanoparticles and Organic UV Filters. Int. J. Pharm. 2011, 414, 276–284. [Google Scholar] [CrossRef]
- Shah, S.; Dhawan, V.; Holm, R.; Nagarsenker, M.S.; Perrie, Y. Liposomes: Advancements and Innovation in the Manufacturing Process. Adv. Drug Deliv. Rev. 2020, 154–155, 102–122. [Google Scholar] [CrossRef]
- Puglia, C.; Bonina, F.; Rizza, L.; Blasi, P.; Schoubben, A.; Perrotta, R.; Tarico, M.S.; Damiani, E. Lipid Nanoparticles as Carrier for Octyl-Methoxycinnamate: In Vitro Percutaneous Absorption and Photostability Studies. J. Pharm. Sci. 2012, 101, 301–311. [Google Scholar] [CrossRef]
- Martins, R.M.; Martins, S.d.S.; Barbosa, G.L.F.; Silva, E.G.d.N.e.; Fonseca, M.J.V.; Freitas, L.A.P.d. Natural Component and Solid Lipid Microparticles of Solar Filter in Sunscreen: Photoprotective and Photostability Effect Enhancement. J. Drug Deliv. Sci. Technol. 2023, 88, 104860. [Google Scholar] [CrossRef]
- Scalia, S.; Mezzena, M. Incorporation in Lipid Microparticles of the UVA Filter, Butyl Methoxydibenzoylmethane Combined with the UVB Filter, Octocrylene: Effect on Photostability. AAPS PharmSciTech 2009, 10, 384–390. [Google Scholar] [CrossRef]
- Souto, E.B.; Fangueiro, J.F.; Fernandes, A.R.; Cano, A.; Sanchez-Lopez, E.; Garcia, M.L.; Severino, P.; Paganelli, M.O.; Chaud, M.V.; Silva, A.M. Physicochemical and Biopharmaceutical Aspects Influencing Skin Permeation and Role of SLN and NLC for Skin Drug Delivery. Heliyon 2022, 8, e08938. [Google Scholar] [CrossRef]
- Junyaprasert, V.B.; Teeranachaideekul, V.; Souto, E.B.; Boonme, P.; Müller, R.H. Q10-Loaded NLC versus Nanoemulsions: Stability, Rheology and in Vitro Skin Permeation. Int. J. Pharm. 2009, 377, 207–214. [Google Scholar] [CrossRef]
- Directorate-General for Health and Food Safety (European Commission). Scientific Committee on Consumer Safety SCCS Guidance on the Safety Assessment of Nanomaterials in Cosmetics; European Union: Maastricht, The Netherlands, 2020. [Google Scholar] [CrossRef]
- COLIPA In Vitro UV Protection Method Task Force. Method for In Vitro Determination of In Vitro Method for the Determination of the UVA Protection Factor and “Critical Wavelength” Values of Sunscreen Products; Guideline Prepared by the COLIPA In Vitro UV Protection Method Task Force; The European Cosmetic and Perfumery Association (COLIPA): Brussels, Belgium, 2009. [Google Scholar]
- Food and Drug Administration. Contains Nonbinding Recommendations—Guidance for Industry Safety of Nanomaterials in Cosmetic Products; Food and Drug Administration: Rockville, MD, USA, 2014. [Google Scholar]
- Shah, S.; Nene, S.; Rangaraj, N.; Raghuvanshi, R.S.; Singh, S.B.; Srivastava, S. Bridging the Gap: Academia, Industry and FDA Convergence for Nanomaterials. Drug Dev. Ind. Pharm. 2020, 46, 1735–1746. [Google Scholar] [CrossRef] [PubMed]
- Rambaran, T.; Schirhagl, R. Nanotechnology from Lab to Industry—A Look at Current Trends. Nanoscale Adv. 2022, 4, 3664–3675. [Google Scholar] [CrossRef] [PubMed]
- Souza, C.; Maia Campos, P.M.B.G. Development of a HPLC Method for Determination of Four UV Filters in Sunscreen and Its Application to Skin Penetration Studies. Biomed. Chromatogr. 2017, 31, e4029. [Google Scholar] [CrossRef] [PubMed]
- Caldas, A.R.; Faria, M.J.; Ribeiro, A.; Machado, R.; Gonçalves, H.; Gomes, A.C.; Soares, G.M.B.; Lopes, C.M.; Lúcio, M. Avobenzone-Loaded and Omega-3-Enriched Lipid Formulations for Production of UV Blocking Sunscreen Gels and Textiles. J. Mol. Liq. 2021, 342, 116965. [Google Scholar] [CrossRef]
- ICH Expert Working Group. International Conference on Harmonisation of Technical Requirements for Registration of Pharmaceuticals for Human Use—ICH Harmonised Tripartite Guidelines of Stability Testeing of New Drug Substances and Products Q1A(R2); ICH: Geneva, Switzerland, 2003. [Google Scholar]
- Brazilian Health Regulatory Agency (ANVISA) Health Ministry. 1 Cosmetic Series Quality Cosmetic Products Stability Guide; ANVISA: Brasília, Brazil, 2004.
- OECD. Guideline for the Testing of Chemicals. OECD Guideline 428. Skin Absorption: In Vitro Method; OECD: Paris, France, 2004. [Google Scholar]
- ISO 24444:2019; Cosmetics-Sun Protection Test Methods—In Vivo Determination of the Sun Protection Factor (SPF). ISO International Standard: Geneva, Switzerland, 2019.
- ISO 24442:2022; Cosmetics-Sun Protection Test Methods—In Vivo Determination of Sunscreen UVA Protection. ISO International Standard: Geneva, Switzerland, 2022.
- Bernstein, E.F.; Sarkas, H.W.; Boland, P. Iron Oxides in Novel Skin Care Formulations Attenuate Blue Light for Enhanced Protection against Skin Damage. J. Cosmet. Dermatol. 2021, 20, 532–537. [Google Scholar] [CrossRef]
- Badea, G.; Lăcătuşu, I.; Badea, N.; Ott, C.; Meghea, A. Use of Various Vegetable Oils in Designing Photoprotective Nanostructured Formulations for UV Protection and Antioxidant Activity. Ind. Crops Prod. 2015, 67, 18–24. [Google Scholar] [CrossRef]
- Ispiryan, A.; Viškelis, J.; Viškelis, P. Red Raspberry (Rubus idaeus L.) Seed Oil: A Review. Plants 2021, 10, 944. [Google Scholar] [CrossRef]
- Niculae, G.; Lacatusu, I.; Badea, N.; Stan, R.; Vasile, B.S.; Meghea, A. Rice Bran and Raspberry Seed Oil-Based Nanocarriers with Self-Antioxidative Properties as Safe Photoprotective Formulations. Photochem. Photobiol. Sci. 2014, 13, 703–716. [Google Scholar] [CrossRef] [PubMed]
- Rufino, M.S.M.; Alves, R.E.; Fernandes, F.A.N.; Brito, E.S. Free Radical Scavenging Behavior of Ten Exotic Tropical Fruits Extracts. Food Res. Int. 2011, 44, 2072–2075. [Google Scholar] [CrossRef]
- Oomah, B.D.; Ladet, S.; Godfrey, D.V.; Liang, J.; Girard, B. Characteristics of Raspberry (Rubus idaeus L.) Seed Oil. Food Chem. 2000, 69, 187–193. [Google Scholar] [CrossRef]
- Lindoso, J.V.D.S.; Alencar, S.R.; Dos Santos, A.A.; Mello Neto, R.S.; Mendes, A.V.d.S.; Furtado, M.M.; da Silva, M.G.; da Silva Brito, A.K.; Batista, E.K.F.; Baêta, S.d.A.F.; et al. Effects of “Bacuri” Seed Butter (Platonia insignis Mart.), a Brazilian Amazon Fruit, on Oxidative Stress and Diabetes Mellitus-Related Parameters in STZ-Diabetic Rats. Biol. 2022, 11, 562. [Google Scholar] [CrossRef] [PubMed]
- DSM. DSM Sunscreen OptimizerTM. Available online: https://www.sunscreen-optimizer.com/index.html. (accessed on 22 June 2020).
- Osterwalder, U.; Herzog, B. The Long Way towards the Ideal Sunscreen—Where We Stand and What Still Needs to Be Done. Photochem. Photobiol. Sci. 2010, 9, 470–481. [Google Scholar] [CrossRef]
- Zhao, S.; Yang, X.; Garamus, V.M.; Handge, U.A.; Bérengère, L.; Zhao, L.; Salamon, G.; Willumeit, R.; Zou, A.; Fan, S. Mixture of Nonionic/Ionic Surfactants for the Formulation of Nanostructured Lipid Carriers: Effects on Physical Properties. Langmuir 2014, 30, 6920–6928. [Google Scholar] [CrossRef]
- Witayaudom, P.; Klinkesorn, U. Effect of Surfactant Concentration and Solidification Temperature on the Characteristics and Stability of Nanostructured Lipid Carrier (NLC) Prepared from Rambutan (Nephelium lappaceum L.) Kernel Fat. J. Colloid. Interface Sci. 2017, 505, 1082–1092. [Google Scholar] [CrossRef]
- Ortiz, A.C.; Yañez, O.; Salas-Huenuleo, E.; Morales, J.O. Development of a Nanostructured Lipid Carrier (NLC) by a Low-Energy Method, Comparison of Release Kinetics and Molecular Dynamics Simulation. Pharmaceutics 2021, 13, 531. [Google Scholar] [CrossRef]
- Tamjidi, F.; Shahedi, M.; Varshosaz, J.; Nasirpour, A. Design and Characterization of Astaxanthin-Loaded Nanostructured Lipid Carriers. Innov. Food Sci. Emerg. Technol. 2014, 26, 366–374. [Google Scholar] [CrossRef]
- Shi, L.; Li, Z.; Yu, L.; Jia, H.; Zheng, L. Effects of Surfactants and Lipids on the Preparation of Solid Lipid Nanoparticles Using Double Emulsion Method. J. Dispers. Sci. Technol. 2011, 32, 254–259. [Google Scholar] [CrossRef]
- Martins, S.; Tho, I.; Souto, E.; Ferreira, D.; Brandl, M. Multivariate Design for the Evaluation of Lipid and Surfactant Composition Effect for Optimisation of Lipid Nanoparticles. Eur. J. Pharm. Sci. 2012, 45, 613–623. [Google Scholar] [CrossRef] [PubMed]
- de Araujo, M.M.; Borgheti-Cardoso, L.N.; Praça, F.G.; Marcato, P.D.; Bentley, M.V.L.B. Solid Lipid–Polymer Hybrid Nanoplatform for Topical Delivery of SiRNA: In Vitro Biological Activity and Permeation Studies. J. Funct. Biomater. 2023, 14, 374. [Google Scholar] [CrossRef]
- Ribeiro, L.N.M.; Breitkreitz, M.C.; Guilherme, V.A.; da Silva, G.H.R.; Couto, V.M.; Castro, S.R.; de Paula, B.O.; Machado, D.; de Paula, E. Natural Lipids-Based NLC Containing Lidocaine: From Pre-Formulation to in Vivo Studies. Eur. J. Pharm. Sci. 2017, 106, 102–112. [Google Scholar] [CrossRef]
- Andreozzi, E.; Wang, P.; Valenzuela, A.; Tu, C.; Gorin, F.; Dhenain, M.; Louie, A. Size-Stable Solid Lipid Nanoparticles Loaded with Gd-DOTA for Magnetic Resonance Imaging. Bioconjug Chem. 2013, 24, 1455–1467. [Google Scholar] [CrossRef] [PubMed]
- Yadav, K.S.; Kale, K. High Pressure Homogenizer in Pharmaceuticals: Understanding Its Critical Processing Parameters and Applications. J. Pharm. Innov. 2020, 15, 690–701. [Google Scholar] [CrossRef]
- Hübner, A.A.; Sarruf, F.D.; Oliveira, C.A.; Neto, A.V.; Fischer, D.C.H.; Kato, E.T.M.; Lourenço, F.R.; Baby, A.R.; Bacchi, E.M. Safety and Photoprotective Efficacy of a Sunscreen System Based on Grape Pomace (Vitis vinifera L.) Phenolics from Winemaking. Pharmaceutics 2020, 12, 1148. [Google Scholar] [CrossRef]
- Khater, D.; Nsairat, H.; Odeh, F.; Saleh, M.; Jaber, A.; Alshaer, W.; Al Bawab, A.; Mubarak, M.S. Design, Preparation, and Characterization of Effective Dermal and Transdermal Lipid Nanoparticles: A Review. Cosmetics 2021, 8, 39. [Google Scholar] [CrossRef]
- Johnson, M.E.; Blankschtein, X.D.; Langer, R. Evaluation of Solute Permeation through the Stratum Corneum: Lateral Bilayer Diffusion as the Primary Transport Mechanism. J. Pharm. Sci. 1997, 86, 1162–1172. [Google Scholar] [CrossRef]
- Baspinar, Y.; Borchert, H.H. Penetration and Release Studies of Positively and Negatively Charged Nanoemulsions—Is There a Benefit of the Positive Charge? Int. J. Pharm. 2012, 430, 247–252. [Google Scholar] [CrossRef]
- Czajkowska-Kośnik, A.; Szekalska, M.; Winnicka, K. Nanostructured Lipid Carriers: A Potential Use for Skin Drug Delivery Systems. Pharmacol. Rep. 2019, 71, 156–166. [Google Scholar] [CrossRef]
- Tampucci, S.; Tofani, G.; Chetoni, P.; Di Gangi, M.; Mezzetta, A.; Paganini, V.; Burgalassi, S.; Pomelli, C.S.; Monti, D. Sporopollenin Microcapsule: Sunscreen Delivery System with Photoprotective Properties. Pharmaceutics 2022, 14, 2041. [Google Scholar] [CrossRef] [PubMed]
- Stiefel, C.; Schwack, W. Photoprotection in Changing Times—UV Filter Efficacy and Safety, Sensitization Processes and Regulatory Aspects. Int. J. Cosmet. Sci. 2015, 37, 2–30. [Google Scholar] [CrossRef] [PubMed]
- Bos, J.D.; Meinardi, M.M.H.M. The 500 Dalton Rule for the Skin Penetration of Chemical Compounds and Drugs. Exp. Dermatol. 2000, 9, 165–169. [Google Scholar] [CrossRef] [PubMed]
- Wang, K.; Zhang, Q.J.; Miao, Y.L.; Luo, S.Q.; Wang, H.C.; Zhang, W.P. Effect of Solid Lipid’s Structure on Nanostructured Lipid Carriers Encapsulated with Sun Filter: Characterisation, Photo-Stability and in Vitro Release. J. Microencapsul. 2017, 34, 104–110. [Google Scholar] [CrossRef] [PubMed]
- Wissing, S.A.; Mü, R.H. The Development of an Improved Carrier System for Sunscreen Formulations Based on Crystalline Lipid Nanoparticles. Int. J. Pharm. 2002, 242, 373–375. [Google Scholar] [CrossRef] [PubMed]
- Vitorino, C.; Alves, L.; Antunes, F.E.; Sousa, J.J.; Pais, A.A.C.C. Design of a Dual Nanostructured Lipid Carrier Formulation Based on Physicochemical, Rheological, and Mechanical Properties. J. Nanopart. Res. 2013, 15, 1993. [Google Scholar] [CrossRef]
- Beck, R.; Guterres, S.; Pohlmann, A. Nanocosmetics and Nanomedicines: New Approaches for Skin Care, 1st ed.; Beck, R., Guterres, S., Pohlmann, A., Eds.; Springer: Porto Alegre, Brazil, 2011. [Google Scholar]
- Souto, E.B.; Müller, R.H. Rheological and in Vitro Release Behaviour of Clotrimazole-Containing Aqueous SLN Dispersions and Commercial Creams. Pharmazie 2007, 62, 505–509. [Google Scholar] [CrossRef] [PubMed]
- Passeron, T.; Lim, H.W.; Goh, C.L.; Kang, H.Y.; Ly, F.; Morita, A.; Ocampo Candiani, J.; Puig, S.; Schalka, S.; Wei, L.; et al. Photoprotection According to Skin Phototype and Dermatoses: Practical Recommendations from an Expert Panel. J. Eur. Acad. Dermatol. Venereol. 2021, 35, 1460–1469. [Google Scholar] [CrossRef] [PubMed]

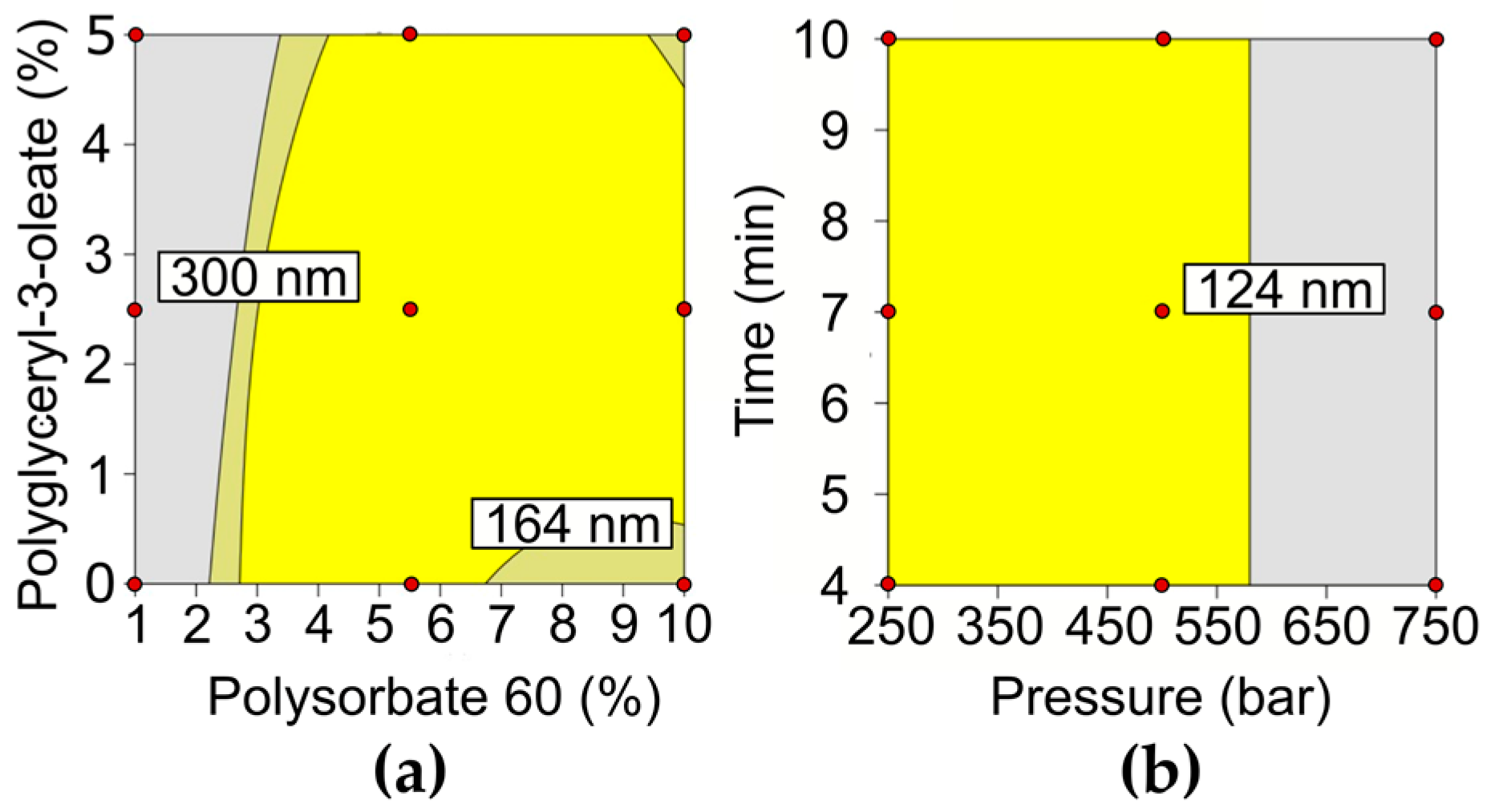



| Factor | −1 | 0 | +1 |
|---|---|---|---|
| CCF-Formulation | |||
| Polysorbate 60 (%) | 1.0 | 5.5 | 10.0 |
| Polyglyceryl-3-dioleate (%) | 0.0 | 2.5 | 5.0 |
| CCF-Process | |||
| Pressure (bar) | 250 | 500 | 750 |
| Time (min) | 4 | 7 | 10 |
| Sample | Run | x1 | x2 |
|---|---|---|---|
| SC-NLC-1 | 2 | −1 | −1 |
| SC-NLC-2 | 1 | +1 | −1 |
| SC-NLC-3 | 7 | −1 | +1 |
| SC-NLC-4 | 3 | +1 | +1 |
| SC-NLC-5 | 6 | −1 | 0 |
| SC-NLC-6 | 13 | +1 | 0 |
| SC-NLC-7 | 8 | 0 | −1 |
| SC-NLC-8 | 5 | 0 | +1 |
| SC-NLC-PC1 | 4 | 0 | 0 |
| SC-NLC-PC2 | 12 | 0 | 0 |
| SC-NLC-PC3 | 11 | 0 | 0 |
| SC-NLC-PC4 | 9 | 0 | 0 |
| SC-NLC-PC5 | 10 | 0 | 0 |
| Filter | Epidermis (µg/cm²) | Dermis (µg/cm²) | Receptor Liquid (µg/cm²) | |||
|---|---|---|---|---|---|---|
| SC-NLC | Emulsion | SC-NLC | Emulsion | SC-NLC | Emulsion | |
| Uvinul® A Plus | 15.86 | 12.95 | 4.31 | 4.24 | 0.00 | 0.00 |
| Tinosorb® S | 8.51 | 3.32 | 2.03 | 1.16 | 0.00 | 0.00 |
| Uvinul® T150 | 0.50 | 0.29 | 0.05 | 0.02 | 0.00 | 0.00 |
| Sample | Clipid aditive (%) | SPF | UVA-PF0 | UVA/UVB |
|---|---|---|---|---|
| CB/SC-NLC | 10 | 64.67 ± 8.50 | 24.67 ± 1.88 | 0.38 |
| 20 | 107.67 ± 17.15 | 28.67 ± 2.05 | 0.27 | |
| 30 | 75.67 ± 10.21 | 18.33 ± 0.47 | 0.24 | |
| CB/emulsion | 10 | 58.34 ± 7.36 | 18.33 ± 0.94 | 0.31 |
| 20 | 57.00 ± 7.26 | 16.67 ± 1.70 | 0.29 | |
| 30 | 49.33 ± 6.94 | 18.00 ± 1.63 | 0.36 | |
| CB | 0 | 111.33 ± 7.41 | 22.00 ± 1.41 | 0.20 |
| SC-NLC | 100 | 13.00 ± 1.41 | 10.67 ± 0.94 | 0.82 |
| Emulsion | 100 | 11.67 ± 1.24 | 8.33 ± 0.47 | 0.71 |
| Formulations | CB 100% | CB 80% 1 | Emulsion 20% 2 | SC-NLC 20% 2 | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| I | II | III | I | II | III | I | II | III | I | II | III | |
| Chemical filter content | ||||||||||||
| Total (%) 3 | 13.5 | 15.9 | 25.4 | 10.8 | 12.7 | 20.3 | 12.0 | 13.9 | 21.5 | 12.0 | 13.9 | 21.5 |
| Filter content reduction (%) 4 | - | - | - | - | 11.1 | 12.4 | 15.3 | 11.1 | 12.4 | 15.3 | ||
| SPF | ||||||||||||
| In vivo | 30 | 30 | 50 | 23.2 ± 0.0 | 21.6 ± 2.8 | 38.1 ± 7.6 | 23.3 ± 5.6 | 29.9 ± 4.5 | 41.7 ± 12.0 | 29.4 ± 2.5 | 39.6 ± 5.2 | 50.3 ± 10.5 |
| UVA-PF0 | ||||||||||||
| In vivo | 10.0 | 10.0 | 17.9 | 6.6 ± 1.6 | 8.1 ± 0.9 | 14.3 ± 3.4 | 8.1 ± 1.0 | 10.0 ± 2.1 | 16.9 ± 2.7 | 12.7 ± 1.5 | 12.2 ± 2.3 | 19.0 ± 3.3 |
| UVA/UVB | ||||||||||||
| In vivo | 0.30 | 0.30 | 0.36 | 0.28 | 0.38 | 0.38 | 0.35 | 0.33 | 0.41 | 0.43 | 0.31 | 0.38 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
de Araújo, M.M.; Schneid, A.C.; Oliveira, M.S.; Mussi, S.V.; de Freitas, M.N.; Carvalho, F.C.; Bernes Junior, E.A.; Faro, R.; Azevedo, H. NLC-Based Sunscreen Formulations with Optimized Proportion of Encapsulated and Free Filters Exhibit Enhanced UVA and UVB Photoprotection. Pharmaceutics 2024, 16, 427. https://doi.org/10.3390/pharmaceutics16030427
de Araújo MM, Schneid AC, Oliveira MS, Mussi SV, de Freitas MN, Carvalho FC, Bernes Junior EA, Faro R, Azevedo H. NLC-Based Sunscreen Formulations with Optimized Proportion of Encapsulated and Free Filters Exhibit Enhanced UVA and UVB Photoprotection. Pharmaceutics. 2024; 16(3):427. https://doi.org/10.3390/pharmaceutics16030427
Chicago/Turabian Stylede Araújo, Margarete M., Andressa C. Schneid, Mariana S. Oliveira, Samuel V. Mussi, Miller N. de Freitas, Flávia C. Carvalho, Edson A. Bernes Junior, Renato Faro, and Hatylas Azevedo. 2024. "NLC-Based Sunscreen Formulations with Optimized Proportion of Encapsulated and Free Filters Exhibit Enhanced UVA and UVB Photoprotection" Pharmaceutics 16, no. 3: 427. https://doi.org/10.3390/pharmaceutics16030427







