Synthesis and Properties of Injectable Hydrogel for Tissue Filling
Abstract
:1. Introduction
2. Synthesis Mechanisms of Injectable Hydrogels
2.1. Chemical Covalent Crosslinking
2.1.1. Dynamic Covalent Chemical Bonds
2.1.2. Radical Polymerization
2.1.3. Delayed Gelatinization

2.2. Physical Crosslinking
2.2.1. Hydrogen Bonding
2.2.2. Hydrophobic Interaction
2.2.3. Host–Guest Interactions
2.2.4. Other Physical Interactions
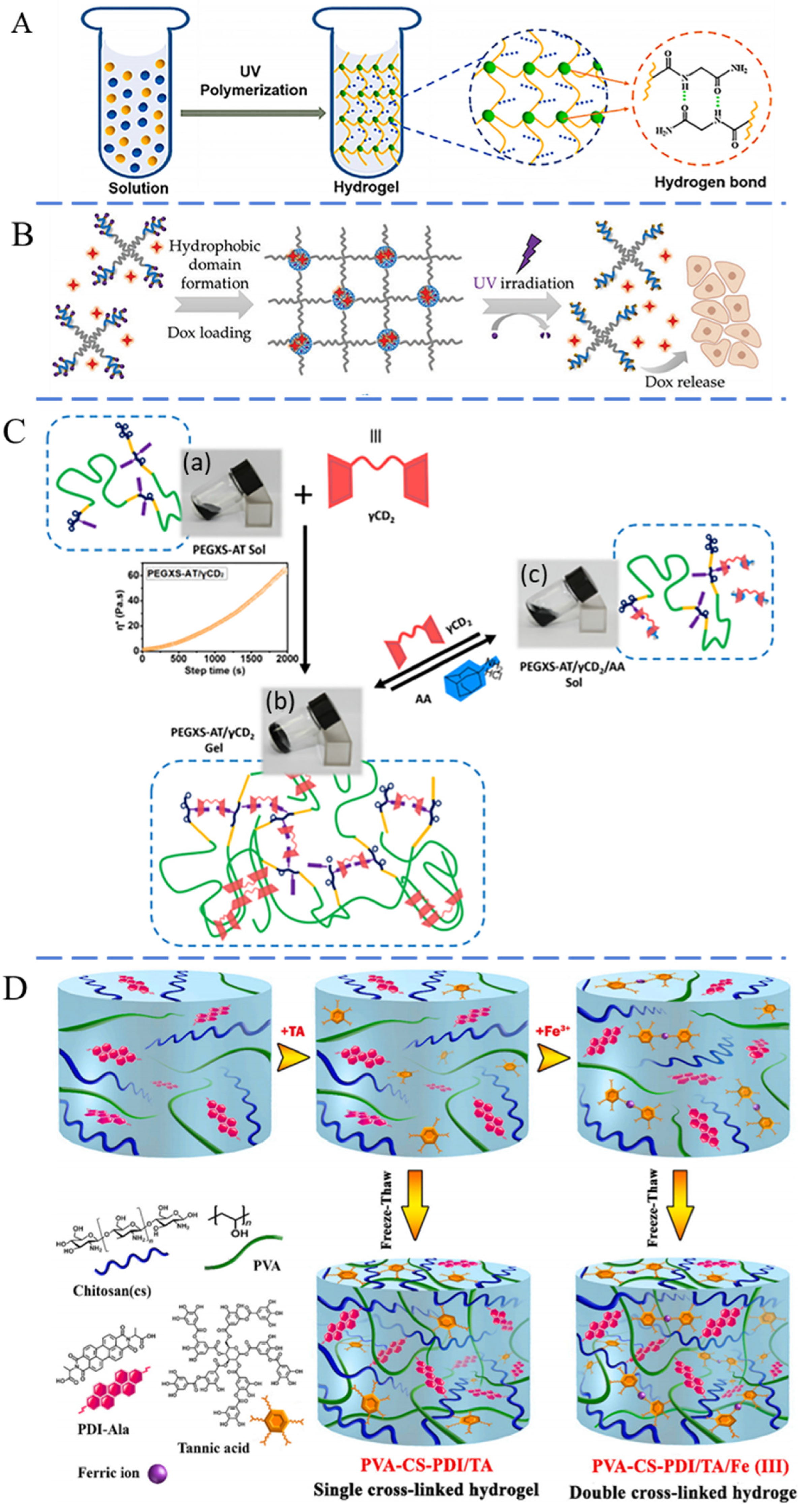
2.3. Biological Crosslinking
3. Composition of Injectable Hydrogels
3.1. Natural Polymer Hydrogels
3.2. Synthetic Polymeric Hydrogels
4. Properties of Injectable Hydrogels
4.1. Mechanical Strength of Injectable Hydrogels
4.2. Degradability of Injectable Hydrogels
4.3. Biological Function of Injectable Hydrogels
5. Injectable Hydrogels for Soft Tissue Fillers
5.1. HA-Based Injectable Hydrogels
5.2. SF-Based Injectable Hydrogel
5.3. Collagen-Based Injectable Hydrogels
5.4. CMC-Based Injectable Hydrogels
5.5. Other Injectable Hydrogels
6. Conclusions and Outlook
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Makrantonaki, E.; Adjaye, J.; Herwig, R.; Brink, T.C.; Groth, D.; Hultschig, C.; Lehrach, H.; Zouboulis, C.C. Age-specific hormonal decline is accompanied by transcriptional changes in human sebocytes in vitro. Aging Cell 2006, 5, 331–344. [Google Scholar] [CrossRef] [PubMed]
- Ralf Paus, L.; Berneburg, M.; Trelles, M.; Friguet, B.; Ogden, S.; Esrefoglu, M.; Kaya, G.; Goldberg, D.J.; Mordon, S.; Calderhead, R.G.; et al. How best to halt and/or revert UV-induced skin ageing: Strategies, facts and fiction. Exp. Dermatol. 2009, 17, 228–229. [Google Scholar]
- Kaoutzanis, C.; Ganesh Kumar, N.; Winocour, J.; Hood, K.; Higdon, K.K. Surgical Site Infections in Aesthetic Surgery. Aesthetic Surg. J. 2019, 39, 1118–1138. [Google Scholar] [CrossRef] [PubMed]
- Chen, Q.; Li, P.; Zhao, Q.; Tu, T.; Lu, H.; Zhang, W. Occurrence and treatment of peripheral nerve injuries after cosmetic surgeries. Front. Neurol. 2023, 14, 1258759. [Google Scholar] [CrossRef]
- Schlager, J.G.; Hartmann, D.; Ruiz San Jose, V.; Patzer, K.; French, L.E.; Kendziora, B. Procedure-Related Risk Factors for Surgical Site Infection in Dermatologic Surgery. Dermatol. Surg. 2022, 48, 1046–1050. [Google Scholar] [CrossRef] [PubMed]
- Winslow, C. Filling the Midface: Injectables. Facial Plast. Surg. 2016, 32, 473–479. [Google Scholar] [CrossRef]
- Pour Mohammad, A.; Gholizadeh Mesgarha, M.; Seirafianpour, F.; Karimi, Y.; Sodagar, S.; Afraie, M.; Goodarzi, A. A systematic review and meta-analysis of efficacy, safety, and satisfaction rates of laser combination treatments vs laser monotherapy in skin rejuvenation resurfacing. Lasers Med. Sci. 2023, 38, 228. [Google Scholar] [CrossRef] [PubMed]
- Sieber, D.A.; Kenkel, J.M. Noninvasive Methods for Lower Facial Rejuvenation. Clin. Plast. Surg. 2018, 45, 571–584. [Google Scholar] [CrossRef]
- Hamilton, M.; Campbell, A.; Holcomb, J.D. Contemporary Laser and Light-Based Rejuvenation Techniques. Facial Plast. Surg. Clin. N. Am. 2018, 26, 113–121. [Google Scholar] [CrossRef]
- Sobanko, J.F.; Dai, J.; Gelfand, J.M.; Sarwer, D.B.; Percec, I. Prospective Cohort Study Investigating Changes in Body Image, Quality of Life, and Self-Esteem Following Minimally Invasive Cosmetic Procedures. Dermatol. Surg. 2018, 44, 1121–1128. [Google Scholar] [CrossRef]
- Zhao, P.; Zhao, W.; Zhang, K.; Lin, H.; Zhang, X. Polymeric injectable fillers for cosmetology: Current status, future trends, and regulatory perspectives. J. Appl. Polym. Sci. 2019, 137, 14. [Google Scholar] [CrossRef]
- Zhao, Y.; Fan, J.; Bai, S. Biocompatibility of injectable hydrogel from decellularized human adipose tissue in vitro and in vivo. J. Biomed. Mater. Res. Part B Appl. Biomater. 2018, 107, 1684–1694. [Google Scholar] [CrossRef] [PubMed]
- Lin, J.H.; Sclafani, A.P. Platelet-Rich Plasma for Skin Rejuvenation and Tissue Fill. Facial Plast. Surg. Clin. N. Am. 2018, 26, 439–446. [Google Scholar] [CrossRef] [PubMed]
- Fan, M.; Liao, J.; Guo, G.; Ding, Q.; Yang, Y.; Luo, F.; Qian, Z. Dexamethasone-Loaded Poly(D, L-lactic acid) Microspheres/Poly(ethylene glycol)-Poly(ε-caprolactone)-Poly(ethylene glycol) Micelles Composite for Skin Augmentation. J. Biomed. Nanotechnol. 2014, 10, 592–602. [Google Scholar] [CrossRef] [PubMed]
- Geckil, H.; Xu, F.; Zhang, X.H.; Moon, S.; Demirci, U. Engineering hydrogels as extracellular matrix mimics. Nanomedicine 2010, 5, 469–484. [Google Scholar] [CrossRef] [PubMed]
- Mantha, S.; Pillai, S.; Khayambashi, P.; Upadhyay, A.; Zhang, Y.; Tao, O.; Pham, H.M.; Tran, S.D. Smart Hydrogels in Tissue Engineering and Regenerative Medicine. Materials 2019, 12, 3323. [Google Scholar] [CrossRef]
- Laeschke, K. Biocompatibility of microparticles into soft tissue fillers. Semin. Cutan. Med. Surg. 2004, 23, 214–217. [Google Scholar] [CrossRef]
- Guo, J.H.; Fang, W.; Wang, F.F. Injectable fillers: Current status, physicochemical properties, function mechanism, and perspectives. Rsc Adv. 2023, 13, 23841–23858. [Google Scholar] [CrossRef]
- Lin, Z.Y.; Shah, V.; Dhinakar, A.; Yildirimer, L.; Cui, W.-G.; Zhao, X. Intradermal fillers for minimally invasive treatment of facial aging. Plast. Aesthetic Res. 2016, 3, 72–82. [Google Scholar] [CrossRef]
- Saunders, L.; Ma, P.X. Self-Healing Supramolecular Hydrogels for Tissue Engineering Applications. Macromol. Biosci. 2019, 19, 1800313. [Google Scholar] [CrossRef]
- Xu, J.; Liu, X.; Ren, X.; Gao, G. The role of chemical and physical crosslinking in different deformation stages of hybrid hydrogels. Eur. Polym. J. 2018, 100, 86–95. [Google Scholar] [CrossRef]
- Yan, J.; Li, S.; Chen, G.; Ma, C.; McClements, D.J.; Liu, X.; Liu, F. Formation, physicochemical properties, and comparison of heat- and enzyme-induced whey protein-gelatin composite hydrogels. Food Hydrocoll. 2023, 137, 12. [Google Scholar] [CrossRef]
- Li, Z.; Lu, F.; Liu, Y. A Review of the Mechanism, Properties, and Applications of Hydrogels Prepared by Enzymatic Crosslinking. J. Agric. Food Chem. 2023, 71, 10238–10249. [Google Scholar] [CrossRef] [PubMed]
- Kumar Sharma, A.; Priya; Singh Kaith, B.; Bajaj, S.; Bhatia, J.K.; Panchal, S.; Sharma, N.; Tanwar, V. Efficient capture of eosin yellow and crystal violet with high performance xanthan-acacia hybrid super-adsorbent optimized using response surface methodology. Colloids Surf. B Biointerfaces 2019, 175, 314–323. [Google Scholar] [CrossRef] [PubMed]
- Sharma, A.K.; Priya; Kaith, B.S.; Sharma, N.; Bhatia, J.K.; Tanwar, V.; Panchal, S.; Bajaj, S. Selective removal of cationic dyes using response surface methodology optimized gum acacia-sodium alginate blended superadsorbent. Int. J. Biol. Macromol. 2019, 124, 331–345. [Google Scholar] [CrossRef] [PubMed]
- Kaith, B.S.; Singh, A.; Sharma, A.K.; Sud, D. Hydrogels: Synthesis, Classification, Properties and Potential Applications—A Brief Review. J. Polym. Environ. 2021, 29, 3827–3841. [Google Scholar] [CrossRef]
- Zhao, Q.S.; Cong, Y.W. Michael reaction acceptor molecules in chemical biology. Prog. Chem. 2007, 19, 1972–1976. [Google Scholar]
- Sun, Y.N.; Nan, D.; Jin, H.Q.; Qu, X.Z. Recent advances of injectable hydrogels for drug delivery and tissue engineering applications. Polym. Test. 2020, 81, 13. [Google Scholar] [CrossRef]
- Yang, X.; He, S.; Wang, J.; Liu, Y.; Ma, W.; Yu, C.-Y.; Wei, H. Hyaluronic acid-based injectable nanocomposite hydrogels with photo-thermal antibacterial properties for infected chronic diabetic wound healing. Int. J. Biol. Macromol. 2023, 242, 124872. [Google Scholar] [CrossRef] [PubMed]
- Fu, H.; Yu, C.; Li, X.; Bao, H.; Zhang, B.; Chen, Z.; Zhang, Z. Facile engineering of ECM-mimetic injectable dual crosslinking hydrogels with excellent mechanical resilience, tissue adhesion, and biosafety. J. Mater. Chem. B 2021, 9, 10003–10014. [Google Scholar] [CrossRef] [PubMed]
- Adams, J.P. Imines, enamines and oximes. J. Chem. Soc. Perkin Trans. 2000, 1, 125–139. [Google Scholar] [CrossRef]
- Meyer, C.D.; Joiner, C.S.; Stoddart, J.F. Template-directed synthesis employing reversible imine bond formation. Chem. Soc. Rev. 2007, 36, 1705–1723. [Google Scholar] [CrossRef]
- Yue, S.; He, H.; Li, B.; Hou, T. Hydrogel as a Biomaterial for Bone Tissue Engineering: A Review. Nanomaterials 2020, 10, 1511. [Google Scholar] [CrossRef]
- Lü, S.; Gao, C.; Xu, X.; Bai, X.; Duan, H.; Gao, N.; Feng, C.; Xiong, Y.; Liu, M. Injectable and Self-Healing Carbohydrate-Based Hydrogel for Cell Encapsulation. ACS Appl. Mater. Interfaces 2015, 7, 13029–13037. [Google Scholar] [CrossRef]
- Wei, Z.; Zhao, J.; Chen, Y.M.; Zhang, P.; Zhang, Q. Self-healing polysaccharide-based hydrogels as injectable carriers for neural stem cells. Sci. Rep. 2016, 6, 37841. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Wang, X.; Wang, Y.; Hao, J. Rapid-Forming and Self-Healing Agarose-Based Hydrogels for Tissue Adhesives and Potential Wound Dressings. Biomacromolecules 2018, 19, 980–988. [Google Scholar] [CrossRef] [PubMed]
- Talebian, S.; Mehrali, M.; Taebnia, N.; Pennisi, C.P.; Kadumudi, F.B.; Foroughi, J.; Hasany, M.; Nikkhah, M.; Akbari, M.; Orive, G.; et al. Self-Healing Hydrogels: The Next Paradigm Shift in Tissue Engineering? Adv. Sci. 2019, 6, 1801664. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.; Liu, C.; Wen, J.; Wu, Y.; Shan, Y.; Liao, J. The design, mechanism and biomedical application of self-healing hydrogels. Chin. Chem. Lett. 2017, 28, 1857–1874. [Google Scholar] [CrossRef]
- Wang, Y.N.; Adokoh, C.K.; Narain, R. Recent development and biomedical applications of self-healing hydrogels. Expert Opin. Drug Deliv. 2018, 15, 77–91. [Google Scholar] [CrossRef]
- Uman, S.; Dhand, A.; Burdick, J.A. Recent advances in shear-thinning and self-healing hydrogels for biomedical applications. J. Appl. Polym. Sci. 2019, 137, 20. [Google Scholar] [CrossRef]
- Ghanian, M.H.; Mirzadeh, H.; Baharvand, H. In Situ Forming, Cytocompatible, and Self-Recoverable Tough Hydrogels Based on Dual Ionic and Click Crosslinked Alginate. Biomacromolecules 2018, 19, 1646–1662. [Google Scholar] [CrossRef] [PubMed]
- Rollas, S.; Küçükgüzel, S.G. Biological activities of hydrazone derivatives. Molecules 2007, 12, 1910–1939. [Google Scholar] [CrossRef]
- Apostolides, D.E.; Patrickios, C.S. Dynamic covalent polymer hydrogels and organogels crosslinked through acylhydrazone bonds: Synthesis, characterization and applications. Polymer Int. 2018, 67, 627–649. [Google Scholar] [CrossRef]
- Jiang, X.; Zeng, F.; Yang, X.; Jian, C.; Zhang, L.; Yu, A.; Lu, A. Injectable self-healing cellulose hydrogel based on host-guest interactions and acylhydrazone bonds for sustained cancer therapy. Acta Biomater. 2022, 141, 102–113. [Google Scholar] [CrossRef] [PubMed]
- Sun, F.; Yao, Q.; Wang, Y.; Lou, J.; Li, Q.; Tao, Q.; Li, G. Self-healing injectable stimuli responsive polysaccharide hydrogel constructed with dynamic covalent bonds. J. Appl. Polym. Sci. 2023, 140, e54304. [Google Scholar] [CrossRef]
- Nguyen, K.T.; West, J.L. Photopolymerizable hydrogels for tissue engineering applications. Biomaterials 2002, 23, 4307–4314. [Google Scholar] [CrossRef] [PubMed]
- Burdick, J.A.; Peterson, A.J.; Anseth, K.S. Conversion and temperature profiles during the photoinitiated polymerization of thick orthopaedic biomaterials. Biomaterials 2001, 22, 1779–1786. [Google Scholar] [CrossRef]
- Noshadi, I.; Hong, S.; Sullivan, K.E.; Shirzaei Sani, E.; Portillo-Lara, R.; Tamayol, A.; Shin, S.R.; Gao, A.E.; Stoppel, W.L.; Black Iii, L.D.; et al. In vitro and in vivo analysis of visible light crosslinkable gelatin methacryloyl (GelMA) hydrogels. Biomater. Sci. 2017, 5, 2093–2105. [Google Scholar] [CrossRef]
- Yuan, L.; Wu, Y.; Gu, Q.-s.; El-Hamshary, H.; El-Newehy, M.; Mo, X. Injectable photo crosslinked enhanced double-network hydrogels from modified sodium alginate and gelatin. Int. J. Biol. Macromol. 2017, 96, 569–577. [Google Scholar] [CrossRef]
- Bordbar-Khiabani, A.; Gasik, M. Smart Hydrogels for Advanced Drug Delivery Systems. Int. J. Mol. Sci. 2022, 23, 3665. [Google Scholar] [CrossRef]
- Fan, L.; Ge, X.; Qian, Y.; Wei, M.; Zhang, Z.; Yuan, W.-E.; Ouyang, Y. Advances in Synthesis and Applications of Self-Healing Hydrogels. Front. Bioeng. Biotechnol. 2020, 8, 654. [Google Scholar] [CrossRef]
- Deng, S.; Li, X.; Yang, W.; He, K.; Ye, X. Injectable in situ crosslinking hyaluronic acid/carboxymethyl cellulose based hydrogels for drug release. J. Biomater. Sci. 2018, 29, 1643–1655. [Google Scholar] [CrossRef]
- Li, R.; Cai, Z.; Li, Z.; Zhang, Q.; Zhang, S.; Deng, L.; Lu, L.; Li, L.; Zhou, C. Synthesis of in-situ formable hydrogels with collagen and hyaluronan through facile Michael addition. Mater. Sci. Eng. C 2017, 77, 1035–1043. [Google Scholar] [CrossRef]
- Wang, W.; Shi, D.; Zhang, Y.; Li, W.; Li, F.; Feng, H.; Ma, L.; Yang, C.; Peng, Z.; Song, G.; et al. An injectable hydrogel based on hyaluronic acid prepared by Schiff base for long-term controlled drug release. Int. J. Biol. Macromol. 2023, 245, 125341. [Google Scholar] [CrossRef] [PubMed]
- Koshy, S.T.; Desai, R.M.; Joly, P.; Li, J.; Bagrodia, R.K.; Lewin, S.A.; Joshi, N.S.; Mooney, D.J. Click-Crosslinked Injectable Gelatin Hydrogels. Adv. Healthc. Mater. 2016, 5, 541–547. [Google Scholar] [CrossRef] [PubMed]
- Bi, B.; Ma, M.; Lv, S.; Zhuo, R.; Jiang, X. In-situ forming thermosensitive hydroxypropyl chitin-based hydrogel crosslinked by Diels-Alder reaction for three dimensional cell culture. Carbohydr. Polym. 2019, 212, 368–377. [Google Scholar] [CrossRef] [PubMed]
- Wang, G.; Cao, X.; Dong, H.; Zeng, L.; Yu, C.; Chen, X. A Hyaluronic Acid Based Injectable Hydrogel Formed via Photo-Crosslinking Reaction and Thermal-Induced Diels-Alder Reaction for Cartilage Tissue Engineering. Polymers 2018, 10, 949. [Google Scholar] [CrossRef]
- Han, Y.Y.; Cao, Y.; Lei, H. Dynamic Covalent Hydrogels: Strong yet Dynamic. Gels 2022, 8, 577. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Wang, Z.; Wen, L.; Nie, J.; Yang, S.; Xu, J.; Cheng, S.Z.D. Highly Elastic Fibers Made from Hydrogen-Bonded Polymer Complex. ACS Macro Lett. 2016, 5, 814–818. [Google Scholar] [CrossRef] [PubMed]
- Guadagno, L.; Vertuccio, L.; Naddeo, C.; Calabrese, E.; Barra, G.; Raimondo, M.; Sorrentino, A.; Binder, W.H.; Michael, P.; Rana, S. Self-healing epoxy nanocomposites via reversible hydrogen bonding. Compos. Part B-Eng. 2019, 157, 1–13. [Google Scholar] [CrossRef]
- Wang, X.Y.; Zhang, H.J.; Yang, Y.X.; Chen, Y.M.; Zhu, X.L.; You, X.Y. Biopolymer-based self-healing hydrogels: A short review. Giant 2023, 16, 100188. [Google Scholar] [CrossRef]
- Zhang, G.; Ngai, T.; Deng, Y.; Wang, C. An Injectable Hydrogel with Excellent Self-Healing Property Based on Quadruple Hydrogen Bonding. Macromol. Chem. Phys. 2016, 217, 2172–2181. [Google Scholar] [CrossRef]
- Zhao, X.; Yang, J.; Liu, Y.; Gao, J.; Wang, K.; Liu, W. An injectable and antifouling self-fused supramolecular hydrogel for preventing postoperative and recurrent adhesions. Chem. Eng. J. 2021, 404, 127096. [Google Scholar] [CrossRef]
- Tuncaboylu, D.C.; Sahin, M.; Argun, A.; Oppermann, W.; Okay, O. Dynamics and Large Strain Behavior of Self-Healing Hydrogels with and without Surfactants. Macromolecules 2012, 45, 1991–2000. [Google Scholar] [CrossRef]
- Xu, K.; An, H.; Lu, C.; Tan, Y.; Li, P.; Wang, P. Facile fabrication method of hydrophobic-associating crosslinking hydrogel with outstanding mechanical performance and self-healing property in the absence of surfactants. Polymer 2013, 54, 5665–5672. [Google Scholar] [CrossRef]
- Quan, L.; Xin, Y.; Wu, X.X.; Ao, Q. Mechanism of Self-Healing Hydrogels and Application in Tissue Engineering. Polymers 2022, 14, 2184. [Google Scholar] [CrossRef]
- Jiang, H.C.; Duan, L.J.; Ren, X.Y.; Gao, G.H. Hydrophobic association hydrogels with excellent mechanical and self-healing properties. Eur. Polym. J. 2019, 112, 660–669. [Google Scholar] [CrossRef]
- Chiu, Y.-L.; Chen, S.-C.; Su, C.-J.; Hsiao, C.-W.; Chen, Y.-M.; Chen, H.-L.; Sung, H.-W. pH-triggered injectable hydrogels prepared from aqueous N-palmitoyl chitosan: In vitro characteristics and in vivo biosafety. Biomaterials 2009, 30, 4877–4888. [Google Scholar] [CrossRef]
- Liu, X.; Wu, H.; Pan, P.; Wang, M.; Zhang, W.; Hu, C.; Lu, S.; Li, M.; Liu, Y. Preparation of Thermosensitive Hydroxybutyl Chitosan/Silk Fibroin Hybrid Hydrogels. Macromol. Mater. Eng. 2022, 307, 2200415. [Google Scholar] [CrossRef]
- Zhao, D.; Tang, Q.; Zhou, Q.; Peng, K.; Yang, H.; Zhang, X. A photo-degradable injectable self-healing hydrogel based on star poly(ethylene glycol)-b-polypeptide as a potential pharmaceuticals delivery carrier. Soft Matter 2018, 14, 7420–7428. [Google Scholar] [CrossRef]
- Yang, X.; Yu, H.; Wang, L.; Tong, R.; Akram, M.; Chen, Y.; Zhai, X. Self-healing polymer materials constructed by macrocycle-based host–guest interactions. Soft Matter 2015, 11, 1242–1252. [Google Scholar] [CrossRef]
- Jin, J.; Cai, L.; Jia, Y.-G.; Liu, S.; Chen, Y.; Ren, L. Progress in self-healing hydrogels assembled by host–guest interactions: Preparation and biomedical applications. J. Mater. Chem. B 2019, 7, 1637–1651. [Google Scholar] [CrossRef] [PubMed]
- Harada, A.; Takashima, Y.; Nakahata, M. Supramolecular Polymeric Materials via Cyclodextrin–Guest Interactions. Acc. Chem. Res. 2014, 47, 2128–2140. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.Z.; Wu, Y.; Feng, K.; Liu, H.; Qin, C.X.; Cai, M.R.; Pei, X.W.; Zhou, F. State transition of macroscopic supramolecular hydrogel by the host-guest coverage effect. J. Appl. Polym. Sci. 2024, 141, e55264. [Google Scholar] [CrossRef]
- Wu, Y.; Guo, B.; Ma, P.X. Injectable Electroactive Hydrogels Formed via Host–Guest Interactions. ACS Macro Lett. 2014, 3, 1145–1150. [Google Scholar] [CrossRef] [PubMed]
- Rodell, C.B.; MacArthur, J.W.; Dorsey, S.M.; Wade, R.J.; Wang, L.L.; Woo, Y.J.; Burdick, J.A. Shear-Thinning Supramolecular Hydrogels with Secondary Autonomous Covalent Crosslinking to Modulate Viscoelastic Properties In Vivo. Adv. Funct. Mater. 2014, 25, 636–644. [Google Scholar] [CrossRef] [PubMed]
- Oshita, H.; Shimazaki, Y. π–π Stacking Interaction of Metal Phenoxyl Radical Complexes. Molecules 2022, 27, 1135. [Google Scholar] [CrossRef]
- Zhuang, W.-R.; Wang, Y.; Cui, P.-F.; Xing, L.; Lee, J.; Kim, D.; Jiang, H.-L.; Oh, Y.-K. Applications of π-π stacking interactions in the design of drug-delivery systems. J. Control. Release 2019, 294, 311–326. [Google Scholar] [CrossRef] [PubMed]
- Yang, B.; Song, J.; Jiang, Y.; Li, M.; Wei, J.; Qin, J.; Peng, W.; López Lasaosa, F.; He, Y.; Mao, H.; et al. Injectable Adhesive Self-Healing Multicrosslinked Double-Network Hydrogel Facilitates Full-Thickness Skin Wound Healing. ACS Appl. Mater. Interfaces 2020, 12, 57782–57797. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Heilshorn, S.C. Adaptable Hydrogel Networks with Reversible Linkages for Tissue Engineering. Adv. Mater. 2015, 27, 3717–3736. [Google Scholar] [CrossRef]
- Sun, Z.; Liu, H.; Dai, D.; Lyu, H.; Huang, R.; Wang, W.; Guo, C. Injectable cell-laden silk acid hydrogel. Chem. Commun. 2024, 60, 316–319. [Google Scholar] [CrossRef]
- Rybak, D.; Rinoldi, C.; Nakielski, P.; Du, J.T.; Bayan, M.A.H.; Zargarian, S.S.; Pruchniewski, M.; Li, X.R.; Strojny-Cieslak, B.; Ding, B.; et al. Injectable and self-healable nano-architectured hydrogel for NIR-light responsive chemo- and photothermal bacterial eradication. J. Mater. Chem. B 2024, 12, 1905–1925. [Google Scholar] [CrossRef]
- Nilforoushzadeh, M.A.; Yazdi, M.K.; Ghavami, S.B.; Farokhimanesh, S.; Amirabad, L.M.; Zarrintaj, P.; Saeb, M.R.; Hamblin, M.R.; Zare, M.; Mozafari, M. Mesenchymal Stem Cell Spheroids Embedded in an Injectable Thermosensitive Hydrogel: An In Situ Drug Formation Platform for Accelerated Wound Healing. Acs Biomater. Sci. Eng. 2020, 6, 5096–5109. [Google Scholar] [CrossRef] [PubMed]
- Kobayashi, Y.; Takashima, Y.; Hashidzume, A.; Yamaguchi, H.; Harada, A. Reversible self-assembly of gels through metal-ligand interactions. Sci. Rep. 2013, 3, 4. [Google Scholar] [CrossRef]
- Azadikhah, F.; Karimi, A.R. Injectable photosensitizing supramolecular hydrogels: A robust physically crosslinked system based on polyvinyl alcohol/chitosan/tannic acid with self-healing and antioxidant properties. React. Funct. Polym. 2022, 173, 105212. [Google Scholar] [CrossRef]
- Mirabelli, V.; Majidi Salehi, S.; Angiolillo, L.; Belviso, B.D.; Conte, A.; Del Nobile, M.A.; Di Profio, G.; Caliandro, R. Enzyme Crystals and Hydrogel Composite Membranes as New Active Food Packaging Material. Glob. Chall. 2018, 2, 1700089. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Lin, S.; Zhang, X.; Liu, X.; Wang, J.; Lu, F. A novel approach for improving the yield of Bacillus subtilis transglutaminase in heterologous strains. J. Ind. Microbiol. Biotechnol. 2014, 41, 1227–1235. [Google Scholar] [CrossRef] [PubMed]
- Sperinde, J.J.; Griffith, L.G. Synthesis and Characterization of Enzymatically-Crosslinked Poly(ethylene glycol) Hydrogels. Macromolecules 1997, 30, 5255–5264. [Google Scholar] [CrossRef]
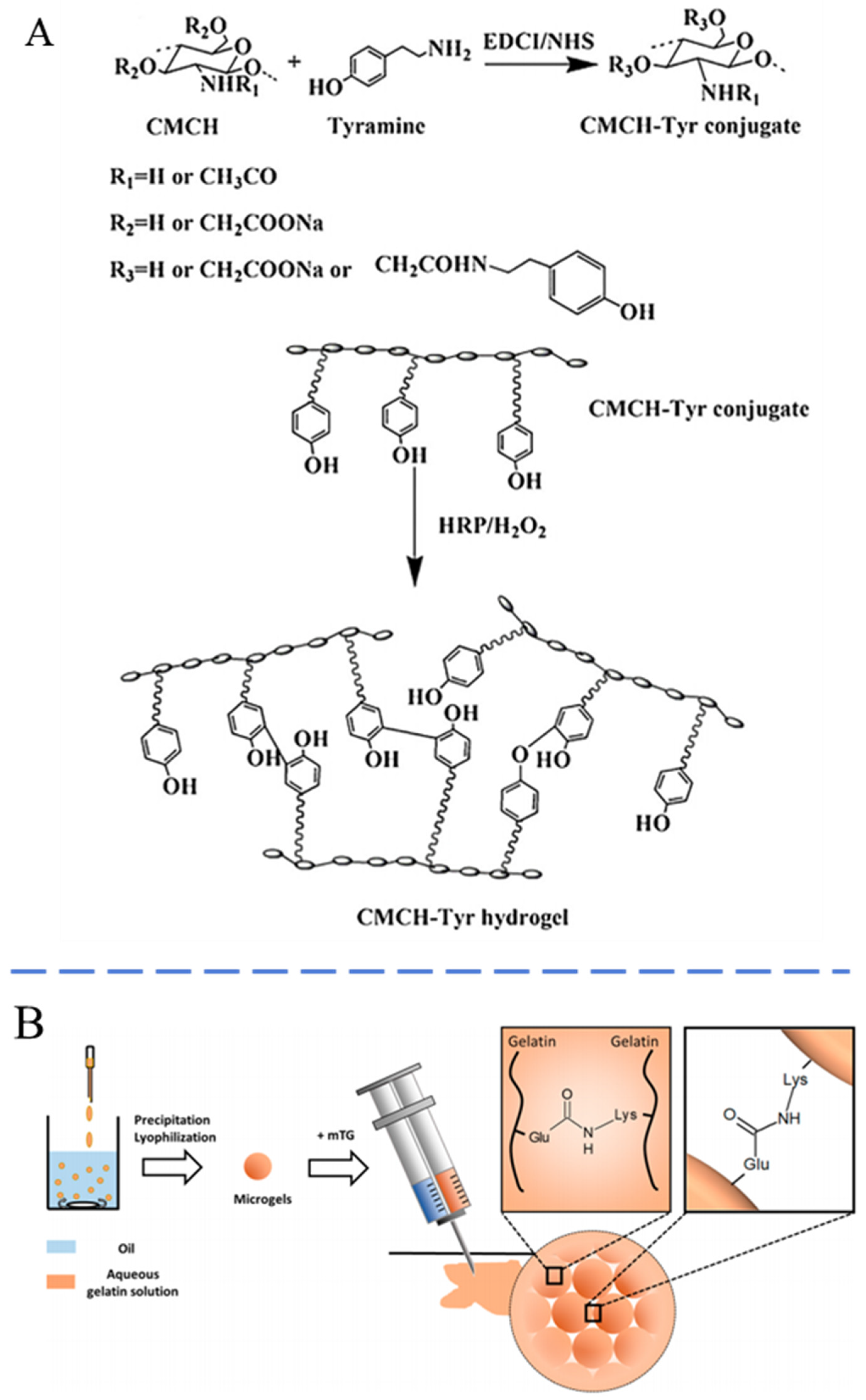
- Bi, B.; Liu, H.; Kang, W.; Zhuo, R.; Jiang, X. An injectable enzymatically crosslinked tyramine-modified carboxymethyl chitin hydrogel for biomedical applications. Colloids Surf. B Biointerfaces 2019, 175, 614–624. [Google Scholar] [CrossRef] [PubMed]
- Tang, Y.; Ding, J.; Zhou, X.; Ma, X.; Zhao, Y.; Mu, Q.; Huang, Z.; Tao, Q.; Liu, F.; Wang, L.; et al. Injectable hydrogels of enzyme-catalyzed crosslinked tyramine-modified gelatin for drug delivery. Aust. J. Chem. 2023, 76, 88–99. [Google Scholar] [CrossRef]
- Hou, S.; Lake, R.; Park, S.; Edwards, S.; Jones, C.; Jeong, K.J. Injectable Macroporous Hydrogel Formed by Enzymatic Crosslinking of Gelatin Microgels. ACS Appl. Bio Mater. 2018, 1, 1430–1439. [Google Scholar] [CrossRef]
- Elham, B.; Hosseini, M.; Mohajer, M.; Hassanzadeh, S.; Saghati, S.; Hilborn, J.; Khanmohammadi, M. Enzymatic Crosslinked Hydrogels for Biomedical Application. Polym. Sci. Ser. A 2022, 63, S1–S22. [Google Scholar] [CrossRef]
- Qu, X.H.; Yan, L.; Liu, S.; Tan, Y.F.; Xiao, J.; Cao, Y.; Chen, K.; Xiao, W.Q.; Li, B.; Liao, X.L. Preparation of silk fibroin/hyaluronic acid hydrogels with enhanced mechanical performance by a combination of physical and enzymatic crosslinking. J. Biomater. Sci.-Polym. Ed. 2021, 32, 1635–1653. [Google Scholar] [CrossRef] [PubMed]
- Dimatteo, R.; Darling, N.J.; Segura, T. In situ forming injectable hydrogels for drug delivery and wound repair. Adv. Drug Deliv. Rev. 2018, 127, 167–184. [Google Scholar] [CrossRef] [PubMed]
- Draghi, L. Injectable Hydrogels for 3D Bioprinting; Noh, I., Wang, X., Van Vlierberghe, S., Eds.; Royal Soc Chemistry: Cambridge, UK, 2021; Volume 8, pp. 77–111. [Google Scholar]
- Li, Y.; Rodrigues, J.; Tomás, H. Injectable and biodegradable hydrogels: Gelation, biodegradation and biomedical applications. Chem. Soc. Rev 2012, 41, 2193–2221. [Google Scholar] [CrossRef]
- Huynh, A.; Priefer, R. Hyaluronic acid applications in ophthalmology, rheumatology, and dermatology. Carbohydr. Res. 2020, 489, 107950. [Google Scholar] [CrossRef]
- Oh, H.; Lee, S.; Na, J.; Kim, J.H. Comparative Evaluation of Safety and Efficacy of a Novel Hyaluronic Acid-polynucleotide/Poly-L-lactic Acid Composite Dermal Filler. Aesthetic Plast. Surg. 2021, 45, 1792–1801. [Google Scholar] [CrossRef] [PubMed]
- Latifi, N.; Asgari, M.; Vali, H.; Mongeau, L. A tissue-mimetic nano-fibrillar hybrid injectable hydrogel for potential soft tissue engineering applications. Sci. Rep. 2018, 8. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Xue, W.J.; Liu, Y.N.; Fan, D.D.; Zhu, C.H.; Ma, X.X. Novel multifunctional PB and PBH hydrogels as soft filler for tissue engineering. J. Mater. Chem. B 2015, 3, 4742–4755. [Google Scholar] [CrossRef] [PubMed]
- Xie, C.; Yang, X.; Zheng, F.; Shi, J.; Huo, C.; Wang, Z.; Reis, R.L.; Kundu, S.C.; Xiao, B.; Duan, L. Facilely printed silk fibroin hydrogel microparticles as injectable long-lasting fillers. Biomater. Sci. 2024, 12, 375–386. [Google Scholar] [CrossRef]
- Onder, O.C.; Batool, S.R.; Nazeer, M.A. Self-assembled silk fibroin hydrogels: From preparation to biomedical applications. Mater. Adv. 2022, 3, 6920–6949. [Google Scholar] [CrossRef]
- Mushtaq, F.; Raza, Z.A.; Batool, S.R.; Zahid, M.; Onder, O.C.; Rafique, A.; Nazeer, M.A. Preparation, properties, and applications of gelatin-based hydrogels (GHs) in the environmental, technological, and biomedical sectors. Int. J. Biol. Macromol. 2022, 218, 601–633. [Google Scholar] [CrossRef] [PubMed]
- Jin, S.G. Production and Application of Biomaterials Based on Polyvinyl alcohol (PVA) as Wound Dressing. Chem. Asian J. 2022, 17, e202200595. [Google Scholar] [CrossRef] [PubMed]
- Jung, K.; Corrigan, N.; Wong, E.H.H.; Boyer, C. Bioactive Synthetic Polymers. Adv. Mater. 2021, 34, 28. [Google Scholar] [CrossRef] [PubMed]
- Asti, A.; Gioglio, L. Natural and Synthetic Biodegradable Polymers: Different Scaffolds for Cell Expansion and Tissue Formation. Int. J. Artif. Organs 2018, 37, 187–205. [Google Scholar] [CrossRef]
- Maadani, A.M.; Salahinejad, E. Performance comparison of PLA- and PLGA-coated porous bioceramic scaffolds: Mechanical, biodegradability, bioactivity, delivery and biosafety assessments. J. Control. Release 2022, 351, 1–7. [Google Scholar] [CrossRef]
- Pan, Y.; Xiao, Y.; Hao, Y.; Shi, K.; Pan, M.; Qian, Z. An injectable mPEG-PDLLA microsphere/PDLLA-PEG-PDLLA hydrogel composite for soft tissue augmentation. Chin. Chem. Lett. 2022, 33, 2486–2490. [Google Scholar] [CrossRef]
- Engler, A.J.; Sen, S.; Sweeney, H.L.; Discher, D.E. Matrix Elasticity Directs Stem Cell Lineage Specification. Cell 2006, 126, 677–689. [Google Scholar] [CrossRef] [PubMed]
- Joodaki, H.; Panzer, M.B. Skin mechanical properties and modeling: A review. Proc. Inst. Mech. Part H Eng. J. Eng. Med. 2018, 232, 323–343. [Google Scholar] [CrossRef] [PubMed]
- Hendriks, F.M.; Brokken, D.; Oomens, C.W.J.; Bader, D.L.; Baaijens, F.P.T. The relative contributions of different skin layers to the mechanical behavior of human skin in vivo using suction experiments. Med. Eng. Phys. 2006, 28, 259–266. [Google Scholar] [CrossRef]
- Gold, G.T.; Varma, D.M.; Taub, P.J.; Nicoll, S.B. Development of crosslinked methylcellulose hydrogels for soft tissue augmentation using an ammonium persulfate-ascorbic acid redox system. Carbohydr. Polym. 2015, 134, 497–507. [Google Scholar] [CrossRef]
- Lee, J.S.; Cho, J.H.; An, S.; Shin, J.; Choi, S.; Jeon, E.J.; Cho, S.-W. In Situ Self-Crosslinkable, Long-Term Stable Hyaluronic Acid Filler by Gallol Autoxidation for Tissue Augmentation and Wrinkle Correction. Chem. Mater. 2019, 31, 9614–9624. [Google Scholar] [CrossRef]
- Christensen, L.; Breiting, V.; Janssen, M.; Vuust, J.; Hogdall, E. Adverse Reactions to Injectable Soft Tissue Permanent Fillers. Aesthetic Plast. Surg. 2005, 29, 34–48. [Google Scholar] [CrossRef]
- Gold, G.T.; Varma, D.M.; Harbottle, D.; Gupta, M.S.; Stalling, S.S.; Taub, P.J.; Nicoll, S.B. Injectable redox-polymerized methylcellulose hydrogels as potential soft tissue filler materials. J. Biomed. Mater. Res. Part A 2014, 102, 4536–4544. [Google Scholar] [CrossRef]
- Hirsch, R.J.; Brody, H.J.; Carruthers, J.D. Hyaluronidase in the office: A necessity for every dermasurgeon that injects hyaluronic acid. J. Cosmet. Laser Ther. 2007, 9, 182–185. [Google Scholar] [CrossRef]
- Rzany, B.; Becker-Wegerich, P.; Bachmann, F.; Erdmann, R.; Wollina, U. Hyaluronidase in the correction of hyaluronic acid-based fillers: A review and a recommendation for use. J. Cosmet. Dermatol. 2009, 8, 317–323. [Google Scholar] [CrossRef] [PubMed]
- Hong, B.M.; Hong, G.L.; Gwak, M.A.; Kim, K.H.; Jeong, J.E.; Jung, J.Y.; Park, S.A.; Park, W.H. Self-crosslinkable hyaluronate-based hydrogels as a soft tissue filler. Int. J. Biol. Macromol. 2021, 185, 98–110. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.N.; Fan, D.D. Novel hyaluronic acid-tyrosine/collagen-based injectable hydrogels as soft filler for tissue engineering. Int. J. Biol. Macromol. 2019, 141, 700–712. [Google Scholar] [CrossRef] [PubMed]
- Tsou, Y.-H.; Khoneisser, J.; Huang, P.-C.; Xu, X. Hydrogel as a bioactive material to regulate stem cell fate. Bioact. Mater. 2016, 1, 39–55. [Google Scholar] [CrossRef] [PubMed]
- Hahn, H.M.; Lee, W.B.; Lee, I.J. The Effects of Subcutaneously Injected Novel Biphasic Crosslinked Hyaluronic Acid Filler: In Vivo Study. Aesthetic Plast. Surg. 2020, 45, 322–331. [Google Scholar] [CrossRef] [PubMed]
- Ding, Q.X.; Zha, R.Y.; He, X.Y.; Sun, A.; Shi, K.; Qu, Y.; Qian, Z.Y. A novel injectable fibroblasts-porous PDLLA microspheres/collagen gel composite for soft-tissue augmentation. Prog. Nat. Sci.-Mater. Int. 2020, 30, 651–660. [Google Scholar] [CrossRef]
- Kijanska, M.; Marmaras, A.; Hegglin, A.; Kurtcuoglu, V.; Giovanoli, P.; Lindenblatt, N. In vivo characterization of the integration and vascularization of a silk-derived surgical scaffold. J. Plast. Reconstr. Aesthetic Surg. 2016, 69, 1141–1150. [Google Scholar] [CrossRef]
- Ahearne, M. Introduction to cell–hydrogel mechanosensing. Interface Focus 2014, 4, 20130038. [Google Scholar] [CrossRef]
- Ma, J.R.; Huang, C.Y. Composition and Mechanism of Three-Dimensional Hydrogel System in Regulating Stem Cell Fate. Tissue Eng. Part B-Rev. 2020, 26, 498–518. [Google Scholar] [CrossRef] [PubMed]
- Saez, A.; Ghibaudo, M.; Buguin, A.; Silberzan, P.; Ladoux, B. Rigidity-driven growth and migration of epithelial cells on microstructured anisotropic substrates. Proc. Natl. Acad. Sci. USA 2007, 104, 8281–8286. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.; Koh, W.-G.; Lee, H.J. Effects of basic fibroblast growth factor combined with an injectable in situ crosslinked hyaluronic acid hydrogel for a dermal filler. React. Funct. Polym. 2021, 164, 104933. [Google Scholar] [CrossRef]
- Brown, J.E.; Gulka, C.P.; Giordano, J.E.M.; Montero, M.P.; Hoang, A.; Carroll, T.L. Injectable Silk Protein Microparticle-based Fillers: A Novel Material for Potential Use in Glottic Insufficiency. J. Voice 2019, 33, 773–780. [Google Scholar] [CrossRef] [PubMed]
- Chircov, C.; Grumezescu, A.M.; Bejenaru, L.E. Hyaluronic acid-based scaffolds for tissue engineering. Rom. J. Morphol. Embryol. 2018, 59, 71–76. [Google Scholar] [PubMed]
- Fakhari, A.; Berkland, C. Applications and emerging trends of hyaluronic acid in tissue engineering, as a dermal filler and in osteoarthritis treatment. Acta Biomater. 2013, 9, 7081–7092. [Google Scholar] [CrossRef] [PubMed]
- Sharma, P.; Sharma, S. Comparative study of a new dermal filler Uma Jeunesse® and Juvederm®. J. Cosmet. Dermatol. 2011, 10, 118–125. [Google Scholar] [CrossRef]
- Hong, B.M.; Kim, H.C.; Jeong, J.E.; Park, S.A.; Park, W.H. Visible-light-induced hyaluronate hydrogel for soft tissue fillers. Int. J. Biol. Macromol. 2020, 165, 2834–2844. [Google Scholar] [CrossRef]
- Vepari, C.; Kaplan, D.L. Silk as a biomaterial. Prog. Polym. Sci. 2007, 32, 991–1007. [Google Scholar] [CrossRef]
- Wang, Y.; Kim, H.-J.; Vunjak-Novakovic, G.; Kaplan, D.L. Stem cell-based tissue engineering with silk biomaterials. Biomaterials 2006, 27, 6064–6082. [Google Scholar] [CrossRef]
- Wang, H.Y.; Zhang, Y.Q. Processing silk hydrogel and its applications in biomedical materials. Biotechnol. Prog. 2015, 31, 630–640. [Google Scholar] [CrossRef] [PubMed]
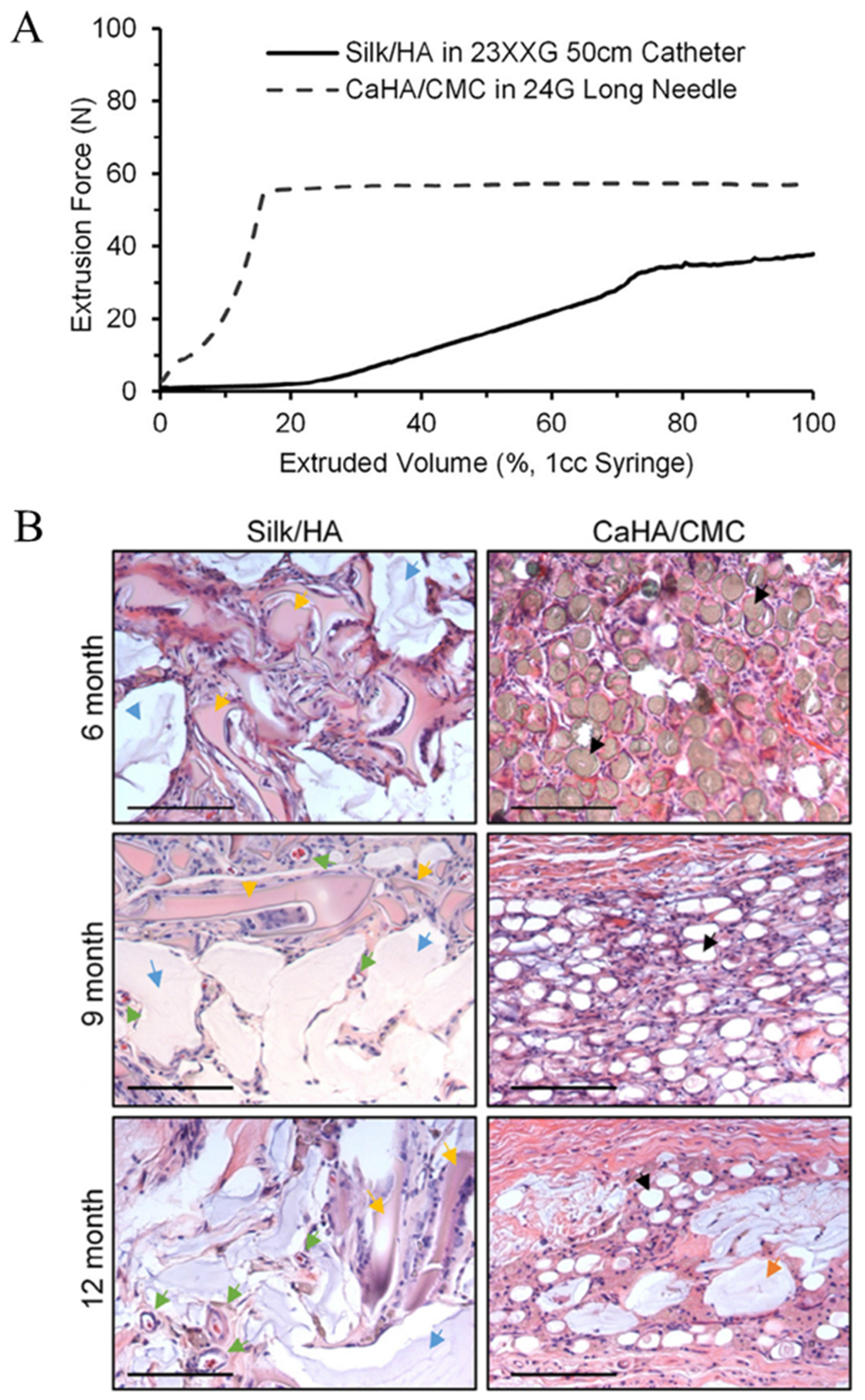
- Zeplin, P.H.; Sukhova, I.; Kranz, A.; Nürnberger, T.; Mihalceanu, S.; Beescho, C.; Schacht, K.; Vleugels, M.; Römer, L.; Machens, H.-G.; et al. Recombinant Silk Hydrogel as a Novel Dermal Filler Component: Preclinical Safety and Efficacy Studies of a New Class of Tissue Fillers. Aesthetic Surg. J. 2020, 40, NP511–NP518. [Google Scholar] [CrossRef]
- Salvatore, L.; Gallo, N.; Natali, M.L.; Terzi, A.; Sannino, A.; Madaghiele, M. Mimicking the Hierarchical Organization of Natural Collagen: Toward the Development of Ideal Scaffolding Material for Tissue Regeneration. Front. Bioeng. Biotechnol. 2021, 9, 644595. [Google Scholar] [CrossRef] [PubMed]
- Varnavides, C.K.; Forster, R.A.; Cunliffe, W.J. The role of bovine collagen in the treatment of acne scars. Br. J. Dermatol. 1987, 116, 199–206. [Google Scholar] [CrossRef] [PubMed]
- Smith, K.C. Repair of Acne Scars with Dermicol-P35. Aesthetic Surg. J. 2009, 29, S16–S18. [Google Scholar] [CrossRef] [PubMed]
- Solomon, P.; Sklar, M.; Zener, R. Facial soft tissue augmentation with Artecoll®: A review of eight years of clinical experience in 153 patients. Can. J. Plast. Surg. 2012, 20, 28–32. [Google Scholar] [CrossRef]
- Maloney, B.P.; Murphy, B.A.; Cole, H.P. Cymetra. Facial Plast. Surg. 2004, 20, 129–134. [Google Scholar] [CrossRef]
- Liu, B.W.; Ma, X.X.; Zhu, C.H.; Mi, Y.; Fan, D.D.; Li, X.; Chen, L. Study of a novel injectable hydrogel of human-like collagen and carboxymethylcellulose for soft tissue augmentation. e-Polymers 2013, 13, 035. [Google Scholar] [CrossRef]
- Ogushi, Y.; Sakai, S.; Kawakami, K. Synthesis of enzymatically-gellable carboxymethylcellulose for biomedical applications. J. Biosci. Bioeng. 2007, 104, 30–33. [Google Scholar] [CrossRef]
- Alijotas-Reig, J.; Garda-Gimenez, V. Delayed immune-mediated adverse effects related to hyaluronic acid and acrylic hydrogel dermal fillers: Clinical findings, long-term follow-up and review of the literature. J. Eur. Acad. Dermatol. Venereol. 2008, 22, 150–161. [Google Scholar] [CrossRef]
- Allemann, I.B.; Baumann, L. Hyaluronic acid gel (Juvederm™) preparations in the treatment of facial wrinkles and folds. Clin. Interv. Aging 2008, 3, 629–634. [Google Scholar]
- de Melo, F.; Marijnissen-Hofste, J. Investigation of physical properties of a polycaprolactone dermal filler when mixed with lidocaine and lidocaine/epinephrine. Dermatol. Ther. 2012, 2, 13. [Google Scholar] [CrossRef]
- Novel synthetic dermal fillers based on sodium carboxymethylcellulose: Comparison with crosslinked hyaluronic acid-based dermal fillers. Dermatol. Surg. 2007, 33, S136–S143.
- Varma, D.M.; Gold, G.T.; Taub, P.J.; Nicoll, S.B. Injectable carboxymethylcellulose hydrogels for soft tissue filler applications. Acta Biomater. 2014, 10, 4996–5004. [Google Scholar] [CrossRef] [PubMed]
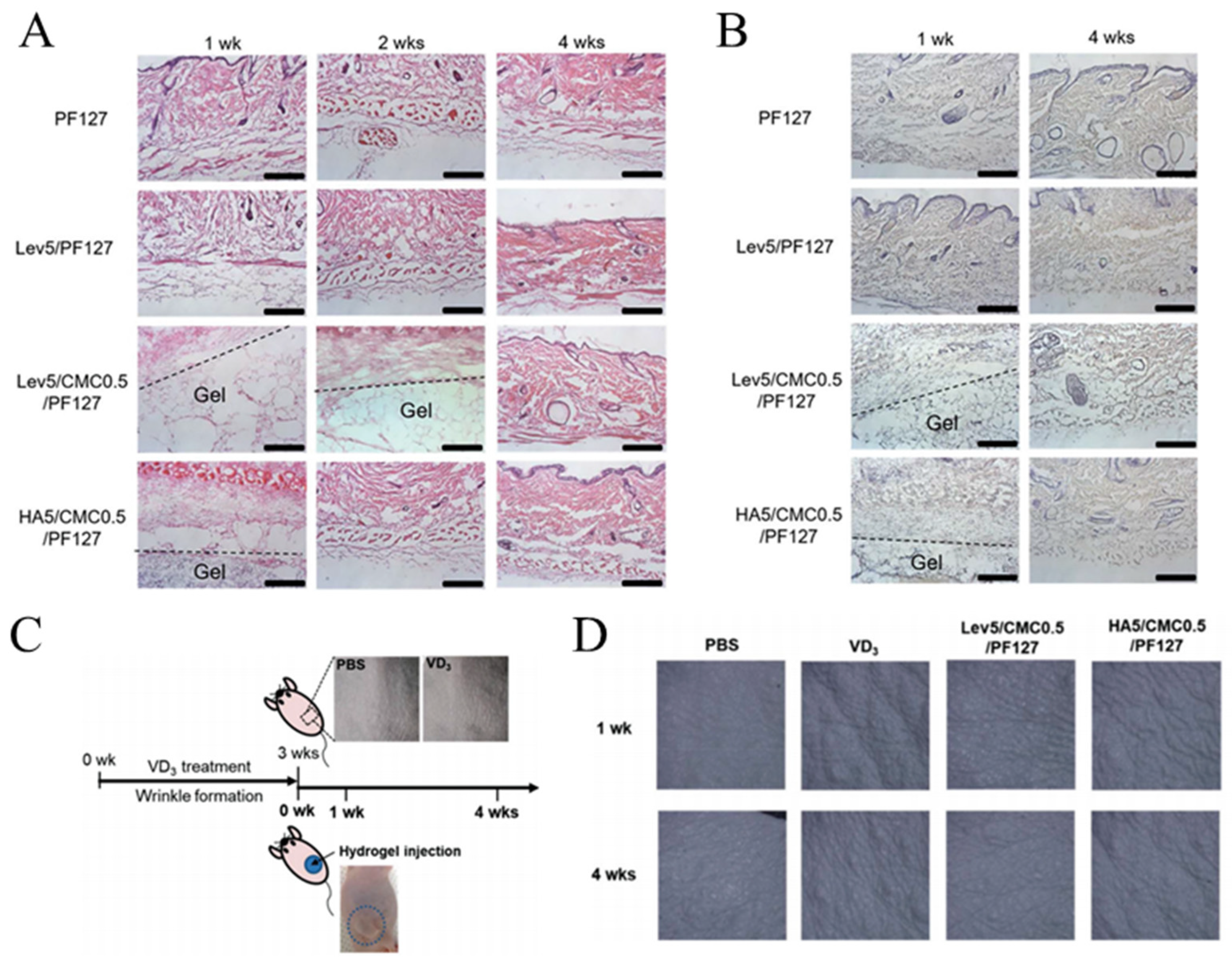
- Choi, W.I.; Hwang, Y.; Sahu, A.; Min, K.; Sung, D.; Tae, G.; Chang, J.H. An injectable and physical levan-based hydrogel as a dermal filler for soft tissue augmentation. Biomater. Sci. 2018, 6, 2627–2638. [Google Scholar] [CrossRef] [PubMed]
- Xiao, X.; Yu, L.; Dong, Z.; Mbelek, R.; Xu, K.; Lei, C.; Zhong, W.; Lu, F.; Xing, M. Adipose stem cell-laden injectable thermosensitive hydrogel reconstructing depressed defects in rats: Filler and scaffold. J. Mater. Chem. B 2015, 3, 5635–5644. [Google Scholar] [CrossRef] [PubMed]
- Jacovella, P.F. Calcium Hydroxylapatite Facial Filler (Radiesse™): Indications, Technique, and Results. Clin. Plast. Surg. 2006, 33, 511–523. [Google Scholar] [CrossRef]
- Johl, S.S.; Burgett, R.A. Dermal filler agents: A practical review. Curr. Opin. Ophthalmol. 2006, 17, 471–479. [Google Scholar] [CrossRef] [PubMed]
- Kadouch, J.A. Calcium hydroxylapatite: A review on safety and complications. J. Cosmet. Dermatol. 2017, 16, 152–161. [Google Scholar] [CrossRef] [PubMed]
- Hwang, Y.; Lee, J.S.; An, H.; Oh, H.; Sung, D.; Tae, G.; Choi, W.I. Hydroxyapatite-embedded levan composite hydrogel as an injectable dermal filler for considerable enhancement of biological efficacy. J. Ind. Eng. Chem. 2021, 104, 491–499. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xie, C.; Liu, G.; Wang, L.; Yang, Q.; Liao, F.; Yang, X.; Xiao, B.; Duan, L. Synthesis and Properties of Injectable Hydrogel for Tissue Filling. Pharmaceutics 2024, 16, 430. https://doi.org/10.3390/pharmaceutics16030430
Xie C, Liu G, Wang L, Yang Q, Liao F, Yang X, Xiao B, Duan L. Synthesis and Properties of Injectable Hydrogel for Tissue Filling. Pharmaceutics. 2024; 16(3):430. https://doi.org/10.3390/pharmaceutics16030430
Chicago/Turabian StyleXie, Chunyu, Ga Liu, Lingshuang Wang, Qiang Yang, Fuying Liao, Xiao Yang, Bo Xiao, and Lian Duan. 2024. "Synthesis and Properties of Injectable Hydrogel for Tissue Filling" Pharmaceutics 16, no. 3: 430. https://doi.org/10.3390/pharmaceutics16030430




