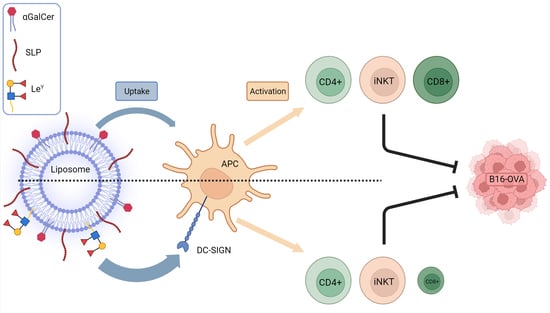
Vaccination with DC-SIGN-Targeting αGC Liposomes Leads to Tumor Control, Irrespective of Suboptimally Activated T-Cells
Abstract
:1. Introduction
2. Material and Methods
2.1. Multi-Color Flow Cytometry
2.2. Mice
2.3. Production of Vaccines
2.4. Binding ELISA
2.5. BMDC Culture
2.6. Uptake in BMDCs
2.7. Uptake in APCs In Vivo
2.8. Immunization Studies
2.9. Ex Vivo Re-Stimulation of Splenocytes
2.10. Tumor Challenge and Therapeutic Vaccination
2.11. Statistics
3. Results
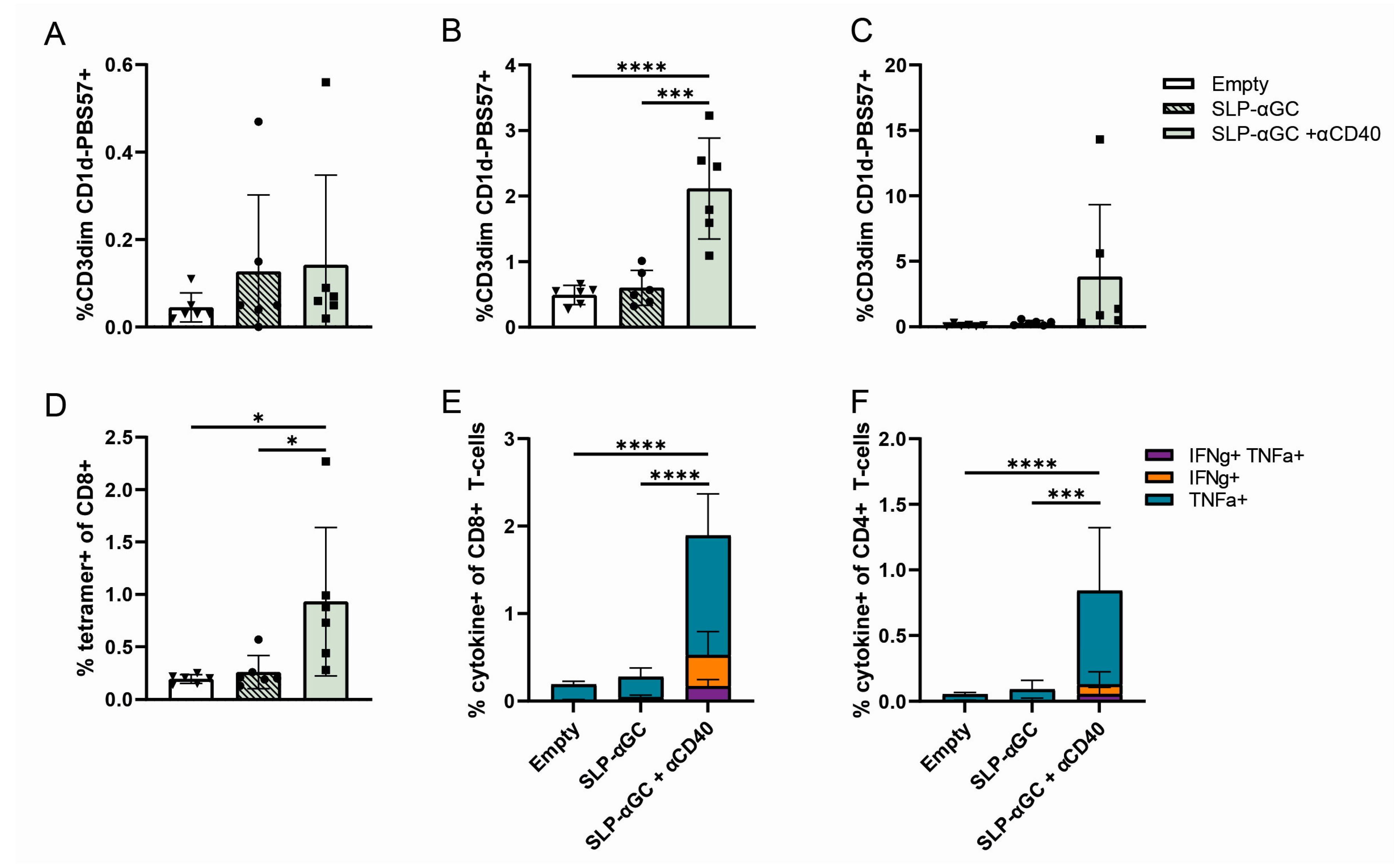
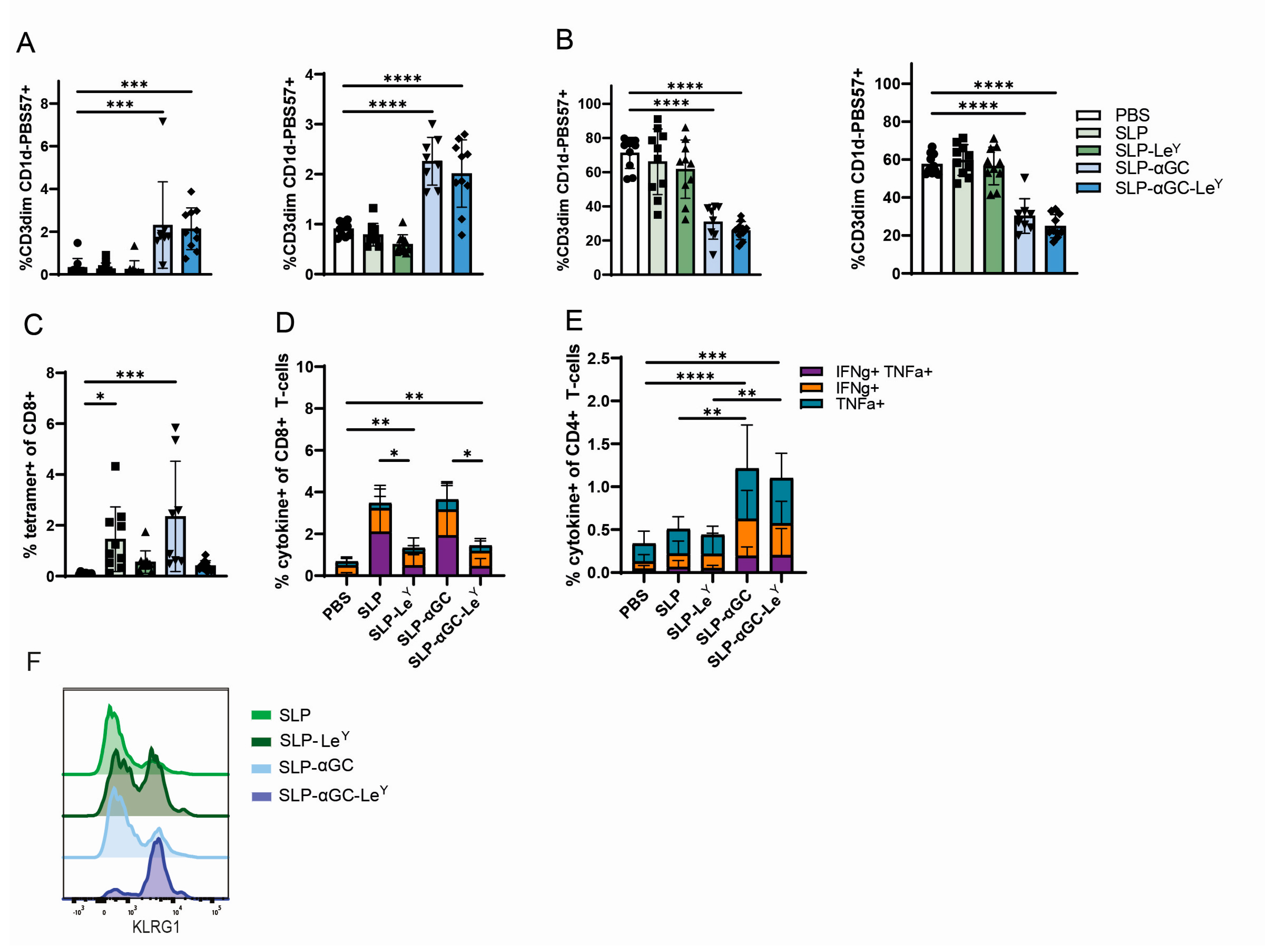
3.1. Supplementation of SLP-αGC Liposomes with αCD40 Is Necessary for iNKT Proliferation and Induction of Antigen-Specific T-Cells
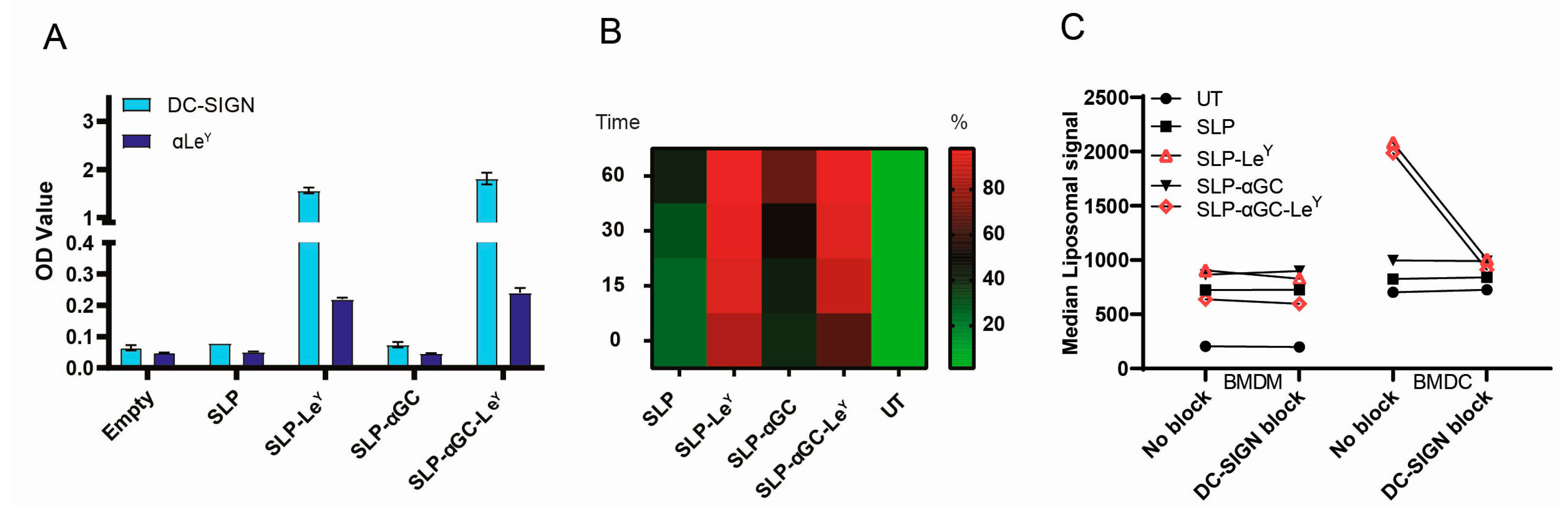
3.2. Addition of LeY to Liposomal Formulations Increases Uptake by DC-SIGN+ APCs In Vitro
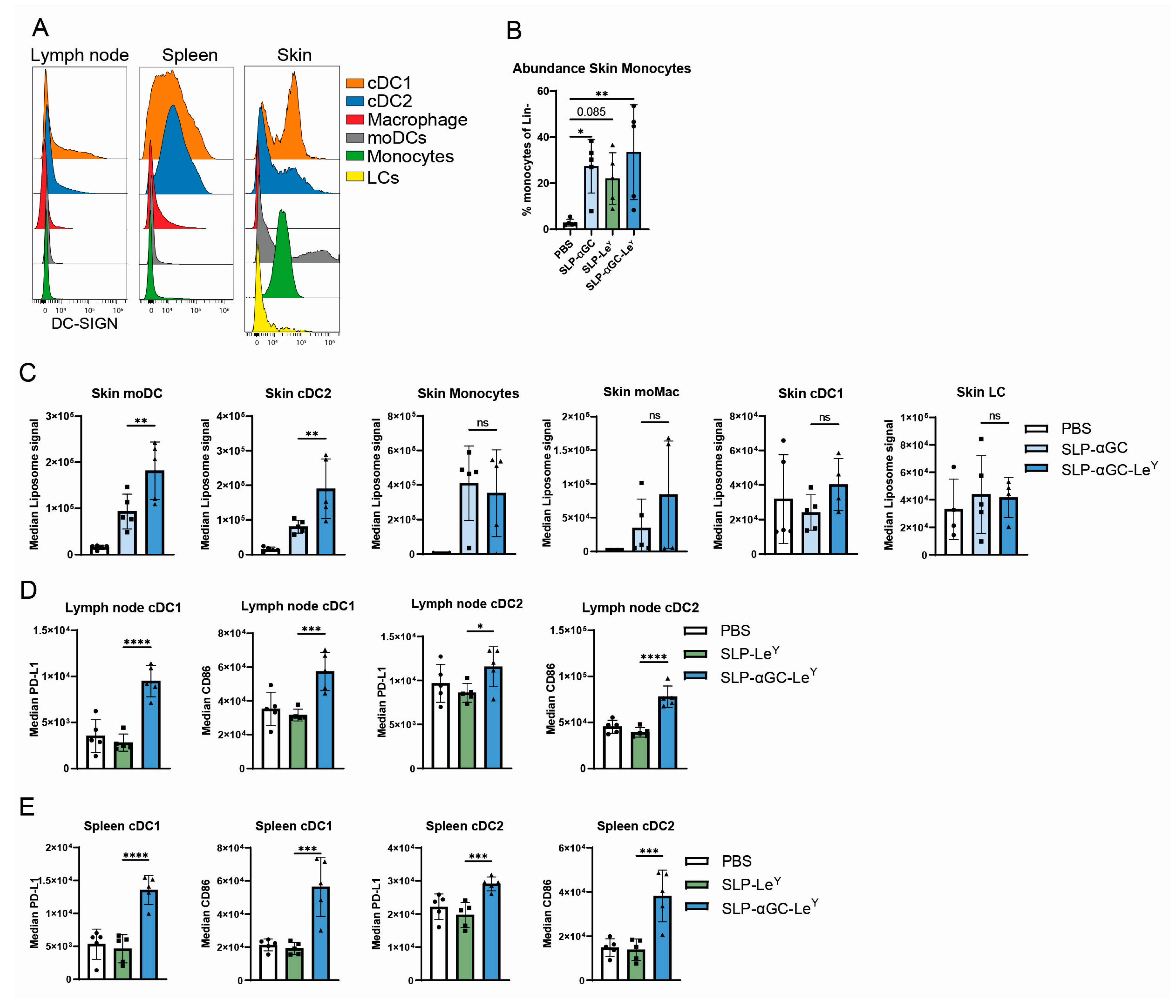
3.3. Injection of LeY-Coated Liposomes s.c. in Mice Specifically Target moDCs and cDC2s in the Skin
3.4. Incorporation of LeY into Liposomal Formulations for Increased DC-SIGN Targeting Fails to Further Increase iNKT, CD4+ T-Cells or Antigen-Specific CD8+ T-Cell Responses In Vivo
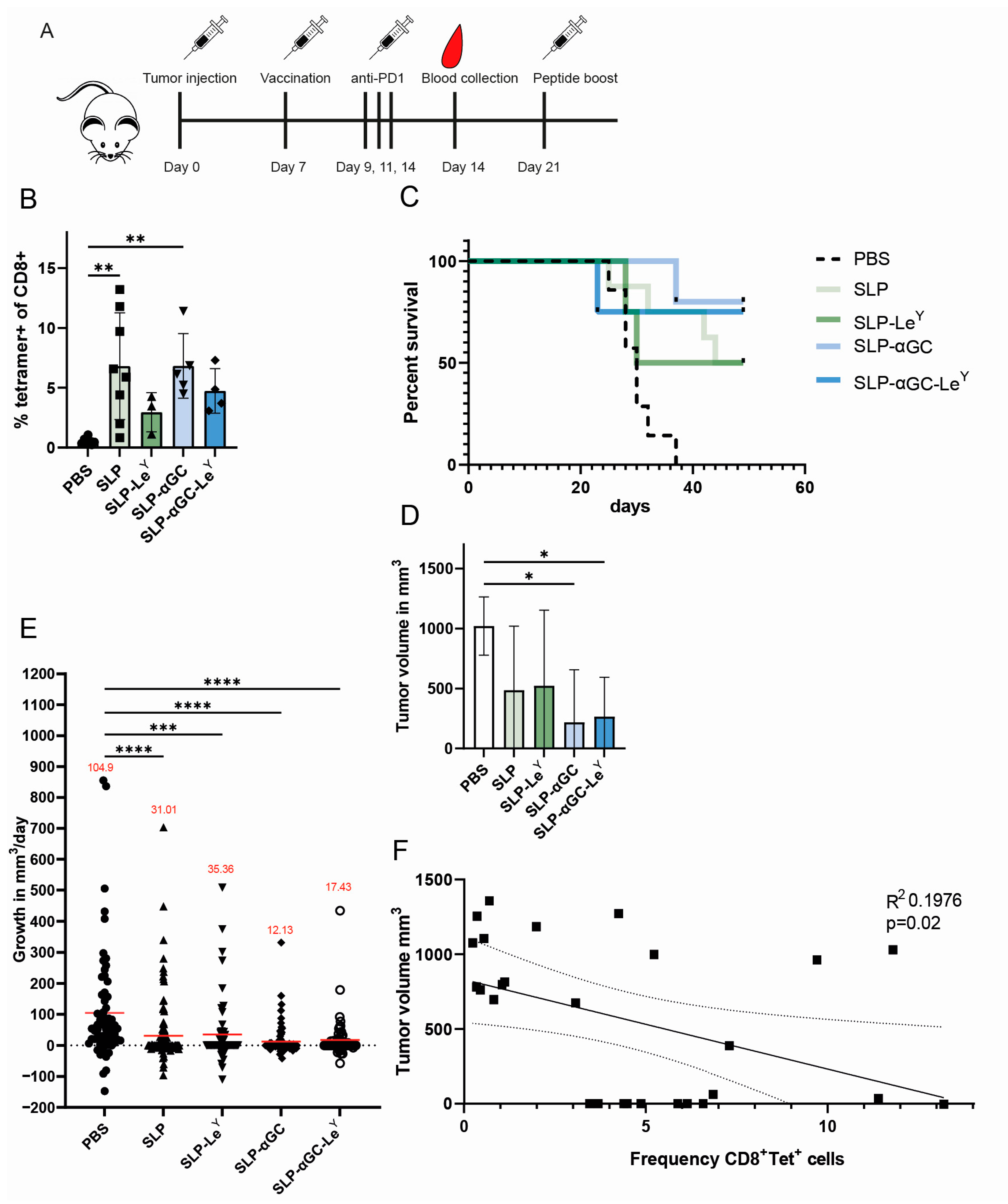
3.5. Liposome Formulations Provide Similar Tumor Control, despite Lower Frequencies of Antigen-Specific CD8+ T-cells in LeY Groups
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Fehleisen, F. Ueber die Züchtung der Erysipelkokken auf künstlichem Nährboden und ihre Uebertragbarkeit auf den Menschen. Dtsch. Med. Wochenschr. 1882, 8, 553–554. [Google Scholar] [CrossRef]
- Busch, W. Aus der Sitzung der medicinischen Section vom 13 November 1867. Berl. Klin. Wochenschr. 1868, 5, 137. [Google Scholar]
- Coley, W.B., II. Contribution to the Knowledge of Sarcoma. Ann. Surg. 1891, 14, 199–220. [Google Scholar] [CrossRef]
- Hargadon, K.M.; Johnson, C.E.; Williams, C.J. Immune checkpoint blockade therapy for cancer: An overview of FDA-approved immune checkpoint inhibitors. Int. Immunopharmacol. 2018, 62, 29–39. [Google Scholar] [CrossRef]
- Robert, C.; Long, G.V.; Brady, B.; Dutriaux, C.; Maio, M.; Mortier, L.; Hassel, J.C.; Rutkowski, P.; McNeil, C.; Kalinka-Warzocha, E.; et al. Nivolumab in previously untreated melanoma without BRAF mutation. N. Engl. J. Med. 2015, 372, 320–330. [Google Scholar] [CrossRef]
- Hou, X.; Zaks, T.; Langer, R.; Dong, Y. Lipid nanoparticles for mRNA delivery. Nat. Rev. Mater. 2021, 6, 1078–1094. [Google Scholar] [CrossRef]
- van Dinther, D.; Stolk, D.A.; van de Ven, R.; van Kooyk, Y.; de Gruijl, T.D.; den Haan, J.M.M. Targeting C-type lectin receptors: A high-carbohydrate diet for dendritic cells to improve cancer vaccines. J. Leukoc. Biol. 2017, 102, 1017–1034. [Google Scholar] [CrossRef]
- Stolk, D.A. Targeting Antigen Presenting Cells with Lipid-Based Vaccines for the Induction of Strong Immune Responses. Ph.D. Thesis, Vrije Universiteit Amsterdam, Amsterdam, The Netherlands, 2022. [Google Scholar]
- van Kooyk, Y.; Unger, W.W.; Fehres, C.M.; Kalay, H.; García-Vallejo, J.J. Glycan-based DC-SIGN targeting vaccines to enhance antigen cross-presentation. Mol. Immunol. 2013, 55, 143–145. [Google Scholar] [CrossRef]
- Duinkerken, S.; Horrevorts, S.K.; Kalay, H.; Ambrosini, M.; Rutte, L.; de Gruijl, T.D.; Garcia-Vallejo, J.J.; van Kooyk, Y. Glyco-Dendrimers as Intradermal Anti-Tumor Vaccine Targeting Multiple Skin DC Subsets. Theranostics 2019, 9, 5797–5809. [Google Scholar] [CrossRef]
- Schaefer, M.; Reiling, N.; Fessler, C.; Stephani, J.; Taniuchi, I.; Hatam, F.; Yildirim, A.O.; Fehrenbach, H.; Walter, K.; Ruland, J.; et al. Decreased pathology and prolonged survival of human DC-SIGN transgenic mice during mycobacterial infection. J. Immunol. 2008, 180, 6836–6845. [Google Scholar] [CrossRef]
- Schetters, S.T.T.; Li, R.J.E.; Kruijssen, L.J.W.; Engels, S.; Ambrosini, M.; Garcia-Vallejo, J.J.; Kalay, H.; Unger, W.W.J.; van Kooyk, Y. Adaptable antigen matrix platforms for peptide vaccination strategies and T-cell-mediated anti-tumor immunity. Biomaterials 2020, 262, 120342. [Google Scholar] [CrossRef]
- Unger, W.W.J.; Mayer, C.T.; Engels, S.; Hesse, C.; Perdicchio, M.; Puttur, F.; Streng-Ouwehand, I.; Litjens, M.; Kalay, H.; Berod, L.; et al. Antigen targeting to dendritic cells combined with transient regulatory T-cell inhibition results in long-term tumor regression. OncoImmunology 2015, 4, e970462. [Google Scholar] [CrossRef]
- Fujii, S.; Shimizu, K.; Smith, C.; Bonifaz, L.; Steinman, R.M. Activation of natural killer T-cells by alpha-galactosylceramide rapidly induces the full maturation of dendritic cells in vivo and thereby acts as an adjuvant for combined CD4 and CD8 T-cell immunity to a coadministered protein. J. Exp. Med. 2003, 198, 267–279. [Google Scholar] [CrossRef]
- Stolk, D.A.; de Haas, A.; Vree, J.; Duinkerken, S.; Lübbers, J.; van de Ven, R.; Ambrosini, M.; Kalay, H.; Bruijns, S.; van der Vliet, H.J.; et al. Lipo-Based Vaccines as an Approach to Target Dendritic Cells for Induction of T- and iNKT-cell Responses. Front. Immunol. 2020, 11, 990. [Google Scholar] [CrossRef]
- Bullock, T.N.J. CD40 stimulation as a molecular adjuvant for cancer vaccines and other immunotherapies. Cell. Mol. Immunol. 2022, 19, 14–22. [Google Scholar] [CrossRef]
- Horrevorts, S.K.; Duinkerken, S.; Bloem, K.; Secades, P.; Kalay, H.; Musters, R.J.; van Vliet, S.J.; García-Vallejo, J.J.; van Kooyk, Y. Toll-Like Receptor 4 Triggering Promotes Cytosolic Routing of DC-SIGN-Targeted Antigens for Presentation on MHC Class I. Front. Immunol. 2018, 9, 1231. [Google Scholar] [CrossRef]
- van Duikeren, S.; Fransen, M.F.; Redeker, A.; Wieles, B.; Platenburg, G.; Krebber, W.J.; Ossendorp, F.; Melief, C.J.; Arens, R. Vaccine-induced effector-memory CD8+ T-cell responses predict therapeutic efficacy against tumors. J. Immunol. 2012, 189, 3397–3403. [Google Scholar] [CrossRef]
- Grabowska, J.; Affandi, A.J.; van Dinther, D.; Nijen Twilhaar, M.K.; Olesek, K.; Hoogterp, L.; Ambrosini, M.; Heijnen, D.A.M.; Klaase, L.; Hidalgo, A.; et al. Liposome induction of CD8+ T-cell responses depends on CD169+ macrophages and Batf3-dependent dendritic cells and is enhanced by GM3 inclusion. J. Control. Release 2021, 331, 309–320. [Google Scholar] [CrossRef]
- Liu, X.; Hogg, G.D.; DeNardo, D.G. Rethinking immune checkpoint blockade: ‘Beyond the T-cell’. J. Immunother. Cancer 2021, 9, e001460. [Google Scholar] [CrossRef]
- Brossart, P. The Role of Antigen Spreading in the Efficacy of Immunotherapies. Clin. Cancer Res. 2020, 26, 4442. [Google Scholar] [CrossRef]
- Herndler-Brandstetter, D.; Ishigame, H.; Shinnakasu, R.; Plajer, V.; Stecher, C.; Zhao, J.; Lietzenmayer, M.; Kroehling, L.; Takumi, A.; Kometani, K.; et al. KLRG1(+) Effector CD8(+) T-cells Lose KLRG1, Differentiate into All Memory T-cell Lineages, and Convey Enhanced Protective Immunity. Immunity 2018, 48, 716–729.e718. [Google Scholar] [CrossRef]
- Renkema, K.R.; Huggins, M.A.; Borges da Silva, H.; Knutson, T.P.; Henzler, C.M.; Hamilton, S.E. KLRG1(+) Memory CD8 T-cells Combine Properties of Short-Lived Effectors and Long-Lived Memory. J. Immunol. 2020, 205, 1059–1069. [Google Scholar] [CrossRef]
- Joshi, N.S.; Cui, W.; Chandele, A.; Lee, H.K.; Urso, D.R.; Hagman, J.; Gapin, L.; Kaech, S.M. Inflammation directs memory precursor and short-lived effector CD8(+) T-cell fates via the graded expression of T-bet transcription factor. Immunity 2007, 27, 281–295. [Google Scholar] [CrossRef]
- Stolk, D.A.; Horrevorts, S.K.; Schetters, S.T.T.; Kruijssen, L.J.W.; Duinkerken, S.; Keuning, E.; Ambrosini, M.; Kalay, H.; van de Ven, R.; Garcia-Vallejo, J.J.; et al. Palmitoylated antigens for the induction of anti-tumor CD8(+) T-cells and enhanced tumor recognition. Mol. Ther. Oncolytics 2021, 21, 315–328. [Google Scholar] [CrossRef]
- Benne, N.; van Duijn, J.; Kuiper, J.; Jiskoot, W.; Slütter, B. Orchestrating immune responses: How size, shape and rigidity affect the immunogenicity of particulate vaccines. J. Control. Release 2016, 234, 124–134. [Google Scholar] [CrossRef]
- Joshi, M.D.; Unger, W.W.; van Beelen, A.J.; Bruijns, S.C.; Litjens, M.; van Bloois, L.; Kalay, H.; van Kooyk, Y.; Storm, G. DC-SIGN mediated antigen-targeting using glycan-modified liposomes: Formulation considerations. Int. J. Pharm. 2011, 416, 426–432. [Google Scholar] [CrossRef]
- Rouser, G.; Fleischer, S.; Yamamoto, A. Two dimensional thin layer chromatographic separation of polar lipids and determination of phospholipids by phosphorus analysis of spots. Lipids 1970, 5, 494–496. [Google Scholar] [CrossRef]
- Nakamura, T.; Yamazaki, D.; Yamauchi, J.; Harashima, H. The nanoparticulation by octaarginine-modified liposome improves α-galactosylceramide-mediated antitumor therapy via systemic administration. J. Control. Release 2013, 171, 216–224. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
de Haas, A.M.; Stolk, D.A.; Schetters, S.T.T.; Goossens-Kruijssen, L.; Keuning, E.; Ambrosini, M.; Boon, L.; Kalay, H.; Storm, G.; van der Vliet, H.J.; et al. Vaccination with DC-SIGN-Targeting αGC Liposomes Leads to Tumor Control, Irrespective of Suboptimally Activated T-Cells. Pharmaceutics 2024, 16, 581. https://doi.org/10.3390/pharmaceutics16050581
de Haas AM, Stolk DA, Schetters STT, Goossens-Kruijssen L, Keuning E, Ambrosini M, Boon L, Kalay H, Storm G, van der Vliet HJ, et al. Vaccination with DC-SIGN-Targeting αGC Liposomes Leads to Tumor Control, Irrespective of Suboptimally Activated T-Cells. Pharmaceutics. 2024; 16(5):581. https://doi.org/10.3390/pharmaceutics16050581
Chicago/Turabian Stylede Haas, Aram M., Dorian A. Stolk, Sjoerd T. T. Schetters, Laura Goossens-Kruijssen, Eelco Keuning, Martino Ambrosini, Louis Boon, Hakan Kalay, Gert Storm, Hans J. van der Vliet, and et al. 2024. "Vaccination with DC-SIGN-Targeting αGC Liposomes Leads to Tumor Control, Irrespective of Suboptimally Activated T-Cells" Pharmaceutics 16, no. 5: 581. https://doi.org/10.3390/pharmaceutics16050581