1. Introduction
The main disadvantage of most oral mass-market drug products is that they do not fit everyone. The reason usually lies in the required personalised approach, including dose personalisation or consumer properties [
1,
2]. Inappropriate consumer properties are usually associated with unpleasant smells, tastes, swallowing discomfort, or even swallowing problems (such as dysphagia) [
3]. Some paediatric age subgroups have contraindications to be treated with tablets and capsules at the same time and, for the geriatric population subgroup, swallowing discomfort is acquired with age because of decreased motor activities [
4,
5]. It is worth mentioning that swallowing discomfort is observed often, even in healthy adults [
6].
The usual real-world approach to solving the swallowing problem is to break or crush the tablet or open the capsule [
7]. The problem with this is that the biopharmaceutical properties of drug products can change drastically due to changes in the dissolution profile and the place of drug release [
8,
9]. In addition, after these unauthorised modifications, the unpleasant smell and taste problems intensify.
One of the drugs that requires both dose personalisation and taste masking [
10] is warfarin [
11]. Warfarin is a coumarin anticoagulant and a vitamin K antagonist used to treat and originally prevent thromboembolic events and the development of the disease in cases of many different health conditions, such as deep venous thrombosis, cardiomyopathy, and pulmonary embolism. It is also used for thrombosis, stroke, and myocardial infarction prophylaxis, among others. Warfarin inhibits the vitamin K-dependent synthesis of coagulation factors (II, VII, IX, and X) by inhibiting vitamin K reductase, vitamin K epoxide reductase, and anticoagulant proteins C and S, therefore depleting the available vitamin K-dependent activated proteins that play a role in the coagulation process. At the beginning of the treatment, a certain loading dose is needed. Due to the mechanism of action, the first effect is seen gradually, and the full clinical effect can be observed 3–4 days after starting the therapy. Based on the indication and considering its narrow therapeutic index, the general oral dose may vary from 2 to 10 mg once a day, depending on age, weight, genetic polymorphism, and the prothrombin time [
12].
Thus, the personalisation of the warfarin dose is demanded. Patients may greatly benefit from changes in dosage using different drug dosage forms as well as oral dosage forms that can still be taste-masked against the bitter taste of warfarin, while keeping the flexibility of personalised dosing [
10,
11,
13].
One of the approaches to solving taste and swallowing problems is to formulate the drug in the form of a liquid suspension or solution. Taste masking is usually achieved by adding taste additives and sweeteners [
10]. Despite the relative effectiveness of this method, solutions and suspensions cannot solve the taste-masking problem completely because they cannot prevent the drugs from come into contact with the taste buds [
10,
14,
15].
To the best of our knowledge, the best alternative approach is represented by taste-masked microparticles [
14,
16,
17]. This approach provides better taste masking due to the microparticles, which are insoluble in oral cavity saliva with a pH of 5.8–7.6 [
18]. Thus, the drug does not come into contact with the taste buds. The insolubility of particles in oral liquids can be achieved by using polymers with pH-dependent solubility, such as Kollicoat
® Smartseal 30D. This polymer is insoluble at the pH of the oral cavity, but dissolves quickly at the pH of the stomach [
16,
19].
The taste-masked microparticles/pellets can be manufactured by coating drug-containing granules or by drug-layering placebo cores and then applying a taste-masking coating [
20,
21]. Even though placebo cores are usually perceived as inert, the cores’ properties can affect the drug release from the pellets. For instance, the density of the particles predetermines the number of particles per gram, the size of the cores predetermines the specific surface area, and the solubility and osmolarity can increase the release rate [
22,
23,
24].
While the application of Kollicoat® Smartseal to obtain taste-masking-coated pellets is known, to the best of our knowledge, this polymer has never been investigated before for the preparation of warfarin-containing pellets. Moreover, the effect of the core type on the release of warfarin from the Kollicoat® Smartseal-coated pellets has not yet been investigated.
The aim of this study is to investigate the effect of microcrystalline cellulose, anhydrous dibasic calcium phosphate, and sodium chloride cores of comparable sizes in terms of their effect on the release of warfarin from the Kollicoat® Smartseal taste-masking-coated pellets.
3. Results and Discussion
In oral dosage forms, the most commonly used form of warfarin is a soluble salt, specifically sodium warfarin, including its sodium warfarin clathrate crystal form. In the sodium warfarin clathrate crystals, the molecule of isopropyl alcohol is trapped in the crystalline structure in a 2:1 molecular proportion, which is equivalent to 92 wt.% of sodium warfarin [
32,
33,
34,
35,
36]. The experimental results obtained via TGA confirmed this by showing the moisture and warfarin sodium contents at 8.4 ± 0.1 and 91.6 ± 0.1 wt.%, respectively (
Figure 2).
In this study, cores with a size that is usually unproblematic upon aqueous polymeric dispersion coating were used in the Wurster fluid-bed coaters [
23]. Soluble NaCl cores had a cubic shape, while PharSQ
® Spheres M, soluble at certain pHs (such as at pH 6.8 in phosphate buffer solution), and insoluble CELPHERE™ CP-507 cores had a spheroid shape (
Figure 2). The cores were successfully drug loaded and coated (
Table 1 and
Table 2,
Appendix A,
Appendix B and
Appendix C).
All pellets were drug-loaded with a 30% warfarin sodium clathrate solution and then coated with the Kollicoat
® Smartseal taste-masking coating (
Figure 3,
Table 3).
The structure of the pellets, including the drug loading of the cores and coating, is illustrated by the cross-section microscopy of NaCl-containing pellets (
Figure 4). Upon drug-loading and coating, the average size of the cores increased from 532 to 570 and to 590 µm for PharSQ
® Spheres M, from 615 to 698 and to 738 µm for CELPHERE™ CP-507, and from 521 to 549 and to 668 µm for NaCl (
Table 4;
Figure 5).
The average thickness of the coating can be assumed based on the difference between the average size of the drug-loaded and taste-masked pellets. Therefore, the assumed average thickness of the taste-masked coating for NaCl, CELPHERE™ CP-507, and PharSQ® Spheres M will comprise 59.5, 20, and 10 µm, respectively.
The particle size (D
50%) of the initial cores measured with the laser diffraction method agreed with the microscopic method, resulting in 500 µm for PharSQ
® Spheres M, 586 µm for CELPHERE™ CP-507, and 602 µm for NaCl (
Figure 6,
Table 5). The span of the core sizes was the smallest at 0.23 for PharSQ
® Spheres M, followed by 0.32 for CELPHERE™ CP-507, with the biggest being 0.46 for the NaCl cores (
Table 5). Considering the cubic structure of the NaCl cores (
Figure 3), further calculations were done via microscopic observations.
The average volume of the cores increased from 0.079 to 0.097 and to 0.108 mm
3 for PharSQ
® Spheres M, from 0.124 to 0.182 and to 0.213 mm
3 for CELPHERE™ CP-507, and from 0.143 to 0.170 and to 0.303 mm
3 for NaCl (
Table 4;
Figure 5).
The apparent density of the initial NaCl, CELPHERE™ CP-507, and PharSQ
® Spheres M cores was different. Due to the sequential drug loading and coating, the PharSQ
® Spheres M- and CELPHERE™ CP-507-containing pellets changed their apparent density from 1.60 to 1.69 and 1.88 g/mL, and from 1.24 to 1.26 and 1.33 g/mL, respectively (
Table 4). The initial apparent density of the NaCl cores was 2.36 g/mL, while after drug loading and coating, the apparent density (calculated based on the cubic geometry) was 2.25 and 1.39 g/mL, respectively. The drastic decrease in the calculated apparent density of NaCl-containing pellets can be explained by the lower density of the drug and taste-masking layers, as well as by the soft edges and the loss of their cubic-like shape (
Figure 3). The shape and apparent density of the cores predetermine the specific surface area of the drug-loaded pellets (
Table 4), whereas the calculated specific surface area predetermines the required amount of taste-masking coatings to achieve a desirable coating level.
Practical drug layering and coating (
Table 3) resulted in different pellets’ compositions (
Table 6). While PharSQ
® Spheres M and CELPHERE™ CP-507 cores are well-known in the pharmaceutical industry, NaCl cores are not unusually used. Thus, safety questions could be raised. In the case of NaCl drug-loaded and taste-masked pellets, following calculations, every 1 mg of warfarin sodium will be accompanied by 3.7 mg of NaCl (
Table 6) which is equivalent to 1.5 mg of sodium. Considering the daily recommended intake of sodium which is between 2000 and 5000 mg/day [
37,
38], 1.5 mg comprises 0.08–0.03%, respectively. So, the intake of therapeutic doses of warfarin with NaCl drug-loaded and taste-masked pellets cannot significantly influence the daily intake of sodium and compromise safety.
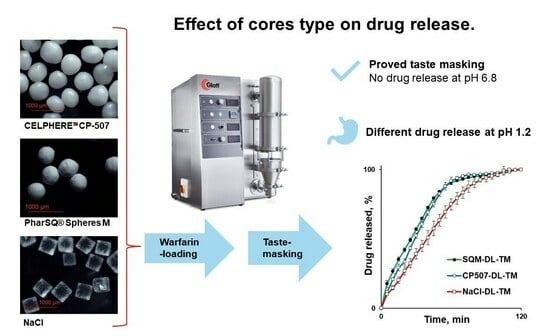
Despite the difference in cores and the resulting properties of the pellets (
Table 4,
Figure 5), none of the pellets demonstrated drug release in the phosphate buffer with a pH of 6.8, which simulates the pH of oral saliva. This confirms the efficiency of the taste masking. The release of warfarin was observed in stomach-simulated conditions (0.1 N HCl solution). The drug release kinetics decreased from PharSQ
® Spheres M to CELPHERE™ CP-507 and to NaCl-containing pellets, and the average amount of drug released in 60 min comprised 91, 92, and 75%, respectively (
Figure 7). Despite the reported incompatibility of calcium phosphate and warfarin [
39], in our study, the complete drug release from the pellets was observed via UV detection. Therefore, the experimental drug release results did not comply with the immediate release requirements for any of the pellets [
11].
The behaviour of single pellets upon exposition to a 0.1 N HCl solution without hydrodynamic forces agreed with the drug release from these pellets (
Figure 8). At a comparable coating level, the taste-masking-coating release was faster for PharSQ
® Spheres M- and CELPHERE™ CP-507- than for NaCl-containing pellets. The drug release kinetic decrease was in full accordance with the increase in the assumed average thickness of the taste-masked coating. Nevertheless, NaCl is known to be osmotically active, and thus faster water absorption and dissolution can be expected due to the osmotically active core.
However, the drug release from the NaCl-coated pellets was the slowest. When the NaCl-coated pellets were put in a 0.1 N HCl solution, the Kollicoat® Smartseal coating turned opalescent instead of clear, and it stayed on the pellets for a much longer time than on the other cores.
To reveal the effect of the NaCl core on the drug release, isolated dry Kollicoat
® Smartseal films were exposed to a 0.1 N solution of HCl with different concentrations of NaCl at room temperature under static conditions. Interestingly, at a 0% concentration of NaCl, the Kollicoat
® Smartseal films dissolved in less than 60 min, while even in 4.5 h, the films remained intact in the solutions with 11 and 23% of NaCl (
Figure 9).
The films exposed to an 11.5% concentration of NaCl were swollen and had opalescence, while those exposed to a 23% concentration of NaCl did not swell and were transparent. Being different compared to the other pellets, the NaCl-coated pellets showed similar opalescence upon single-pellet microscopic observation in a 0.1 N solution of HCl (
Figure 8). This experiment explained the different behaviours of Kollicoat
® Smartseal films in NaCl solution and the slower drug release from NaCl-containing pellets in stomach-mimicking pH conditions by the salting-out effect [
40,
41,
42].
The fluid bed coating method provided here is well-accepted and widespread in the pharmaceutical industry. Laboratory- and industrial-scale fluid bed coaters are present in many manufacturing facilities worldwide. The proposed manufacturing process does not require organic solvents, which could be a limitation for some drug product manufacturers. The proposed cores and other excipients are commercially available. Thus, the proposed technology does not have barriers to industrialisation and can be easily adopted by manufacturers to produce personalised medical products.
Nevertheless, at the current stage, the proposed formulations are not ready to be proposed for clinical implementation. Despite achieving desirable taste-masking properties, the drug release profiles shown here do not meet immediate release [
11]. Thus, considering favourable industrialisation possibilities, further study on formulation and drug release optimisation is reasonable.