Characterization of Oil-in-Water Emulsions Prepared with Triblock Copolymer Poloxamer 407 and Low-Molecular-Mass Surfactant Mixtures as Carriers of Grape Pomace Waste Polyphenols
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Apparatus and Instruments
2.3. Grape Pomace Sample
2.4. Extraction Procedure
2.5. Preparation of Emulsions
2.6. Particle Size and Particle Size Distribution
- S—specific surface area,
- d32—surface-weighted mean (Sauter mean diameter),
- d43—volume-weighted mean,
- particle diameters—d (0.1), d (0.5) and d (0.9), which represent the mean mass diameters of the volume distribution and indicate that 10% of the sample is less than d (0.1), then 50% of the sample is less than d (0.5) and 90% of the sample is less than d (0.9),
- Span—the width of a droplet size distribution, calculated using the following Equation (1):
2.7. Microscopic Analysis
2.8. Rheological Properties
2.9. Creaming Stability
2.10. Determination of the pH Value of the Prepared Emulsions
2.11. Statistical Analysis
3. Results and Discussion
3.1. Dispersion Characteristics
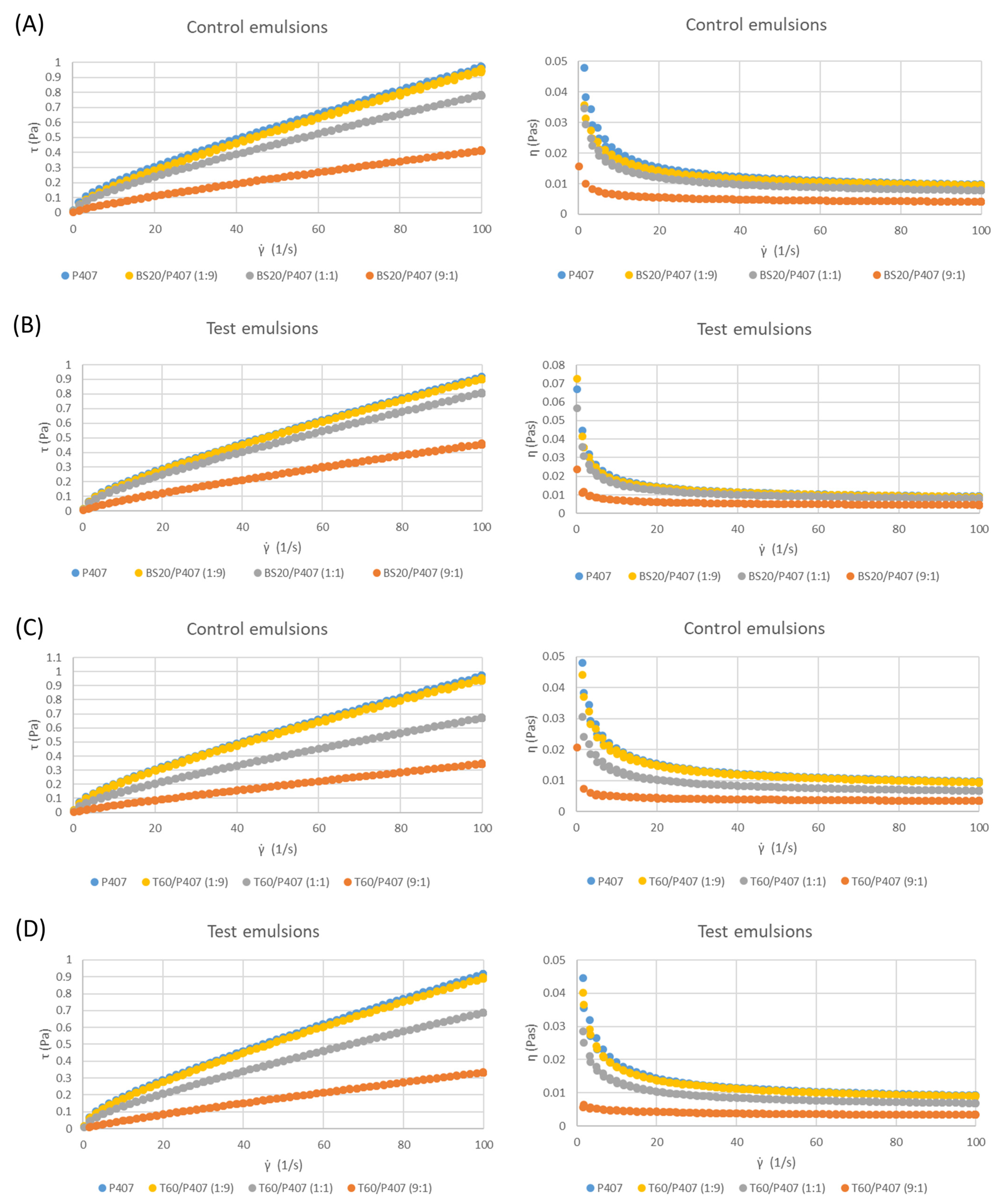
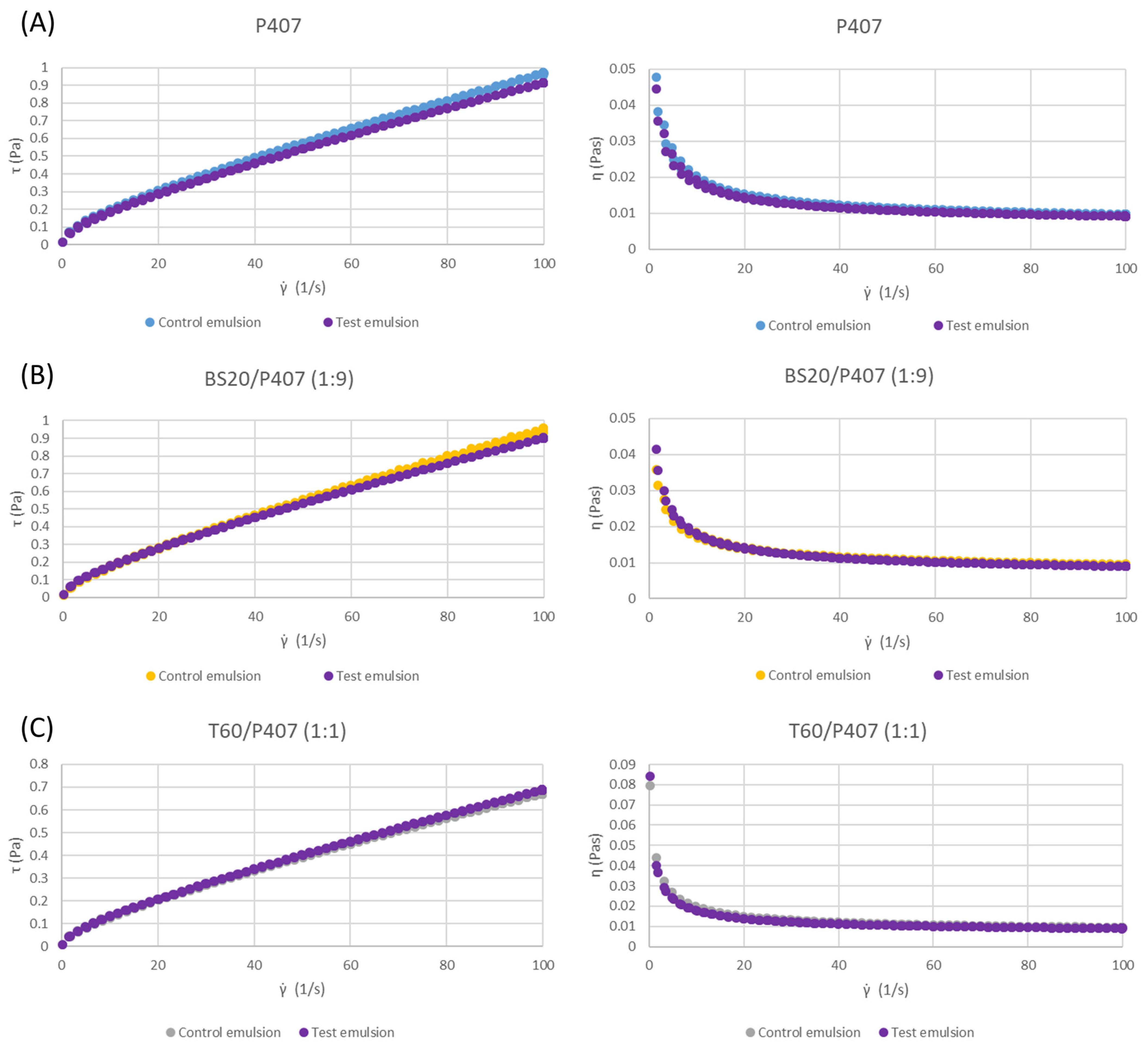
3.2. Rheological Characteristics
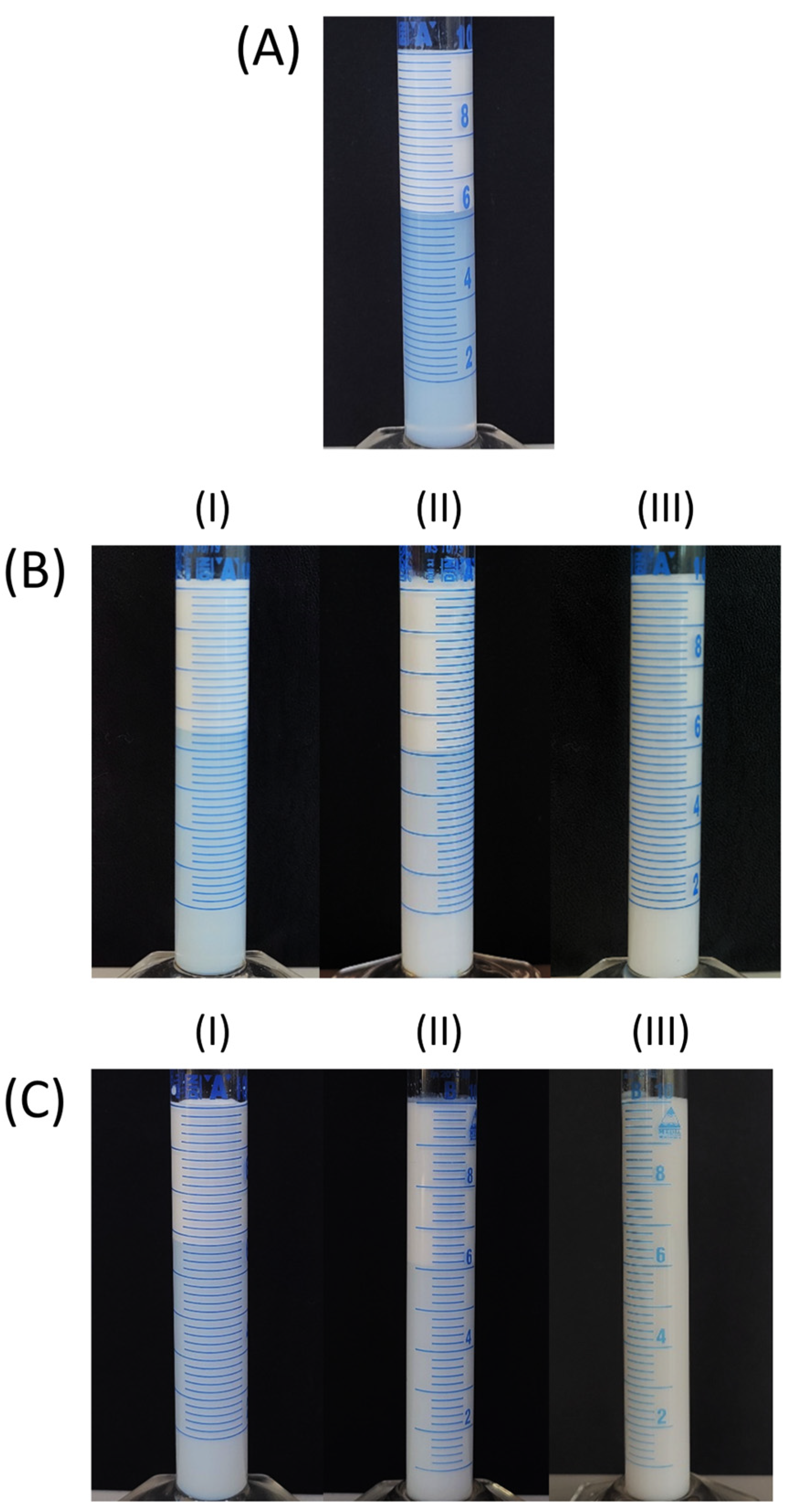
3.3. Emulsion Creaming Stability
3.4. Determination of the pH Value of the Emulsions
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- McClements, D.J.; Rao, J. Food-grade nanoemulsions: Formulation, fabrication, properties, performance, biological fate, and potential toxicity. Crit. Rev. Food Sci. Nutr. 2011, 51, 285–330. [Google Scholar] [CrossRef] [PubMed]
- Jafari, S.M.; Assadpoor, E.; He, Y.; Bhandari, B. Re-coalescence of emulsion droplets during high-energy emulsification. Food Hydrocoll. 2008, 22, 1191–1202. [Google Scholar] [CrossRef]
- Tadros, T.F. Emulsion science and technology: A general introduction. Emuls. Sci. Technol. 2009, 1, 1–55. [Google Scholar]
- Mcclements, D.J. Critical review of techniques and methodologies for characterization of emulsion stability. Crit. Rev. Food Sci. Nutr. 2007, 47, 611–649. [Google Scholar] [CrossRef]
- Wilde, P. Interfaces: Their role in foam and emulsion behaviour. Curr. Opin. Colloid Interface Sci. 2000, 5, 176–181. [Google Scholar] [CrossRef]
- Niraula, B.; King, T.C.; Chun, T.K.; Misran, M. Rheology properties of glucopyranoside stabilized oil–water emulsions: Effect of alkyl chain length and bulk concentration of the surfactant. Colloids Surf. A Physicochem. Eng. Asp. 2004, 251, 117–132. [Google Scholar] [CrossRef]
- Marti-Mestres, G.; Nielloud, F. Emulsions in health care applications—An overview. J. Dispers. Sci. Technol. 2002, 23, 419–439. [Google Scholar] [CrossRef]
- Khan, B.A.; Akhtar, N.; Khan, H.; Braga, V.d.A. Development, characterization and antioxidant activity of polysorbate based O/W emulsion containing polyphenols derived from Hippophae rhamnoides and Cassia fistula. Braz. J. Pharm. Sci. 2013, 49, 763–773. [Google Scholar] [CrossRef]
- Mancuso, A.; Cristiano, M.C.; Pandolfo, R.; Greco, M.; Fresta, M.; Paolino, D. Improvement of ferulic acid antioxidant activity by multiple emulsions: In vitro and in vivo evaluation. Nanomaterials 2021, 11, 425. [Google Scholar] [CrossRef]
- Aditya, N.; Aditya, S.; Yang, H.; Kim, H.W.; Park, S.O.; Ko, S. Co-delivery of hydrophobic curcumin and hydrophilic catechin by a water-in-oil-in-water double emulsion. Food Chem. 2015, 173, 7–13. [Google Scholar] [CrossRef]
- Lu, G.W.; Gao, P. Emulsions and microemulsions for topical and transdermal drug delivery. In Handbook of Non-Invasive Drug Delivery Systems; Elsevier: Amsterdam, The Netherlands, 2010; pp. 59–94. [Google Scholar]
- Glampedaki, P.; Dutschk, V. Stability studies of cosmetic emulsions prepared from natural products such as wine, grape seed oil and mastic resin. Colloids Surf. A Physicochem. Eng. Asp. 2014, 460, 306–311. [Google Scholar] [CrossRef]
- Yarovaya, L.; Waranuch, N.; Wisuitiprot, W.; Khunkitti, W. Effect of grape seed extract on skin fibroblasts exposed to UVA light and its photostability in sunscreen formulation. J. Cosmet. Dermatol. 2021, 20, 1271–1282. [Google Scholar] [CrossRef] [PubMed]
- Bae, J.; Kim, N.; Shin, Y.; Kim, S.-Y.; Kim, Y.-J. Activity of catechins and their applications. Biomed. Dermatol. 2020, 4, 8. [Google Scholar] [CrossRef]
- Yang, S.; Liu, L.; Han, J.; Tang, Y. Encapsulating plant ingredients for dermocosmetic application: An updated review of delivery systems and characterization techniques. Int. J. Cosmet. Sci. 2020, 42, 16–28. [Google Scholar] [CrossRef] [PubMed]
- Lu, W.; Kelly, A.L.; Miao, S. Emulsion-based encapsulation and delivery systems for polyphenols. Trends Food Sci. Technol. 2016, 47, 1–9. [Google Scholar] [CrossRef]
- Wu, T.-H.; Yen, F.-L.; Lin, L.-T.; Tsai, T.-R.; Lin, C.-C.; Cham, T.-M. Preparation, physicochemical characterization, and antioxidant effects of quercetin nanoparticles. Int. J. Pharm. 2008, 346, 160–168. [Google Scholar] [CrossRef] [PubMed]
- Zillich, O.; Schweiggert-Weisz, U.; Eisner, P.; Kerscher, M. Polyphenols as active ingredients for cosmetic products. Int. J. Cosmet. Sci. 2015, 37, 455–464. [Google Scholar] [CrossRef]
- Ramful, D.; Aumjaud, B.; Neergheen, V.; Soobrattee, M.; Googoolye, K.; Aruoma, O.; Bahorun, T. Polyphenolic content and antioxidant activity of Eugenia pollicina leaf extract in vitro and in model emulsion systems. Food Res. Int. 2011, 44, 1190–1196. [Google Scholar] [CrossRef]
- Velderrain-Rodríguez, G.R.; Acevedo-Fani, A.; González-Aguilar, G.A.; Martín-Belloso, O. Encapsulation and stability of a phenolic-rich extract from mango peel within water-in-oil-in-water emulsions. J. Funct. Foods 2019, 56, 65–73. [Google Scholar] [CrossRef]
- Sazdanić, D.; Krstonošić, M.A.; Ćirin, D.; Cvejić, J.; Alamri, A.; Galanakis, C.; Krstonošić, V. Non-ionic surfactants-mediated green extraction of polyphenols from red grape pomace. J. Appl. Res. Med. Aromat. Plants 2022, 32, 100439. [Google Scholar] [CrossRef]
- Krstonošić, M.A.; Sazdanić, D.; Ćirin, D.; Maravić, N.; Mikulić, M.; Cvejić, J.; Krstonošić, V. Aqueous solutions of non-ionic surfactant mixtures as mediums for green extraction of polyphenols from red grape pomace. Sustain. Chem. Pharm. 2023, 33, 101069. [Google Scholar] [CrossRef]
- Krstonošić, V.; Milanović, M.; Dokić, L. Application of different techniques in the determination of xanthan gum-SDS and xanthan gum-Tween 80 interaction. Food Hydrocoll. 2019, 87, 108–118. [Google Scholar] [CrossRef]
- Esumi, K.; Kuwabara, K.; Chiba, T.; Kobayashi, F.; Mizutani, H.; Torigoe, K. Interactions between a hydrophobically modified poly (amidoamine) dendrimer and surfactants in aqueous solutions. Colloids Surf. A Physicochem. Eng. Asp. 2002, 197, 141–146. [Google Scholar] [CrossRef]
- Cao, Y.; Zhao, Q.; Geng, Y.; Li, Y.; Huang, J.; Tian, S.; Ning, P. Interfacial interaction between benzo [a] pyrene and pulmonary surfactant: Adverse effects on lung health. Environ. Pollut. 2021, 287, 117669. [Google Scholar] [CrossRef] [PubMed]
- Stojanović, Z.S.; Marković, S. Merenje raspodele veličina čestica metodom difrakcije laserske svetlosti. Teh. Novi Mater. 2010, 19, 1–15. [Google Scholar]
- Zhang, M.; Yang, B.; Liu, W.; Li, S. Influence of hydroxypropyl methylcellulose, methylcellulose, gelatin, poloxamer 407 and poloxamer 188 on the formation and stability of soybean oil-in-water emulsions. Asian J. Pharm. Sci. 2017, 12, 521–531. [Google Scholar] [CrossRef] [PubMed]
- Bai, L.; Huan, S.; Gu, J.; McClements, D.J. Fabrication of oil-in-water nanoemulsions by dual-channel microfluidization using natural emulsifiers: Saponins, phospholipids, proteins, and polysaccharides. Food Hydrocoll. 2016, 61, 703–711. [Google Scholar] [CrossRef]
- Kabong, M.A.; Focke, W.W.; Du Toit, E.L.; Rolfes, H.; Ramjee, S. Breakdown mechanisms of oil-in-water emulsions stabilised with Pluronic F127 and co-surfactants. Colloids Surf. A Physicochem. Eng. Asp. 2020, 585, 124101. [Google Scholar] [CrossRef]
- Niu, H.; Wang, W.; Dou, Z.; Chen, X.; Chen, X.; Chen, H.; Fu, X. Multiscale combined techniques for evaluating emulsion stability: A critical review. Adv. Colloid Interface Sci. 2023, 311, 102813. [Google Scholar] [CrossRef]
- Papadimitriou, M.K.; Stephanou, P.S. Modeling the rheological behavior of crude oil–water emulsions. Phys. Fluids 2022, 34, 113107. [Google Scholar] [CrossRef]
- Herschel, W.H.; Bulkley, R. Konsistenzmessungen von gummi-benzollösungen. Kolloid-Zeitschrift 1926, 39, 291–300. [Google Scholar] [CrossRef]
- Dokić, P.; Dokić, L.; Dapčević, T.; Krstonošić, V. Colloid characteristics and emulsifying properties of OSA starches. In Colloids for Nano- and Biotechnology; Springer: Berlin/Heidelberg, Germany, 2008; pp. 48–56. [Google Scholar]
- Krstonošić, V.; Dokić, L.; Nikolić, I.; Dapčević, T.; Hadnađev, M. Influence of sodium dodecyl sulphate concentration on disperse and rheological characteristics of oil-in-water emulsions stabilized by OSA starch-SDS mixtures. J. Serbian Chem. Soc. 2012, 77, 83–94. [Google Scholar] [CrossRef]
- Lucia, A.; Guzmán, E.; Rubio, R.G.; Ortega, F. Enhanced solubilization of an insect juvenile hormone (JH) mimetic (piryproxyfen) using eugenol in water nanoemulsions stabilized by a triblock copolymer of poly (ethylenglycol) and poly (propilenglycol). Colloids Surf. A Physicochem. Eng. Asp. 2020, 606, 125513. [Google Scholar] [CrossRef]
- Krstonošić, V. Primena Biopolimera u Proizvodnji Emulzija; Medicinski Fakultet: Novi Sad, Serbia, 2020. [Google Scholar]
- Torcello-Gómez, A.; Wulff-Pérez, M.; Gálvez-Ruiz, M.J.; Martín-Rodríguez, A.; Cabrerizo-Vílchez, M.; Maldonado-Valderrama, J. Block copolymers at interfaces: Interactions with physiological media. Adv. Colloid Interface Sci. 2014, 206, 414–427. [Google Scholar] [CrossRef] [PubMed]
- Dickinson, E. Hydrocolloids as emulsifiers and emulsion stabilizers. Food Hydrocoll. 2009, 23, 1473–1482. [Google Scholar] [CrossRef]
- Krstonošić, V.; Dokić, L.; Nikolić, I.; Milanović, M. Influence of xanthan gum on oil-in-water emulsion characteristics stabilized by OSA starch. Food Hydrocoll. 2015, 45, 9–17. [Google Scholar] [CrossRef]
- Sansone, F.; Esposito, T.; Mencherini, T.; Piccinelli, A.L.; Gazzerro, P.; Picerno, P.; Russo, P.; Del Gaudio, P.; Essolito, M.; Campiglia, P. Annurca peel extract: From the chemical composition, through the functional activity, to the formulation and characterisation of a topical oil-in-water emulsion. Nat. Prod. Res. 2016, 30, 1398–1403. [Google Scholar] [CrossRef] [PubMed]
- Segovia, F.; Lupo, B.; Peiró, S.; Gordon, M.H.; Almajano, M.P. Extraction of antioxidants from borage (Borago officinalis L.) leaves—Optimization by response surface method and application in oil-in-water emulsions. Antioxidants 2014, 3, 339–357. [Google Scholar] [CrossRef] [PubMed]
- Proksch, E. pH in nature, humans and skin. J. Dermatol. 2018, 45, 1044–1052. [Google Scholar] [CrossRef]
- Lukić, M.; Pantelić, I.; Savić, S.D. Towards optimal ph of the skin and topical formulations: From the current state of the art to tailored products. Cosmetics 2021, 8, 69. [Google Scholar] [CrossRef]
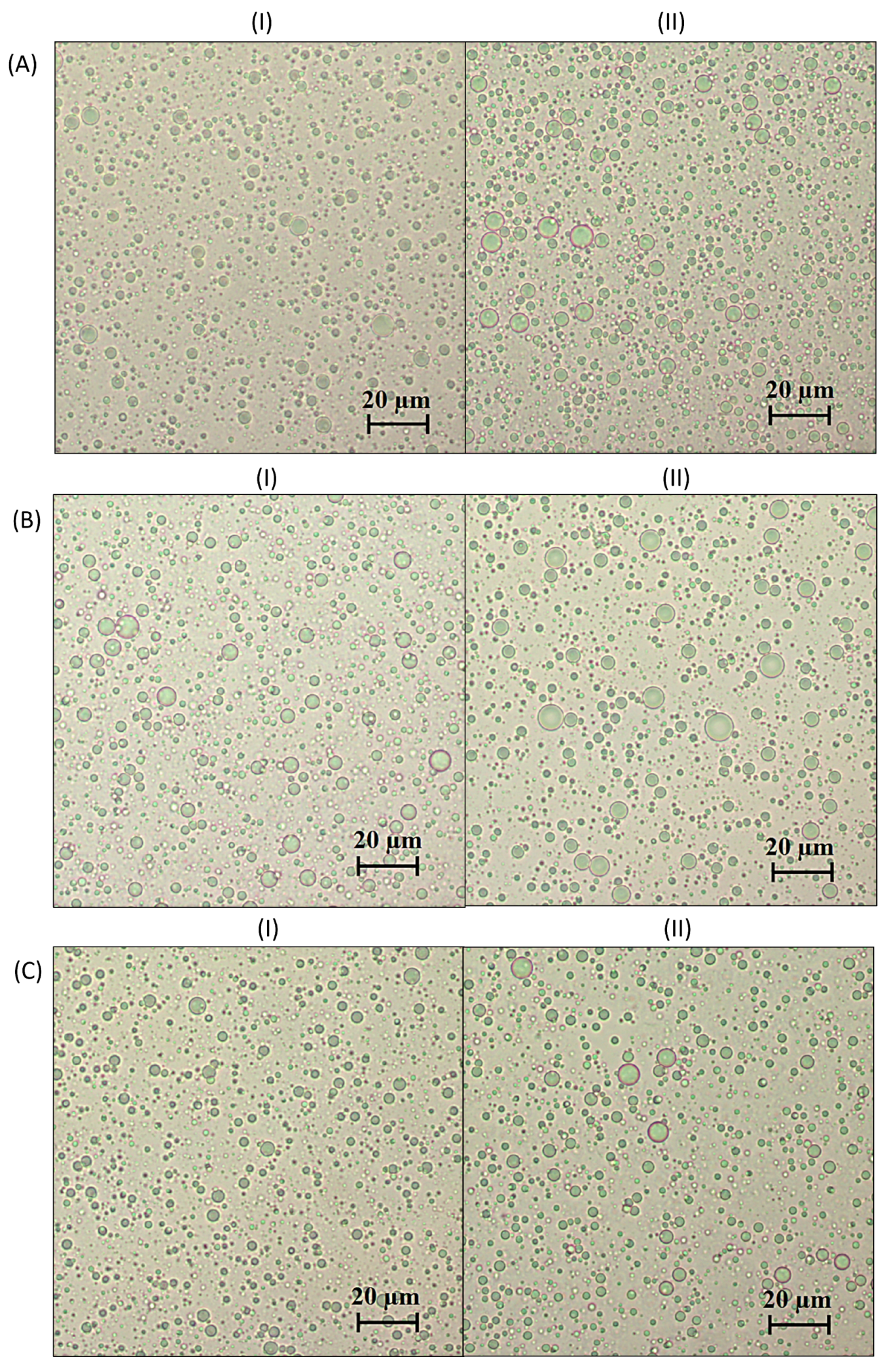



| Emulsion Type | Continuous Phase | Surfactant Concentration (w/w, %) |
|---|---|---|
| Control emulsion | Water | 3 |
| 5 | ||
| Test emulsion | Grape pomace extract | 3 |
| 5 |
| Surfactant System | Specific Surface Area S (m2/g) | Surface-Weighted Mean d32 (μm) | Volume-Weighted Mean d43 (μm) | d (0.1) (μm) | d (0.5) (μm) | d (0.9) (μm) | Span | Uniformity | d32 (μm) Determined Using Microphotography 1 |
|---|---|---|---|---|---|---|---|---|---|
| Control emulsions | |||||||||
| P407 | 3.717 ± 0.023 abd | 1.614 ± 0.011 abd | 3.702 ± 0.084 ab* | 0.788 ± 0.005 ab | 1.945 ± 0.012 abd | 8.973 ± 0.601 ad* | 4.212 ± 0.287 ab* | 1.293 ± 0.038 ac* | 2.25 ± 0.11 |
| BS20/P407 (1:9) | 3.583 ± 0.070 abd* | 1.677 ± 0.033 abd* | 3.557 ± 0.187 ab* | 0.808 ± 0.011 abd* | 2.047 ± 0.049 abd* | 8.793 ± 0.663 b* | 3.898 ± 0.238 ab* | 1.130 ± 0.065 b* | 2.28 ± 0.13 |
| BS20/P407 (1:1) | 3.277 ± 0.099 c* | 1.833 ± 0.054 c* | 4.240 ± 0.150 c* | 0.863 ± 0.019 cd | 2.279 ± 0.088 c* | 10.648 ± 0.485 c* | 4.295 ± 0.044 c* | 1.273 ± 0.011 ac* | 2.34 ± 0.14 |
| BS20/P407 (9:1) | 3.603 ± 0.075 abd* | 1.666 ± 0.033 abd* | 3.034 ± 0.147 d* | 0.836 ± 0.014 bcd* | 2.027 ± 0.039 abd* | 5.973 ± 0.334 ad* | 2.533 ± 0.113 d* | 0.877 ± 0.047 d* | 2.29 ± 0.09 |
| Test emulsions | |||||||||
| P407 | 3.950 ± 0.166 efgh | 1.520 ± 0.062 efgh | 3.010 ± 0.258 efg* | 0.752 ± 0.024 efgh | 1.843 ± 0.079 efgh | 6.341 ± 0.744 efg* | 3.024 ± 0.273 ef* | 1.019 ± 0.083 ef* | 2.23 ± 0.07 |
| BS20/P407 (1:9) | 3.960 ± 0.105 efgh* | 1.516 ± 0.039 efgh* | 2.769 ± 0.129 efg* | 0.756 ± 0.020 efgh* | 1.842 ± 0.053 efgh* | 5.721 ± 0.024 efg* | 2.697 ± 0.090 efg* | 0.890 ± 0.104 efg* | 2.24 ± 0.1 |
| BS20/P407 (1:1) | 3.803 ± 0.150 efgh* | 1.579 ± 0.062 efgh* | 2.682 ± 0.038 efgh* | 0.799 ± 0.038 efgh | 1.915 ± 0.066 efgh* | 5.418 ± 0.187 efg* | 2.412 ± 0.082 fg* | 0.779 ± 0.033 fgh* | 2.24 ± 0.11 |
| BS20/P407 (9:1) | 3.993 ± 0.100 efgh* | 1.503 ± 0.037 efgh* | 2.344 ± 0.074 gh* | 0.769 ± 0.022 efgh* | 1.803 ± 0.076 efgh* | 4.397 ± 0.134 h* | 1.980 ± 0.020 h* | 0.643 ± 0.014 gh* | 2.23 ± 0.12 |
| Surfactant System | Specific Surface Area S (m2/g) | Surface Weighted Mean d32 (μm) | Volume Weighted Mean d43 (μm) | d (0.1) (μm) | d (0.5) (μm) | d (0.9) (μm) | Span | Uniformity | d32 (μm) Determined Using Microphotography 1 |
|---|---|---|---|---|---|---|---|---|---|
| Control emulsions | |||||||||
| P407 | 3.717 ± 0.023 abcd | 1.614 ± 0.011 abc | 3.702 ± 0.084 a* | 0.788 ± 0.005 abcd | 1.945 ± 0.012 abc | 8.973 ± 0.601 a* | 4.212 ± 0.287 a* | 1.293 ± 0.038 a* | 2.25 ± 0.14 |
| T60/P407 (1:9) | 3.937 ± 0.176 abcd | 1.526 ± 0.066 abcd | 2.860 ± 0.196 bc | 0.760 ± 0.028 abcd | 1.843 ± 0.079 abcd | 6.310 ± 0.701 bc | 3.004 ± 0.242 b* | 0.932 ± 0.445 b | 2.23 ± 0.11 |
| T60/P407 (1:1) | 3.770 ± 0.065 abcd | 1.591 ± 0.029 abcd | 2.602 ± 0.147 bcd | 0.817 ± 0.012 abcd | 1.918 ± 0.052 abcd | 5.173 ± 0.365 bcd | 2.270 ± 0.137 cd* | 0.728 ± 0.062 cd* | 2.25 ± 0.12 |
| T60/P407 (9:1) | 3.990 ± 0.101 abcd | 1.504 ± 0.038 bcd | 2.244 ± 0.090 cd* | 0.801 ± 0.039 abcd* | 1.799 ± 0.024 bcd | 3.991 ± 0.188 cd* | 1.775 ± 0.143 cd* | 0.603 ± 0.074 cd* | 2.22 ± 0.12 |
| Test emulsions | |||||||||
| P407 | 3.950 ± 0.166 efgh | 1.520 ± 0.062 efgh | 3.010 ± 0.258 efg* | 0.752 ± 0.024 efgh | 1.843 ± 0.079 efgh | 6.341 ± 0.744 efg* | 3.024 ± 0.273 ef* | 1.019 ± 0.083 e* | 2.23 ± 0.1 |
| T60/P407 (1:9) | 3.933 ± 0.038 efgh | 1.525 ± 0.015 efgh | 2.755 ± 0.055 efgh | 0.766 ± 0.007 efgh | 1.848 ± 0.014 efgh | 5.511 ± 0.135 efgh | 2.567 ± 0.055 fg* | 0.871 ± 0.024 fg | 2.23 ± 0.08 |
| T60/P407 (1:1) | 3.797 ± 0.231 efgh | 1.584 ± 0.093 efgh | 2.851 ± 0.146 efgh | 0.782 ± 0.048 efgh | 1.804 ± 0.367 efgh | 5.930 ± 0.470 efg | 2.630 ± 0.079 fg* | 0.848 ± 0.019 fgh* | 2.24 ± 0.11 |
| T60/P407 (9:1) | 4.017 ± 0.030 efgh | 1.493 ± 0.011 efgh | 2.518 ± 0.134 fgh* | 0.756 ± 0.009 efgh* | 1.832 ± 0.008 efgh | 4.731 ± 0.072 fh* | 2.169 ± 0.029 h* | 0.754 ± 0.010 gh* | 2.20 ± 0.1 |
| Surfactant System | K (Pa sn) | n |
|---|---|---|
| Control emulsions | ||
| P407 | 0.03256 ± 0.00198 a | 0.71847 ± 0.0087 ac |
| BS20/P407 (1:9) | 0.02954 ± 5.42·10−4 b | 0.74470 ± 0.0038 bc |
| BS20/P407 (1:1) | 0.02633 ± 3.65·10−4 c* | 0.73363 ± 0.0015 abc* |
| BS20/P407 (9:1) | 0.00965 ± 7.68·10−4 d | 0.81877 ± 0.0076 d |
| Test emulsions | ||
| P407 | 0.03541 ± 0.00144 e | 0.71503 ± 0.0067 efg |
| BS20/P407 (1:9) | 0.03137 ± 0.0025 fg | 0.72497 ± 0.0121 efg |
| BS20/P407 (1:1) | 0.02878 ± 5.06·10−4 fg* | 0.72393 ± 0.0048 efg* |
| BS20/P407 (9:1) | 0.00978 ± 7.72·10−4 h | 0.82220 ± 0.0072 h |
| Surfactant System | K (Pa sn) | n |
|---|---|---|
| Control emulsions | ||
| P407 | 0.03256 ± 0.00198 ab | 0.71847 ± 0.00866 abc |
| T60/P407 (1:9) | 0.02801 ± 0.00644 abc | 0.72711 ± 0.04786 abc |
| T60/P407 (1:1) | 0.02296 ± 3.76·10−4 bc | 0.72803 ± 0.00404 abc |
| T60/P407 (9:1) | 0.00617 ± 5.71·10−4 d | 0.86697 ± 0.01023 d |
| Test emulsions | ||
| P407 | 0.03541 ± 0.00144 ef | 0.71503 ± 0.00675 efg |
| T60/P407 (1:9) | 0.03297 ± 0.00191 ef | 0.71907 ± 0.00841 efg |
| T60/P407 (1:1) | 0.02274 ± 2.41·10−4 g | 0.73827 ± 0.00352 efg |
| T60/P407 (9:1) | 0.00697 ± 6.62·10−4 h | 0.83987 ± 0.02105 h |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Krstonošić, V.S.; Sazdanić, D.B.; Ćirin, D.M.; Nikolić, I.R.; Hadnađev, M.S.; Atanacković Krstonošić, M.T. Characterization of Oil-in-Water Emulsions Prepared with Triblock Copolymer Poloxamer 407 and Low-Molecular-Mass Surfactant Mixtures as Carriers of Grape Pomace Waste Polyphenols. Pharmaceutics 2024, 16, 578. https://doi.org/10.3390/pharmaceutics16050578
Krstonošić VS, Sazdanić DB, Ćirin DM, Nikolić IR, Hadnađev MS, Atanacković Krstonošić MT. Characterization of Oil-in-Water Emulsions Prepared with Triblock Copolymer Poloxamer 407 and Low-Molecular-Mass Surfactant Mixtures as Carriers of Grape Pomace Waste Polyphenols. Pharmaceutics. 2024; 16(5):578. https://doi.org/10.3390/pharmaceutics16050578
Chicago/Turabian StyleKrstonošić, Veljko S., Darija B. Sazdanić, Dejan M. Ćirin, Ivana R. Nikolić, Miroslav S. Hadnađev, and Milica T. Atanacković Krstonošić. 2024. "Characterization of Oil-in-Water Emulsions Prepared with Triblock Copolymer Poloxamer 407 and Low-Molecular-Mass Surfactant Mixtures as Carriers of Grape Pomace Waste Polyphenols" Pharmaceutics 16, no. 5: 578. https://doi.org/10.3390/pharmaceutics16050578





