Drug Release from Lipid Microparticles—Insights into Drug Incorporation and the Influence of Physiological Factors
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of SLM Dispersions
2.3. Characteristics of SLM Dispersions
2.4. Solubility of Ind in Acceptor Media
2.5. Drug Release Study
2.6. Assessment of SLM after Incubation with Natural Tears
2.7. Analysis of Ind Concentration by HPLC
2.8. Statistical Analysis
3. Results
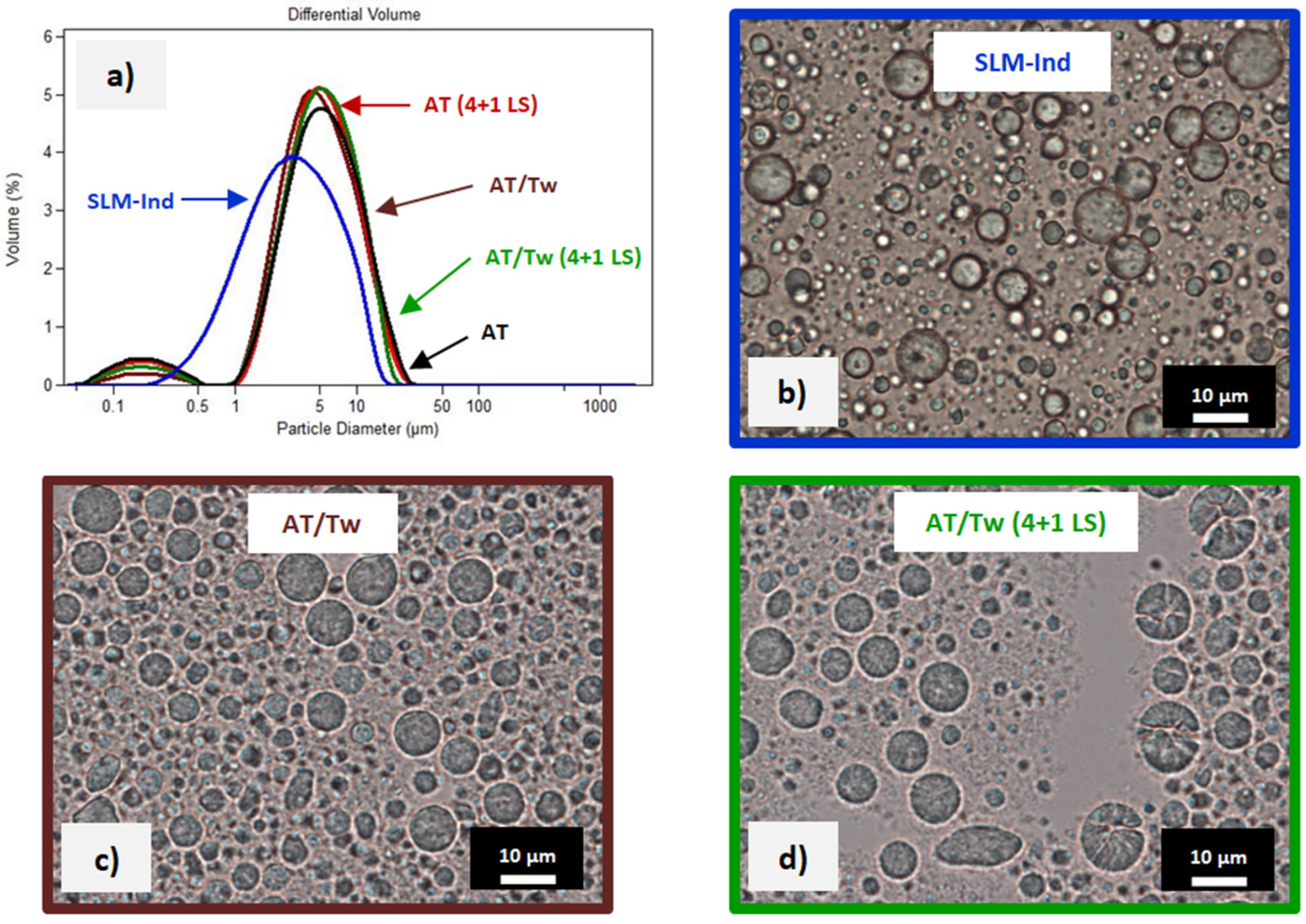
3.1. Characterization of SLM Dispersions
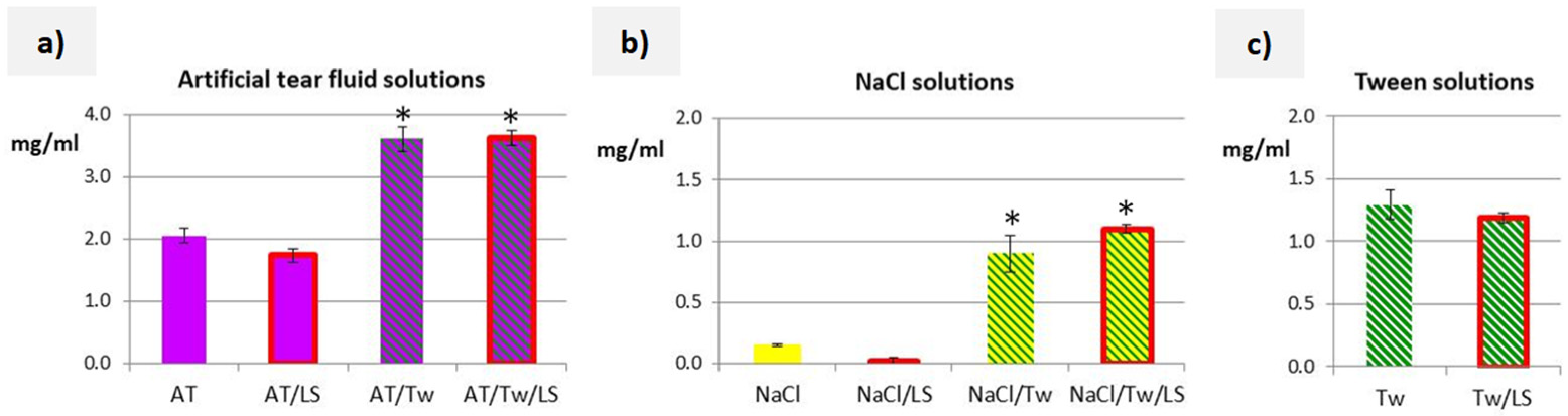
3.2. Solubility Studies and Properties of Acceptor Media
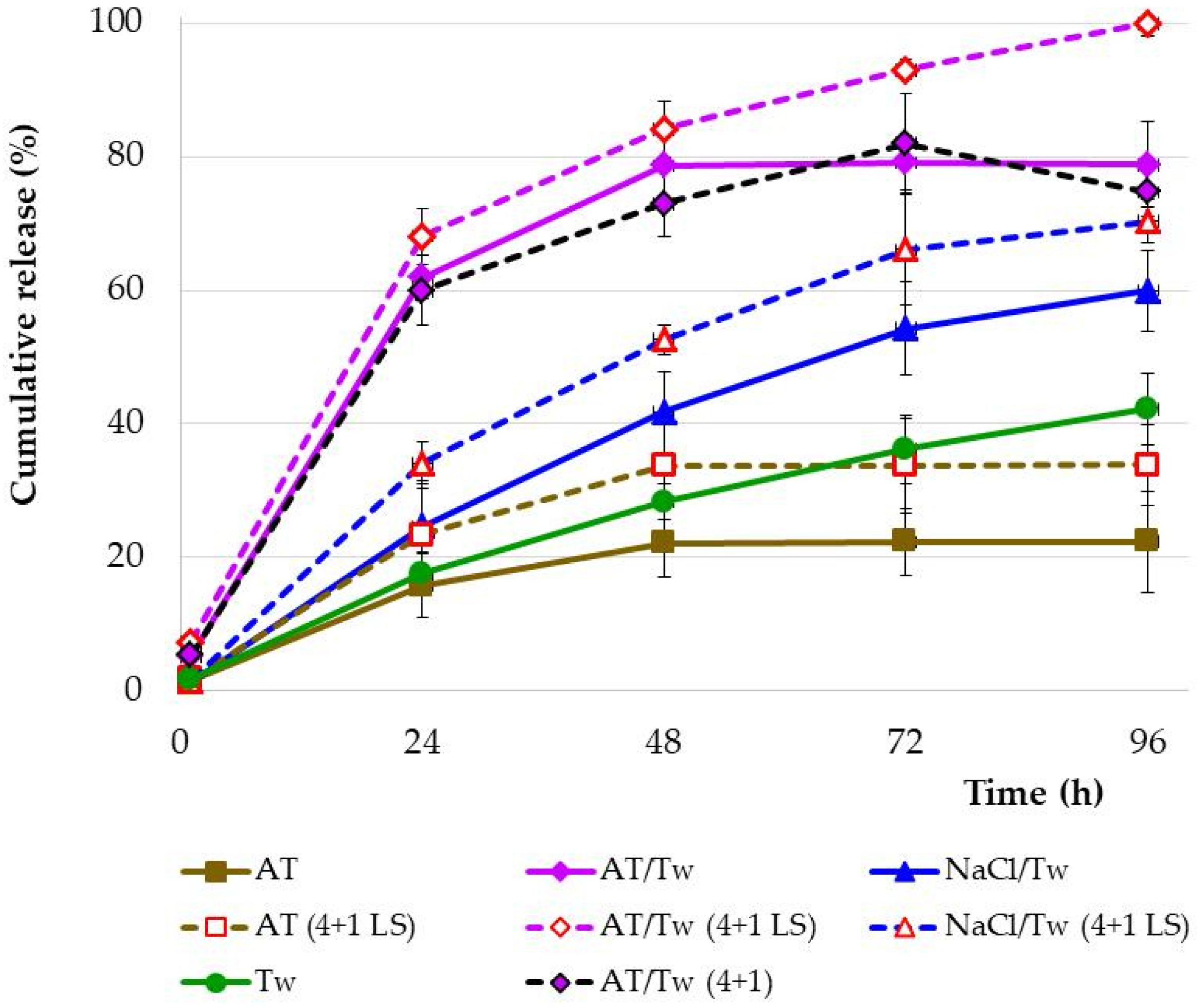
3.3. Drug Release Study
3.4. Assessment of SLM from the Dialysis Bag after the Release Study
3.5. Assessment of SLM after Incubation with Natural Tears
4. Discussion
4.1. Solubility Studies
4.2. Drug Release Studies
4.3. Assessment of SLM from the Dialysis Bag after the Release Study
4.4. Assessment of SLM after Incubation with Natural Tears
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Gugleva, V.; Andonova, V. Recent Progress of Solid Lipid Nanoparticles and Nanostructured Lipid Carriers as Ocular Drug Delivery Platforms. Pharmaceuticals 2023, 16, 474. [Google Scholar] [CrossRef]
- Wolska, E.; Sznitowska, M.; Chorążewicz, J.; Szerkus, O.; Radwańska, A.; Markuszewski, M.J.; Kaliszan, R.; Raczyńska, K. Ocular irritation and cyclosporine A distribution in the eye tissues after administration of Solid Lipid Microparticles in the rabbit model. Eur. J. Pharm. Sci. 2018, 121, 95–105. [Google Scholar] [CrossRef]
- Bertoni, S.; Tedesco, D.; Bartolini, M.; Prata, C.; Passerini, N.; Albertini, B. Solid Lipid Microparticles for Oral Delivery of Catalase: Focus on the Protein Structural Integrity and Gastric Protection. Mol. Pharm. 2020, 17, 3609–3621. [Google Scholar] [CrossRef]
- Gugu, T.; Salome, A.; Chime, S.A.; Attama, A. Solid lipid microparticles: An approach for improving oral bioavailability of aspirin. Asian J. Pharm. Sci. 2015, 10, 425–432. [Google Scholar] [CrossRef]
- Rahimpour, Y.; Javadzadeh, Y.; Hamishehkar, H. Solid lipid microparticles for enhanced dermal delivery of tetracycline HCl. Coll. Surf. Biointerfaces 2016, 145, 14–20. [Google Scholar] [CrossRef]
- Lauterbach, A.; Mueller-Goymann, C.C. Development, formulation, and characterization of an adapalene-loaded solid lipid microparticle dispersion for follicular penetration. Int. J. Pharm. 2014, 466, 122–132. [Google Scholar] [CrossRef]
- Melike, Ü.; Karaman, E.F. Preliminary studies on solid lipid microparticles of loratadine for the treatment of allergic reactions via the nasal route. Trop. J. Pharm. Res. 2013, 12, 287–293. [Google Scholar]
- Dalpiaz, A.; Ferraro, L.; Perrone, D.; Leo, E.; Iannuccelli, V.; Pavan, B.; Paganetto, G.; Beggiato, S.; Scalia, S. Brain uptake of a Zidovudine prodrug after nasal administration of solid lipid microparticles. Mol. Pharm. 2014, 11, 1550–1561. [Google Scholar] [CrossRef]
- Wu, C.; Luo, X.; Baldursdottir, S.G.; Yang, M.; Sun, X.; Mu, H. In vivo evaluation of solid lipid microparticles and hybrid polymer-lipid microparticles for sustained delivery of leuprolide. Eur. J. Pharm. Biopharm. 2019, 142, 315–321. [Google Scholar] [CrossRef]
- Scalia, S.; Haghi, M.; Losi, V.; Trotta, V.; Young, P.M.; Traini, D. Quercetin solid lipid microparticles: A flavonoid for inhalation lung delivery. Eur. J. Pharm. Sci. 2013, 49, 278–285. [Google Scholar] [CrossRef]
- Jaspart, S.; Piel, G.; Delattre, L.; Evrard, B. Solid lipid microparticles: Formulation, preparation, characterization, drug release and applications. Expert Opin. Drug Deliv. 2005, 2, 75–87. [Google Scholar] [CrossRef]
- Scalia, S.; Young, P.M.; Traini, D. Solid lipid microparticles as an approach to drug delivery. Expert Opin. Drug Deliv. 2015, 12, 583–599. [Google Scholar] [CrossRef]
- Müller, R.H.; Radtke, M.; Wissing, S.A. Solid Lipid Nanoparticles (SLN) and Nanostructured Lipid Carriers (NLC) in Cosmetic and Dermatological Preparations. Adv. Drug Deliv. Rev. 2002, 54, S131–S155. [Google Scholar] [CrossRef]
- Wolska, E.; Brach, M. Distribution of drug substances in Solid Lipid Microparticles (SLM)—Methods of analysis and interpretation. Pharmaceutics 2022, 14, 335. [Google Scholar] [CrossRef]
- Long, C.; Zhang, L.; Qian, Y. Mesoscale simulation of drug molecules distribution in the matrix of solid lipid microparticles (SLM). Chem. Eng. J. 2006, 119, 99–106. [Google Scholar] [CrossRef]
- Melike, Ü.; Yener, G. Importance of Solid Lipid Nanoparticles (SLN) in Various Administration Routes and Future Perspective. Int. J. Nanomed. 2007, 2, 289–300. [Google Scholar]
- Zhong, Q.; Zhang, L. Nanoparticles Fabricated from Bulk Solid Lipids: Preparation, Properties, and Potential Food Applications. Adv. Coll. Interface Sci. 2019, 273, 102033. [Google Scholar] [CrossRef]
- Zoubari, G.; Staufenbiel, S.; Volz, P.; Alexiev, U.; Bodmeier, R. Effect of drug solubility and lipid carrier on drug release from lipid nanoparticles for dermal delivery. Eur. J. Pharm. Biopharm. 2017, 110, 39–46. [Google Scholar] [CrossRef]
- Dong, Y.; Hengst, L.; Hunt, R.; Feng, X.; Kozak, D.; Choi, S.; Ashraf, M.; Xu, X. Evaluating Drug Distribution and Release in Ophthalmic Emulsions: Impact of Release Conditions. J. Control. Release 2020, 327, 360–370. [Google Scholar] [CrossRef]
- Wolska, E.; Szymańska, M. Comparison of the In Vitro Drug Release Methods for the Selection of Test Conditions to Characterize Solid Lipid Microparticles. Pharmaceutics 2023, 15, 511. [Google Scholar] [CrossRef]
- Ammar, H.O.; Salama, A.; Ghorab, M.; Mahmoud, A.A. Nanoemulsion as a Potential Ophthalmic Delivery System for Dorzolamide Hydrochloride. AAPS PharmSciTech 2009, 10, 808–819. [Google Scholar] [CrossRef] [PubMed]
- Satyanarayana, S.D.; Abu Lila, A.S.; Moin, A.; Moglad, E.H.; Khafagy, E.-S.; Alotaibi, H.F.; Obaidullah, A.J.; Charyulu, R.N. Ocular Delivery of Bimatoprost-Loaded Solid Lipid Nanoparticles for Effective Management of Glaucoma. Pharmaceuticals 2023, 16, 1001. [Google Scholar] [CrossRef] [PubMed]
- Eid, H.M.; Elkomy, M.; El Menshawe, S.F.; Salem, H. Development, Optimization, and In Vitro/In Vivo Characterization of Enhanced Lipid Nanoparticles for Ocular Delivery of Ofloxacin: The Influence of Pegylation and Chitosan Coating. AAPS PharmSciTech 2019, 20, 183. [Google Scholar] [CrossRef] [PubMed]
- Khames, A.; Khaleel, M.; El-Badawy, M.F.; El-Nezhawy, A.O.H. Natamycin Solid Lipid Nanoparticles—Sustained Ocular Delivery System of Higher Corneal Penetration against Deep Fungal Keratitis: Preparation and Optimization. Int. J. Nanomed. 2019, 14, 2515–2531. [Google Scholar] [CrossRef] [PubMed]
- Khare, A.; Singh, I.; Pawar, P.; Grover, K. Design and Evaluation of Voriconazole Loaded Solid Lipid Nanoparticles for Ophthalmic Application. J. Drug Deliv. 2016, 2016, 6590361. [Google Scholar] [CrossRef] [PubMed]
- Abbas, N.; Sarwar, K.; Irfan, M.; Hussain, A.; Mehmood, R.; Arshad, M.S.; Shah, P.A. Formulation and Evaluation of Indomethacin Loaded Nanosponges for Oral Delivery. Acta Poloniae Pharm. Drug Res. 2018, 75, 1201–1213. [Google Scholar] [CrossRef] [PubMed]
- Comer, J.; Judge, S.; Matthews, D.; Towers, L.; Falcone, B.; Goodman, J.; Dearden, J. The Intrinsic Aqueous Solubility of Indomethacin. ADMET DMPK 2014, 2, 18–32. [Google Scholar] [CrossRef]
- Wolska, E. Fine powder of lipid microparticles—Spray drying process development and optimization. J. Drug Del. Sci. Tech. 2021, 64, 102640. [Google Scholar] [CrossRef]
- Tseng, R.C.; Chen, C.C.; Hsu, S.M.; Chuang, H.S. Contact-Lens Biosensors. Sensors 2018, 18, 2651. [Google Scholar] [CrossRef]
- Villatoro, A.J.; Fernández, V.; Claros, S.; Alcoholado, C.; Cifuentes, M.; Merayo-Lloves, J.; Andrades, J.A.; Becerra, J. Regenerative therapies in dry eye disease: From growth factors to cell therapy. Int. J. Mol. Sci. 2017, 18, 2264. [Google Scholar] [CrossRef]
- Alexeev, V.; Das, S.; Finegold, D.; Asher, S. Photonic crystal glucose-sensing material for noninvasive monitoring of glucose in tear fluid. Clin. Chem. 2005, 50, 2353–2360. [Google Scholar] [CrossRef] [PubMed]
- Vo, A.; Feng, X.; Patel, D.; Mohammad, A.; Patel, M.; Zheng, J.; Kozak, D.; Choi, S.; Ashraf, M.; Xu, X. In vitro physicochemical characterization and dissolution of brinzolamide ophthalmic suspensions with similar composition. Int. J. Pharm. 2020, 588, 119761. [Google Scholar] [CrossRef] [PubMed]
- Wolska, E.; Regdon, G., Jr. Thermal Analysis in the Evaluation of Solid Lipid Microparticles in the Form of Aqueous Dispersion and Fine Powder. Appl. Sci. 2023, 13, 13282. [Google Scholar] [CrossRef]
- The United States Pharmacopoeia Website. Available online: https://www.usp.org/small-molecules/dissolution (accessed on 16 October 2023).
- El-Sabbagh, H.; Ghanem, A.H.; Abdel-Alim, H.M. Solubilization of indometacin. Die Pharm. 1978, 33, 529–531. [Google Scholar]
- Christophersen, P.C.; Zhang, L.; Yang, M.; Nielsen, H.; Müllertz, A.; Mu, H. Solid Lipid Particles for Oral Delivery of Peptide and Protein Drugs I—Elucidating the Release Mechanism of Lysozyme during Lipolysis. Eur. J. Pharm. Biopharm. 2013, 85 Pt A, 473–480. [Google Scholar] [CrossRef]
- Liu, J.; Christophersen, P.C.; Yang, M.; Nielsen, H.M.; Mu, H. The Impact of Particle Preparation Methods and Polymorphic Stability of Lipid Excipients on Protein Distribution in Microparticles. Drug Dev. Ind. Pharm. 2017, 43, 2032–2042. [Google Scholar] [CrossRef] [PubMed]
- Siepmann, J.; Siepmann, F. Sink conditions do not guarantee the absence of saturation effects. Int. J. Pharm. 2020, 577, 119009. [Google Scholar] [CrossRef] [PubMed]
- Tres, F.; Treacher, K.; Booth, J.; Hughes, L.P.; Wren, S.A.C.; Aylott, J.W.; Burley, J.C. Indomethacin-Kollidon VA64 Extrudates: A Mechanistic Study of pH-Dependent Controlled Release. Mol. Pharm. 2016, 13, 1166–1175. [Google Scholar] [CrossRef] [PubMed]
- Committee for Medicinal Products for Human use (CHMP); Committee for Medicinal Products for Veterinary use (CVMP); Quality Working Party. Reflection Paper on the Dissolution Specification for Generic Solid Oral Immediate Release Products with Systemic; European Medicines Agency: Amsterdam, The Netherlands, 2017; p. 44. [Google Scholar]
- Gao, Y.; Glennon, B.; He, Y.; Donnellan, P. Dissolution Kinetics of a BCS Class II Active Pharmaceutical Ingredient: Diffusion-Based Model Validation and Prediction. ACS Omega 2021, 6, 8056–8067. [Google Scholar] [CrossRef]
- Torrent-Burgués, J. Lysozyme Influence on Monolayers of Individual and Mixed Lipids. Coll. Interfaces 2022, 6, 15. [Google Scholar] [CrossRef]
- Sigma-Aldrich. Product Information, Lysozyme from Chicken Egg White, Catalog Number L7651. Available online: https://www.sigmaaldrich.com/deepweb/assets/sigmaaldrich/product/documents/362/900/l7651dat.pdf (accessed on 16 October 2023).


| Formulation | Particle Size (µm) | Ind Distribution (%) | ||||
|---|---|---|---|---|---|---|
| d0.1 | d0.5 | d0.9 | Aqueous Phase | Interphase | Lipid Matrix | |
| SLM placebo | 0.77 ± 0.16 | 2.13 ± 0.08 | 5.95 ± 0.21 | – | – | – |
| SLM-Ind | 0.87 ± 0.25 | 2.87 ± 0.14 | 8.39 ± 0.43 | 1.2 ± 18 | 78.2 ± 5.2 | 20.6 ± 4.8 |
| Acceptor Media | Tw | LS | NaCl | AT | Water | pH | Osmotic Pressure [mOsm/kg] |
|---|---|---|---|---|---|---|---|
| AT | – | – | – | 100.0 | – | 7.41 ± 0.07 | 307 ± 6 |
| AT/LS | – | 0.14 | – | to 100.0 | – | 7.86 ± 0.11 | 318 ± 4 |
| AT/Tw | 5.0 | – | – | to 100.0 | – | 7.52 ± 0.09 | 338 ± 5 |
| AT/Tw/LS | 5.0 | 0.14 | – | to 100.0 | – | 7.99 ± 0.07 | 343 ± 7 |
| NaCl | – | – | 0.9 | – | to 100.0 | 6.14 ± 0.01 | 311 ± 2 |
| NaCl/LS | – | 0.14 | 0.9 | – | to 100.0 | 4.53 ± 0.12 | 319 ± 4 |
| NaCl/Tw | 5.0 | – | 0.9 | – | to 100.0 | 5.74 ± 0.05 | 329 ± 3 |
| NaCl/Tw/LS | 5.0 | 0.14 | 0.9 | – | to 100.0 | 4.85 ± 0.18 | 340 ± 8 |
| Tw | 5.0 | – | – | – | to 100.0 | 6.15 ± 0.11 | 15 ± 7 |
| Tw/LS | 5.0 | 0.14 | – | – | to 100.0 | 4.59 ± 0.16 | 24 ± 4 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wolska, E.; Sadowska, K. Drug Release from Lipid Microparticles—Insights into Drug Incorporation and the Influence of Physiological Factors. Pharmaceutics 2024, 16, 545. https://doi.org/10.3390/pharmaceutics16040545
Wolska E, Sadowska K. Drug Release from Lipid Microparticles—Insights into Drug Incorporation and the Influence of Physiological Factors. Pharmaceutics. 2024; 16(4):545. https://doi.org/10.3390/pharmaceutics16040545
Chicago/Turabian StyleWolska, Eliza, and Karolina Sadowska. 2024. "Drug Release from Lipid Microparticles—Insights into Drug Incorporation and the Influence of Physiological Factors" Pharmaceutics 16, no. 4: 545. https://doi.org/10.3390/pharmaceutics16040545








