QbD Approach-Based Preparation and Optimization of Hydrophobic Ion-Pairing Complex of Lysozyme with Sodium Dodecyl Sulphate to Enhance Stability in Lipid-Based Carriers
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals
2.2. Risk Assessment
2.3. Determination of the Optimum Molar Ratio
2.4. Preparation of the HIP Complex
2.5. Determination of the Dissociable Amount of LYZ from the HIP Complex by NaCl
2.6. Determination of the Complexation Efficiency and Recovered LYZ
2.7. Biological Activity of the Dissociated Lysozyme
2.8. Development and Optimization of the Drying Method
2.9. Fourier Transform Infrared Spectroscopy (FTIR) Analysis
2.10. Differential Scanning Calorimetry (DSC)/Thermogravimetric Analysis (TGA) Measurements
3. Results and Discussion
3.1. Preliminary Study
3.1.1. Determination of Suitable Solvent and LYZ Solution Concentration
3.1.2. HIP Complexation
3.2. Risk Assessment
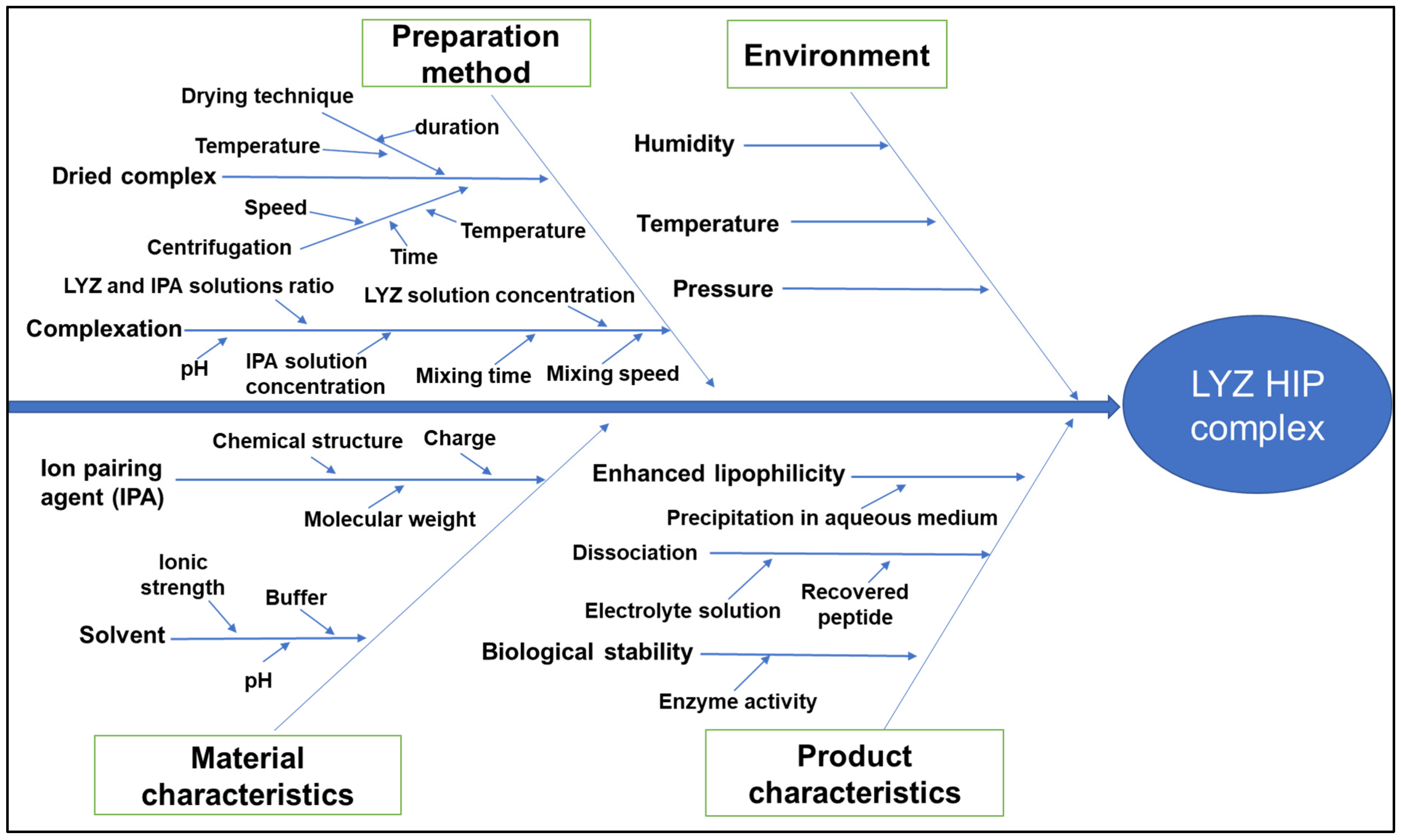
3.2.1. Cause-and-Effect Diagram
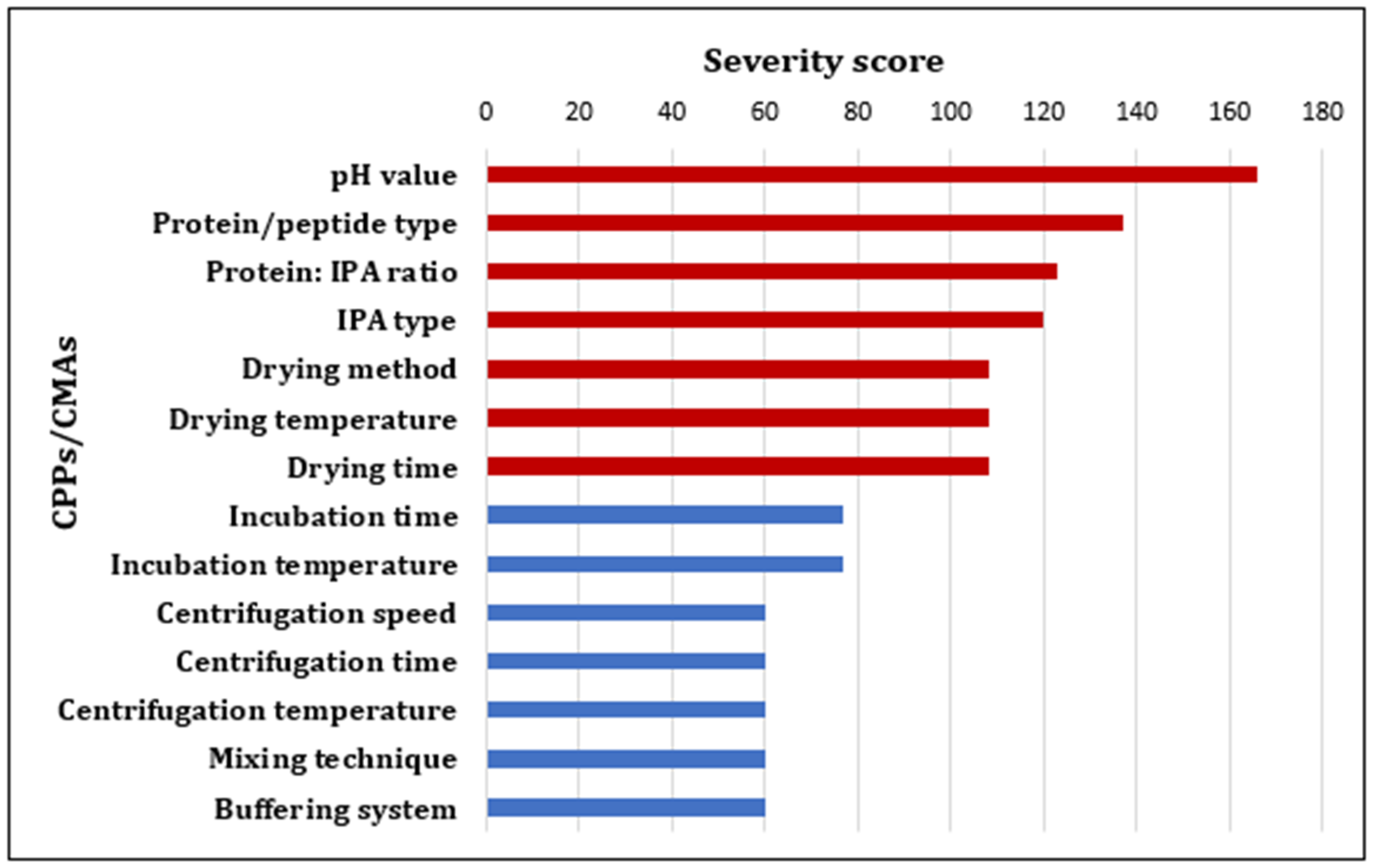
3.2.2. Definition of the QTPPs and Determination of the CQAs and REM
3.2.3. Pareto Analysis
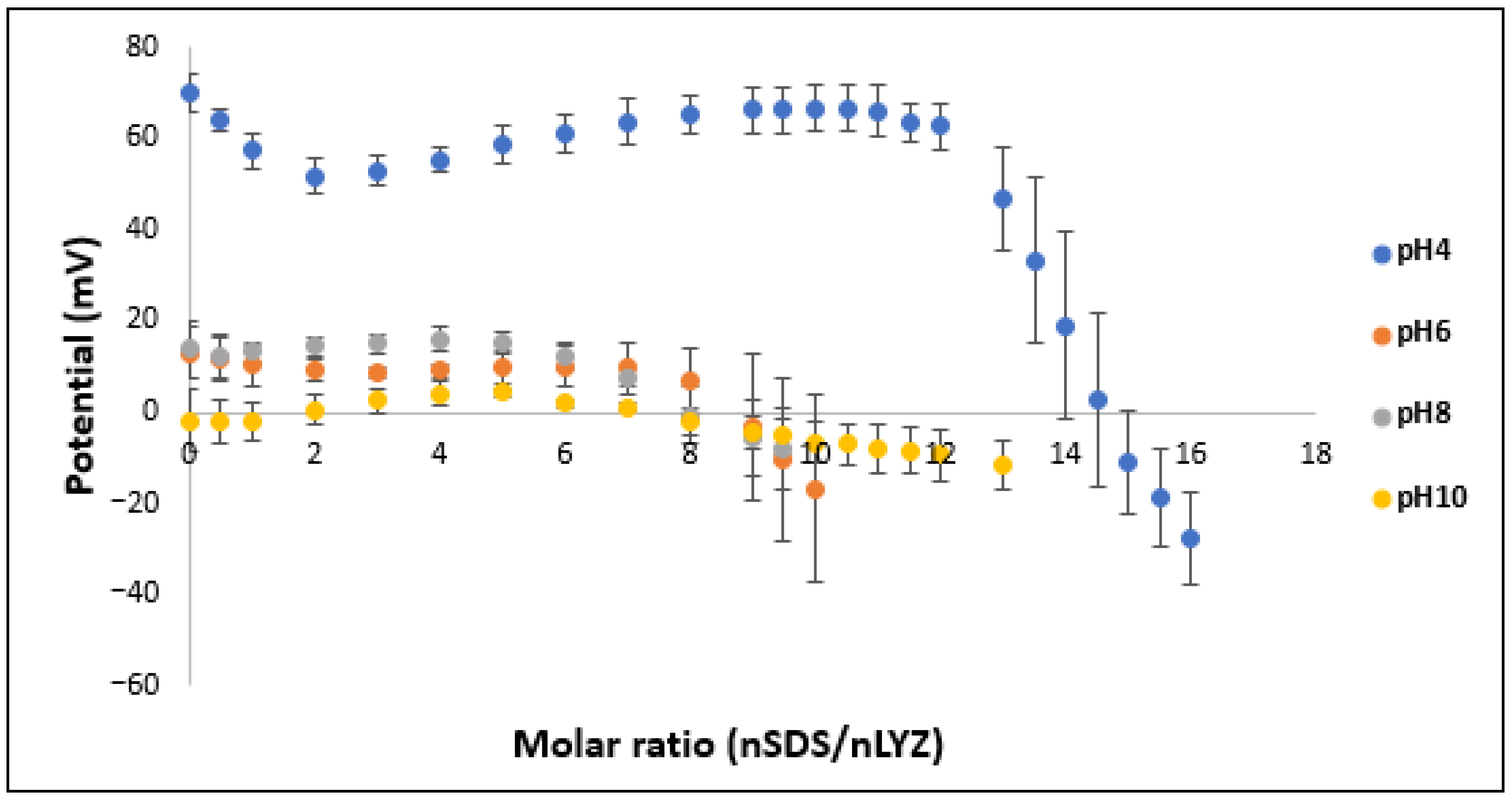
3.3. Optimization of the LYZ/SDS Molar Ratio in the HIP Complex
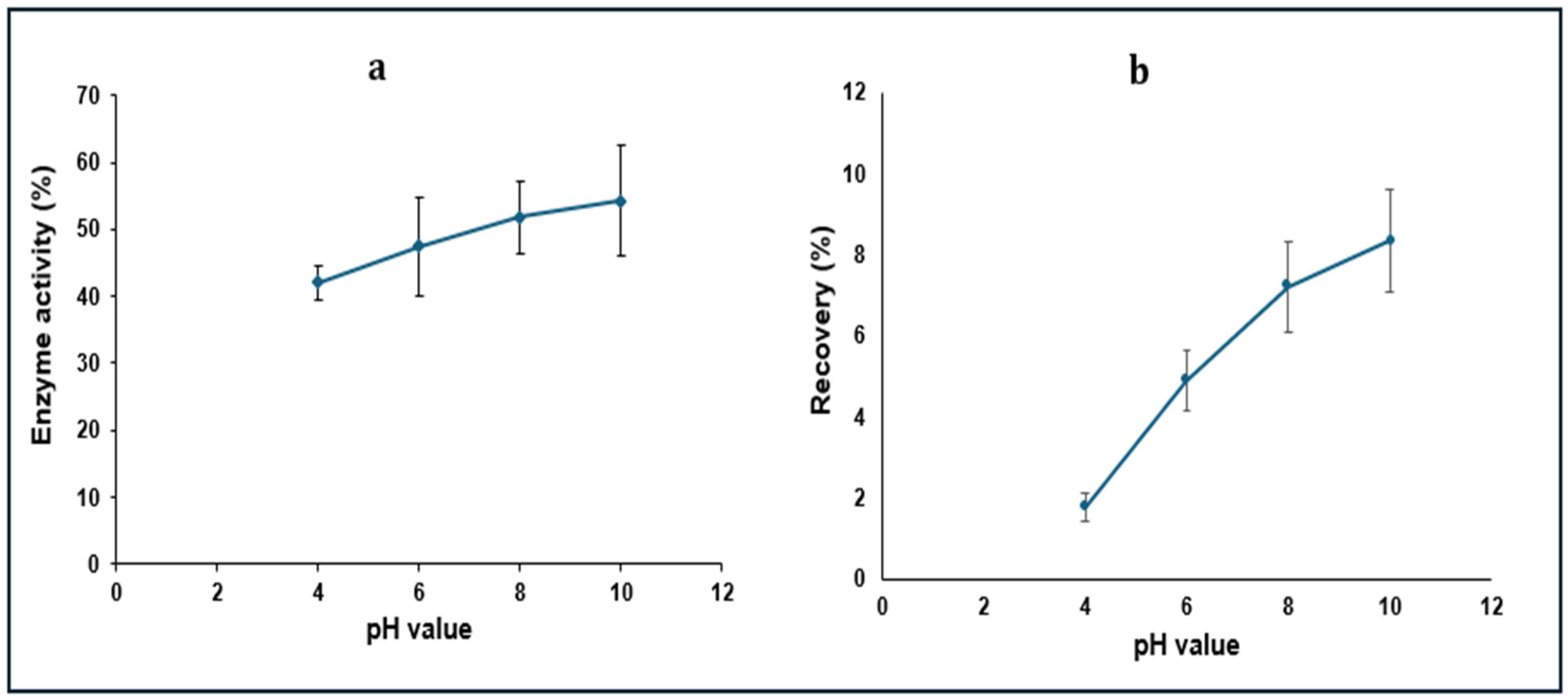
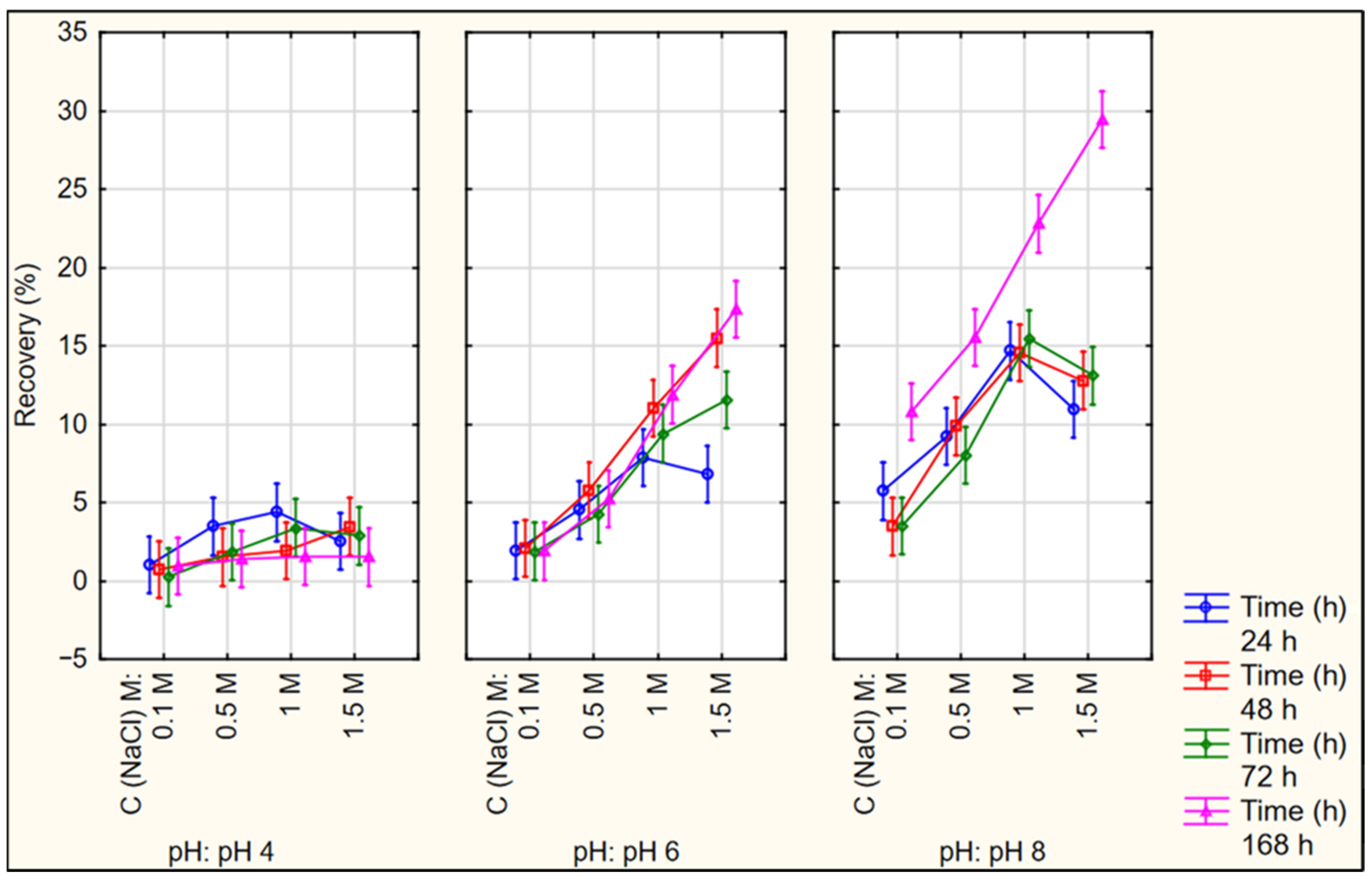
3.4. Optimization of HIP Complex Dissociation Conditions
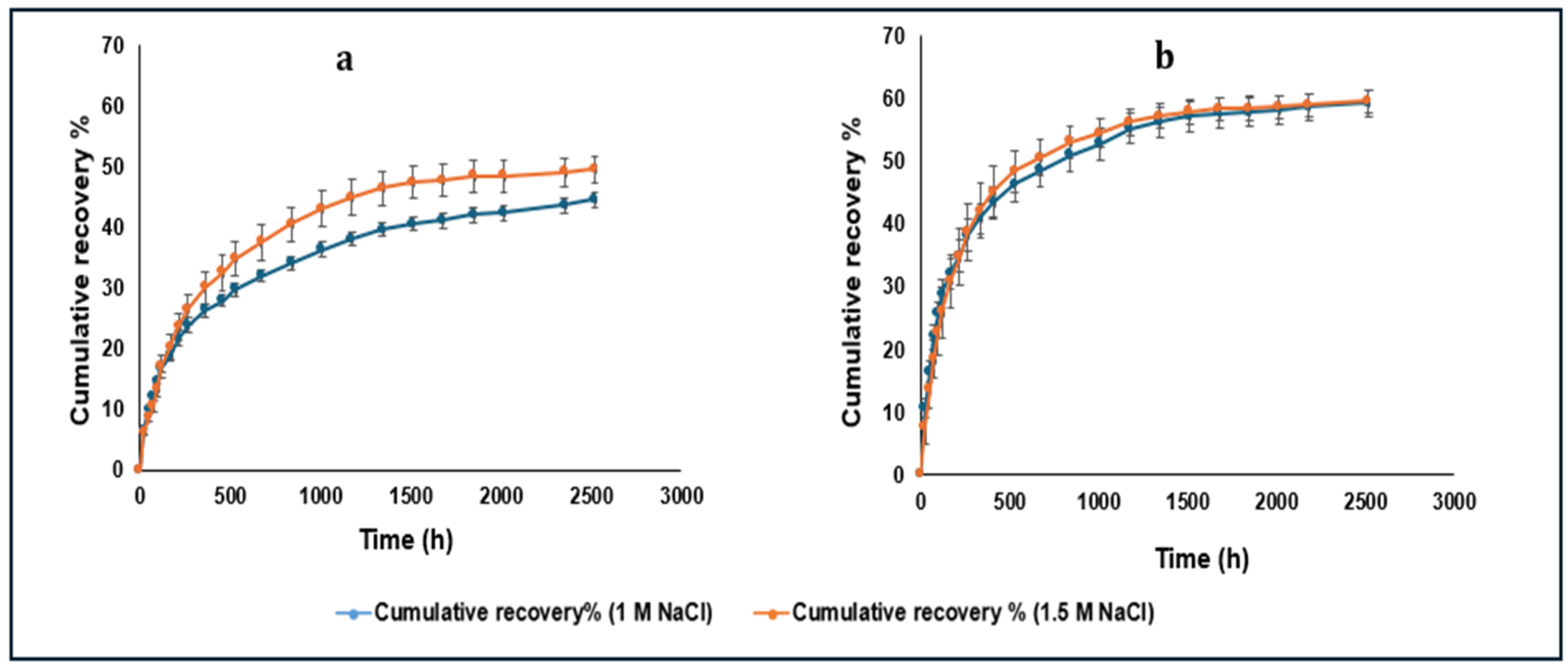
3.5. Cumulative Recovered Amount of LYZ Dissociating from the HIP Complex
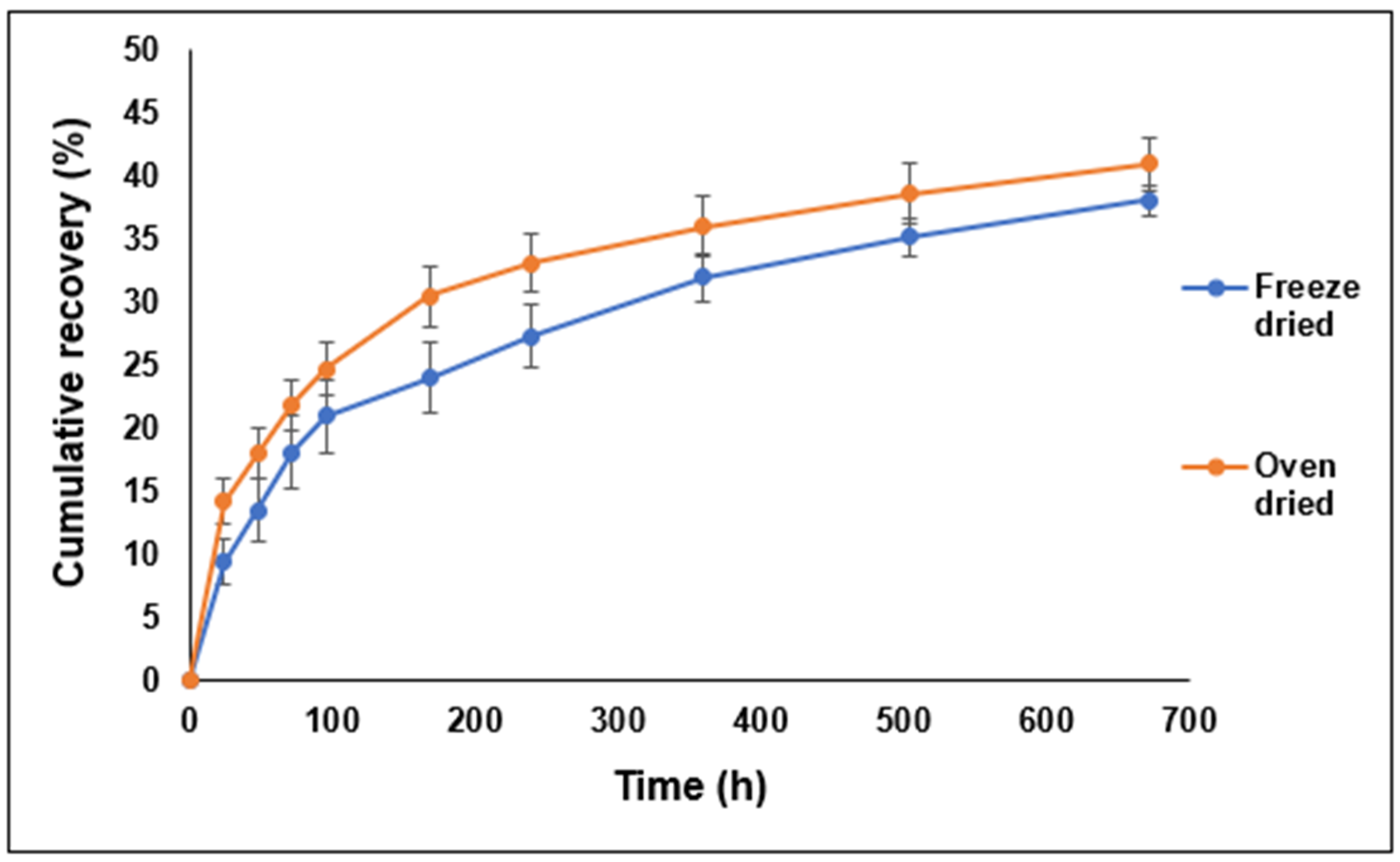
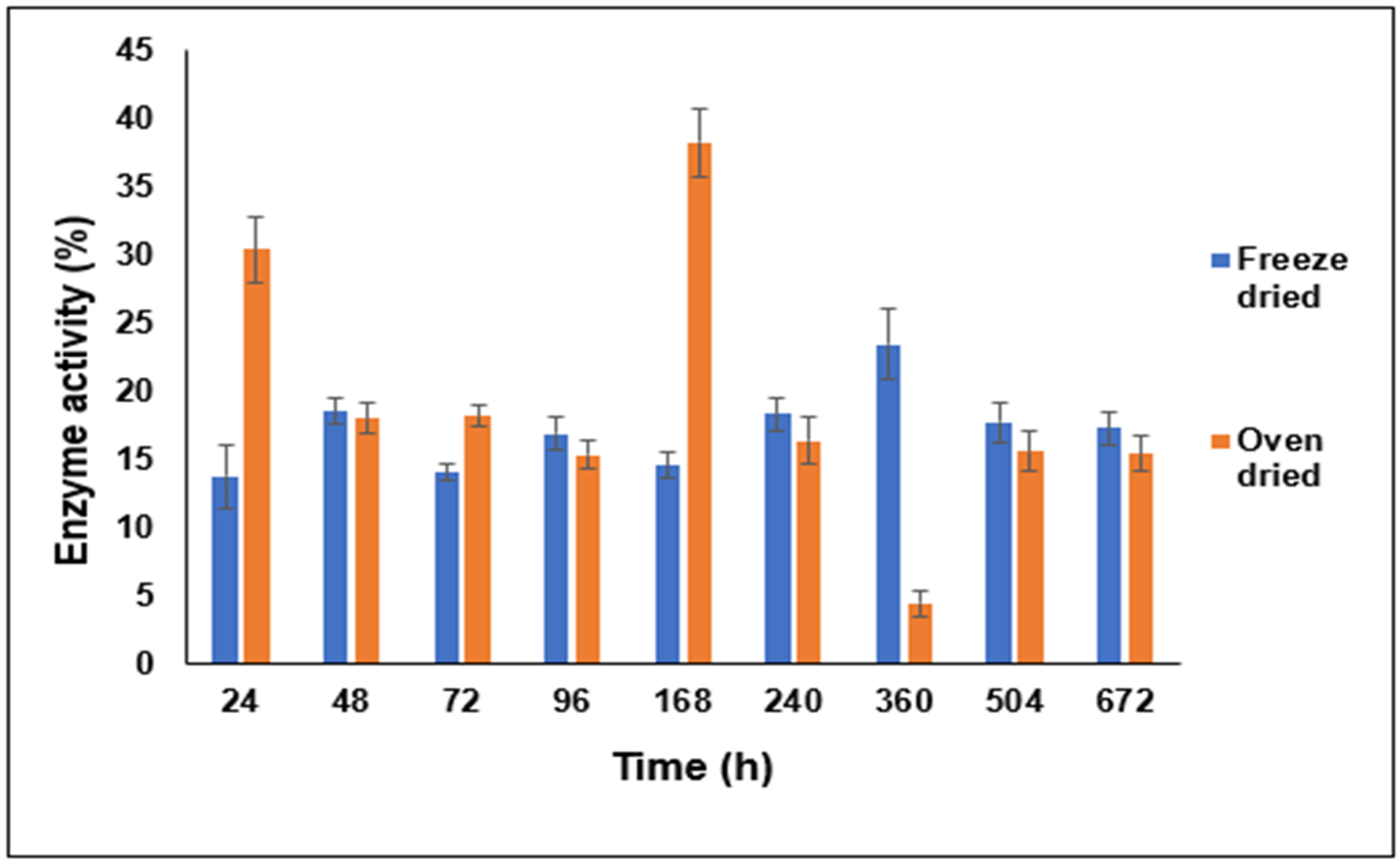
3.6. Effect of the Drying Process on the Characteristics of the LYZ/SDS HIP Complex
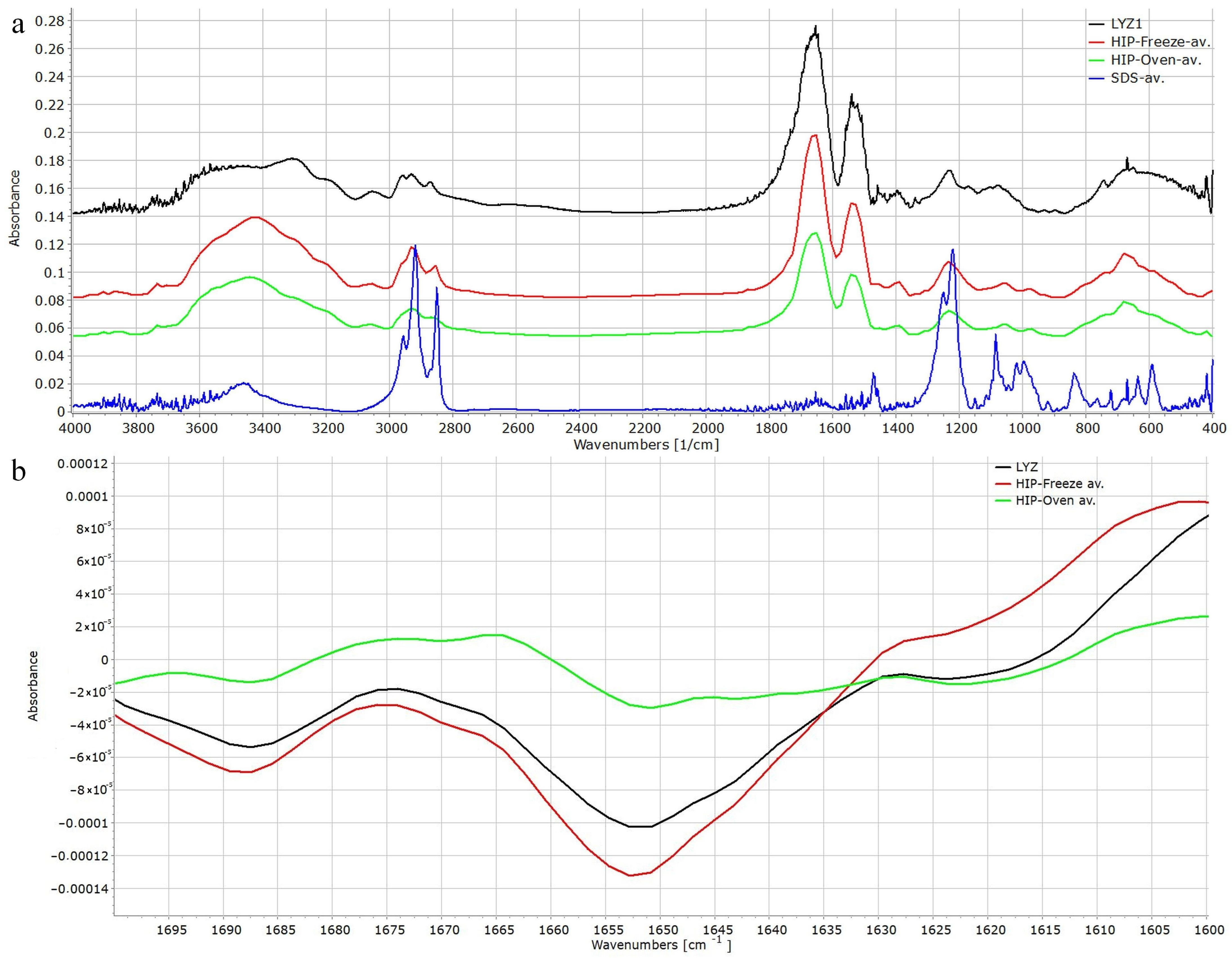
3.6.1. Fourier Transform Infrared Spectroscopy (FTIR) Analysis
3.6.2. Differential Scanning Calorimetry/Thermogravimetric Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Claus, V.; Sandmeier, M.; Hock, N.; Spleis, H.; Lindner, S.; Kalb, M.; Bernkop-Schnürch, A. Counterion Optimization for Hydrophobic Ion Pairing (HIP): Unraveling the Key Factors. Int. J. Pharm. 2023, 647, 123507. [Google Scholar] [CrossRef] [PubMed]
- Efiana, N.A.; Dizdarević, A.; Huck, C.W.; Bernkop-Schnürch, A. Improved Intestinal Mucus Permeation of Vancomycin via Incorporation into Nanocarrier Containing Papain-Palmitate. J. Pharm. Sci. 2019, 108, 3329–3339. [Google Scholar] [CrossRef] [PubMed]
- Gaudana, R.; Gokulgandhi, M.; Khurana, V.; Kwatra, D.; Mitra, A.K. Design and Evaluation of a Novel Nanoparticulate-Based Formulation Encapsulating a HIP Complex of Lysozyme. Pharm. Dev. Technol. 2013, 18, 752–759. [Google Scholar] [CrossRef] [PubMed]
- Kristó, K.; Manteghi, R.; Ibrahim, Y.H.-E.Y.; Ungor, D.; Csapó, E.; Berkesi, D.; Kónya, Z.; Csóka, I. Optimization of Layering Technique and Secondary Structure Analysis during the Formulation of Nanoparticles Containing Lysozyme by Quality by Design Approach. PLoS ONE 2021, 16, e0260603. [Google Scholar] [CrossRef] [PubMed]
- Ristroph, K.D.; Rummaneethorn, P.; Johnson-Weaver, B.; Staats, H.; Prud’homme, R.K. Highly-Loaded Protein Nanocarriers Prepared by Flash NanoPrecipitation with Hydrophobic Ion Pairing. Int. J. Pharm. 2021, 601, 120397. [Google Scholar] [CrossRef] [PubMed]
- Šahinović, M.; Hassan, A.; Kristó, K.; Regdon, G.; Vranić, E.; Sovány, T. Quality by Design-Based Development of Solid Self-Emulsifying Drug Delivery System (SEDDS) as a Potential Carrier for Oral Delivery of Lysozyme. Pharmaceutics 2023, 15, 995. [Google Scholar] [CrossRef] [PubMed]
- Asuman Bozkır, B.D. Design and Evaluation of Hydrophobic Ion-Pairing Complexation of Lysozyme with Sodium Dodecyl Sulfate for Improved Encapsulation of Hydrophilic Peptides/Proteins by Lipid-Polymer Hybrid Nanoparticles. J. Nanomed. Nanotechnol. 2015, 6, 1000259. [Google Scholar] [CrossRef]
- Asuman Bozkır, B.D. Preparation and Characterization of Protein-Loaded Lipid-Polymer Hybrid Nanoparticles with Polycaprolactone as Polymeric Core Material. J. Biomol. Res. Ther. 2014, 3, 2. [Google Scholar] [CrossRef]
- Kalhapure, R.S.; Mocktar, C.; Sikwal, D.R.; Sonawane, S.J.; Kathiravan, M.K.; Skelton, A.; Govender, T. Ion Pairing with Linoleic Acid Simultaneously Enhances Encapsulation Efficiency and Antibacterial Activity of Vancomycin in Solid Lipid Nanoparticles. Colloids Surf. B Biointerfaces 2014, 117, 303–311. [Google Scholar] [CrossRef]
- Nazir, I.; Asim, M.H.; Dizdarević, A.; Bernkop-Schnürch, A. Self-Emulsifying Drug Delivery Systems: Impact of Stability of Hydrophobic Ion Pairs on Drug Release. Int. J. Pharm. 2019, 561, 197–205. [Google Scholar] [CrossRef]
- Yoo, H.S.; Choi, H.; Park, T.G. Protein–Fatty Acid Complex for Enhanced Loading and Stability within Biodegradable Nanoparticles. J. Pharm. Sci. 2001, 90, 194–201. [Google Scholar] [CrossRef] [PubMed]
- Kwiecień, K.; Pudełko, I.; Knap, K.; Reczyńska-Kolman, K.; Krok-Borkowicz, M.; Ochońska, D.; Brzychczy-Włoch, M.; Pamuła, E. Insight in Superiority of the Hydrophobized Gentamycin in Terms of Antibiotics Delivery to Bone Tissue. Int. J. Mol. Sci. 2022, 23, 12077. [Google Scholar] [CrossRef] [PubMed]
- Rotman, S.G.; Thompson, K.; Grijpma, D.W.; Richards, R.G.; Moriarty, T.F.; Eglin, D.; Guillaume, O. Development of Bone Seeker–Functionalised Microspheres as a Targeted Local Antibiotic Delivery System for Bone Infections. J. Orthop. Transl. 2020, 21, 136–145. [Google Scholar] [CrossRef] [PubMed]
- Rotman, S.G.; Moriarty, T.F.; Nottelet, B.; Grijpma, D.W.; Eglin, D.; Guillaume, O. Poly(Aspartic Acid) Functionalized Poly(ϵ-Caprolactone) Microspheres with Enhanced Hydroxyapatite Affinity as Bone Targeting Antibiotic Carriers. Pharmaceutics 2020, 12, 885. [Google Scholar] [CrossRef] [PubMed]
- Ter Boo, G.; Grijpma, D.W.; Richards, R.; Moriarty, T.; Eglin, D. Preparation of Gentamicin Dioctyl Sulfosuccinate Loaded Poly (Trimethylene Carbonate) Matrices Intended for the Treatment of Orthopaedic Infections. Clin. Hemorheol. Microcirc. 2015, 60, 89–98. [Google Scholar] [CrossRef] [PubMed]
- Duan, J.; Kim, K.; Daeschel, M.A.; Zhao, Y. Storability of Antimicrobial Chitosan-Lysozyme Composite Coating and Film-Forming Solutions. J. Food Sci. 2008, 73, M321–M329. [Google Scholar] [CrossRef] [PubMed]
- Mohammad, M.A.; Grimsey, I.M.; Forbes, R.T. Mapping the Solid-State Properties of Crystalline Lysozyme during Pharmaceutical Unit-Operations. J. Pharm. Biomed. Anal. 2015, 114, 176–183. [Google Scholar] [CrossRef] [PubMed]
- Oderinde, B.; Agbede, O.; Iheukwumere, I.; Ghamba, P.; Medugu, J.; Oku, E. Antiviral Activity of Hen Egg-White Lysozyme on Polio Virus. Sokoto J. Med. Lab. Sci. 2017, 2, 109–120. [Google Scholar]
- Oliver, W.T.; Wells, J.E. Lysozyme as an Alternative to Growth Promoting Antibiotics in Swine Production. J. Anim. Sci. Biotechnol. 2015, 6, 35. [Google Scholar] [CrossRef]
- Park, S.; Daeschel, M.; Zhao, Y. Functional Properties of Antimicrobial Lysozyme-chitosan Composite Films. J. Food Sci. 2004, 69, M215–M221. [Google Scholar] [CrossRef]
- Pawar, S.; Raul, K.; Ottoor, D. Investigation of Complexation of Amlodipine with Lysozyme and Its Effect on Lysozyme Crystal Growth. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2020, 227, 117623. [Google Scholar] [CrossRef] [PubMed]
- Devrim, B.; Kara, A.; Vural, İ.; Bozkır, A. Lysozyme-Loaded Lipid-Polymer Hybrid Nanoparticles: Preparation, Characterization and Colloidal Stability Evaluation. Drug Dev. Ind. Pharm. 2016, 42, 1865–1876. [Google Scholar] [CrossRef] [PubMed]
- Vallaster, B.; Engelsing, F.; Grohganz, H. Influence of Water and Trehalose on α- and β-Relaxation of Freeze-Dried Lysozyme Formulations. Eur. J. Pharm. Biopharm. 2024, 194, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Hegg, P.-O. Precipitation of Egg White Proteins below Their Isoelectric Points by Sodium Dodecyl Sulphate and Temperature. Biochim. Biophys. Acta (BBA) Protein Struct. 1979, 579, 73–87. [Google Scholar] [CrossRef]
- ICH. ICH Harmonised Tripartite Guideline: Pharmaceutical Development Q8; ICH: Geneva, Switzerland, 2009; pp. 1–28. [Google Scholar]
- ICH. ICH Harmonised Tripartite Guideline: Pharmaceutical Quality System Q10; ICH: Geneva, Switzerland, 2008; pp. 1–21. [Google Scholar]
- ICH Quality Risk Management Q9. In ICH Harmonized Tripartite Guideline; ICH: Geneva, Switzerland, 2005; pp. 1–23.
- Sipos, B.; Szabó-Révész, P.; Csóka, I.; Pallagi, E.; Dobó, D.G.; Bélteky, P.; Kónya, Z.; Deák, Á.; Janovák, L.; Katona, G. Quality by Design Based Formulation Study of Meloxicam-Loaded Polymeric Micelles for Intranasal Administration. Pharmaceutics 2020, 12, 697. [Google Scholar] [CrossRef] [PubMed]
- Shugar, D. The Measurement of Lysozyme Activity and the Ultra-Violet Inactivation of Lysozyme. Biochim. Biophys. Acta 1952, 8, 302–309. [Google Scholar] [CrossRef] [PubMed]
- Hou, H.; He, H.; Wang, Y. Effects of SDS on the Activity and Conformation of Protein Tyrosine Phosphatase from Thermus Thermophilus HB27. Sci. Rep. 2020, 10, 3195. [Google Scholar] [CrossRef] [PubMed]
- Imoto, T.; Sumi, S.; Tsuru, M.; Yagishita, K. An Analysis of the Interaction of Sodium Dodecyl Sulfate with Lysozyme. Agric. Biol. Chem. 1979, 43, 1809–1815. [Google Scholar]
- Jafari, M.; Mehrnejad, F.; Rahimi, F.; Asghari, S.M. The Molecular Basis of the Sodium Dodecyl Sulfate Effect on Human Ubiquitin Structure: A Molecular Dynamics Simulation Study. Sci. Rep. 2018, 8, 2150. [Google Scholar] [CrossRef]
- Kang, B.; Tang, H.; Zhao, Z.; Song, S. Hofmeister Series: Insights of Ion Specificity from Amphiphilic Assembly and Interface Property. ACS Omega 2020, 5, 6229–6239. [Google Scholar] [CrossRef]
- Yang, H.; Yang, S.; Kong, J.; Dong, A.; Yu, S. Obtaining Information about Protein Secondary Structures in Aqueous Solution Using Fourier Transform IR Spectroscopy. Nat. Protoc. 2015, 10, 382–396. [Google Scholar] [CrossRef]
- Gedawy, A.; Luna, G.; Martinez, J.; Brown, D.; Al-Salami, H.; Dass, C.R. Novel Silicone-Grafted Alginate as a Drug Delivery Scaffold: Pharmaceutical Characterization of Gliclazide-Loaded Silicone-Based Composite Microcapsules. Pharmaceutics 2023, 15, 530. [Google Scholar] [CrossRef]
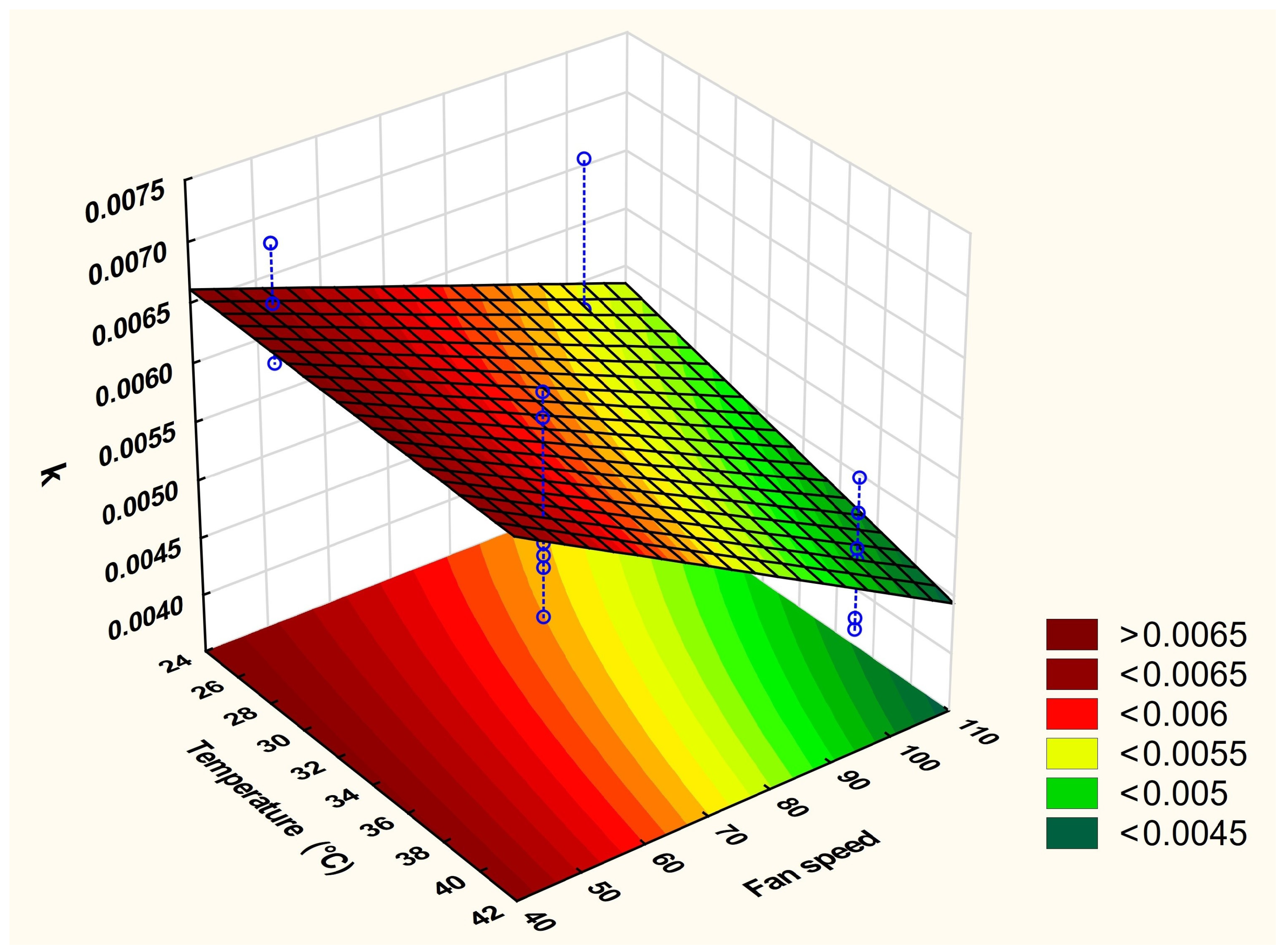

| Run | Temperature (°C) (x1) | Fan Speed * (x2) |
|---|---|---|
| 1 | 25 | 50 |
| 2 | 40 | 50 |
| 3 | 25 | 100 |
| 4 | 40 | 100 |
| QTPP | Target | Justification | CQAs | Target | Justification |
|---|---|---|---|---|---|
| Lipophilicity | Enhanced lipophilicity | Important to enhance the encapsulation of peptides and proteins in lipophilic micro/nanocarriers. Help in the protection of hydrophilic macromolecules from the destructive effect of organic solvents used during the preparation of micro/nanocarriers [5]. | Complexation efficiency | High | To increase the yield of the complexed peptide and consequently the efficiency of the process [11]. |
| Complex stability | Maximum, During preparation and storage | To achieve high encapsulation efficiency in hydrophobic colloidal carriers and reduce losses [5]. | |||
| Biological activity | The maximum possible preserved biological activity | Preserving the secondary and tertiary structures of peptides and proteins is crucial for their biological activity [7]. | Preserving the secondary structure of the peptide | Maximum possible enzymatic activity | It is crucial to achieve biological activity [7]. |
| Reversibility | Reversible dissociation upon contact with the biological fluids | Dissociation of the complex in the biological fluids is important to release peptide/protein to be biologically available [3]. | Recovery (dissociation of the complex) | Maximum dissociation of the peptide | To obtain a free form of the peptide in biological fluids for therapeutic activity [3]. |
| Ingredients | Weight Loss (%) (Heating from 25 °C to 500 °C) | DSC Thermogram Peaks |
|---|---|---|
| SDS | 66.54 | Endothermic dehydration peak at 109.67 °C |
| Sharp endothermic peak at 199.48 °C (melting point) | ||
| Decomposition peaks at 219.34 (endothermic), 238.86 °C (exothermic) and 275.36 °C (endothermic) | ||
| LYZ | 65.42 | Broad endothermic peak from 38.72 °C to 145.45 °C due to water removal |
| Endothermic peak at 201.59 °C due to denaturation (Tm) | ||
| Endothermic decomposition peak at 307.74 °C | ||
| Oven-dried HIP | 58.93 | Broad endothermic peak at 105.40 °C due to water removal |
| Endothermic peak at 196.76 °C | ||
| Decomposition peak at 277.29 °C | ||
| Freeze-dried HIP | 72.23 | Broad endothermic peak at 102.60 °C due to water removal |
| Endothermic peak at 207.72 °C | ||
| Endothermic decomposition peak at 276.43 °C |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hassan, A.A.A.; Sovány, T.; Pamlényi, K.; Deák, M.; Hornok, V.; Csapó, E.; Regdon, G., Jr.; Csóka, I.; Kristó, K. QbD Approach-Based Preparation and Optimization of Hydrophobic Ion-Pairing Complex of Lysozyme with Sodium Dodecyl Sulphate to Enhance Stability in Lipid-Based Carriers. Pharmaceutics 2024, 16, 589. https://doi.org/10.3390/pharmaceutics16050589
Hassan AAA, Sovány T, Pamlényi K, Deák M, Hornok V, Csapó E, Regdon G Jr., Csóka I, Kristó K. QbD Approach-Based Preparation and Optimization of Hydrophobic Ion-Pairing Complex of Lysozyme with Sodium Dodecyl Sulphate to Enhance Stability in Lipid-Based Carriers. Pharmaceutics. 2024; 16(5):589. https://doi.org/10.3390/pharmaceutics16050589
Chicago/Turabian StyleHassan, Alharith A. A., Tamás Sovány, Krisztián Pamlényi, Martin Deák, Viktória Hornok, Edit Csapó, Géza Regdon, Jr., Ildikó Csóka, and Katalin Kristó. 2024. "QbD Approach-Based Preparation and Optimization of Hydrophobic Ion-Pairing Complex of Lysozyme with Sodium Dodecyl Sulphate to Enhance Stability in Lipid-Based Carriers" Pharmaceutics 16, no. 5: 589. https://doi.org/10.3390/pharmaceutics16050589





