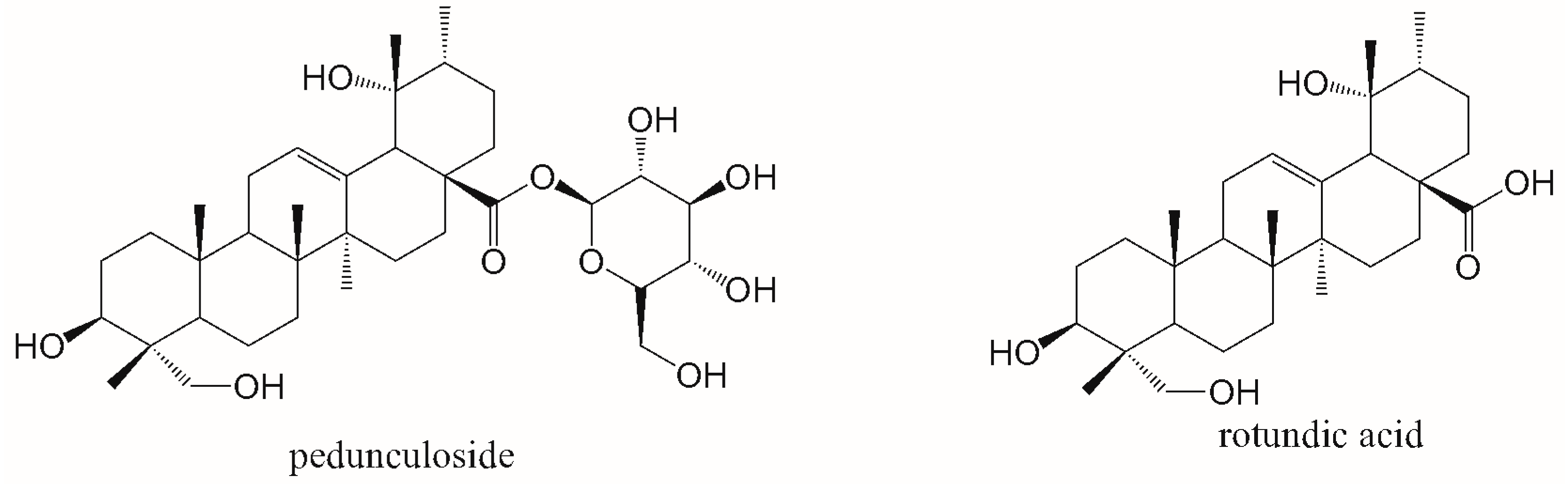
In Vitro Stability and Pharmacokinetic Study of Pedunculoside and Its Beta-CD Polymer Inclusion Complex
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals and Materials
2.2. Chromatography and Mass Spectrometry Conditions
2.3. Sample Preparation
2.4. In Vitro Stability Study of Pedunculoside and Pedunculoside–βCDP in the Gastrointestinal Environment
2.5. Simultaneous Determination of Pedunculoside and Rotundic Acid in Rat Plasma—Sample Pretreatment
2.6. Simultaneous Determination of Pedunculoside and Rotundic Acid in Rat Plasma—Method Validation
2.7. Pharmacokinetic Behavior Study of Pedunculoside after Pedunculoside or Pedunculoside–βCDP Was Intravenously Injected to Rats
2.8. Data Process
3. Results
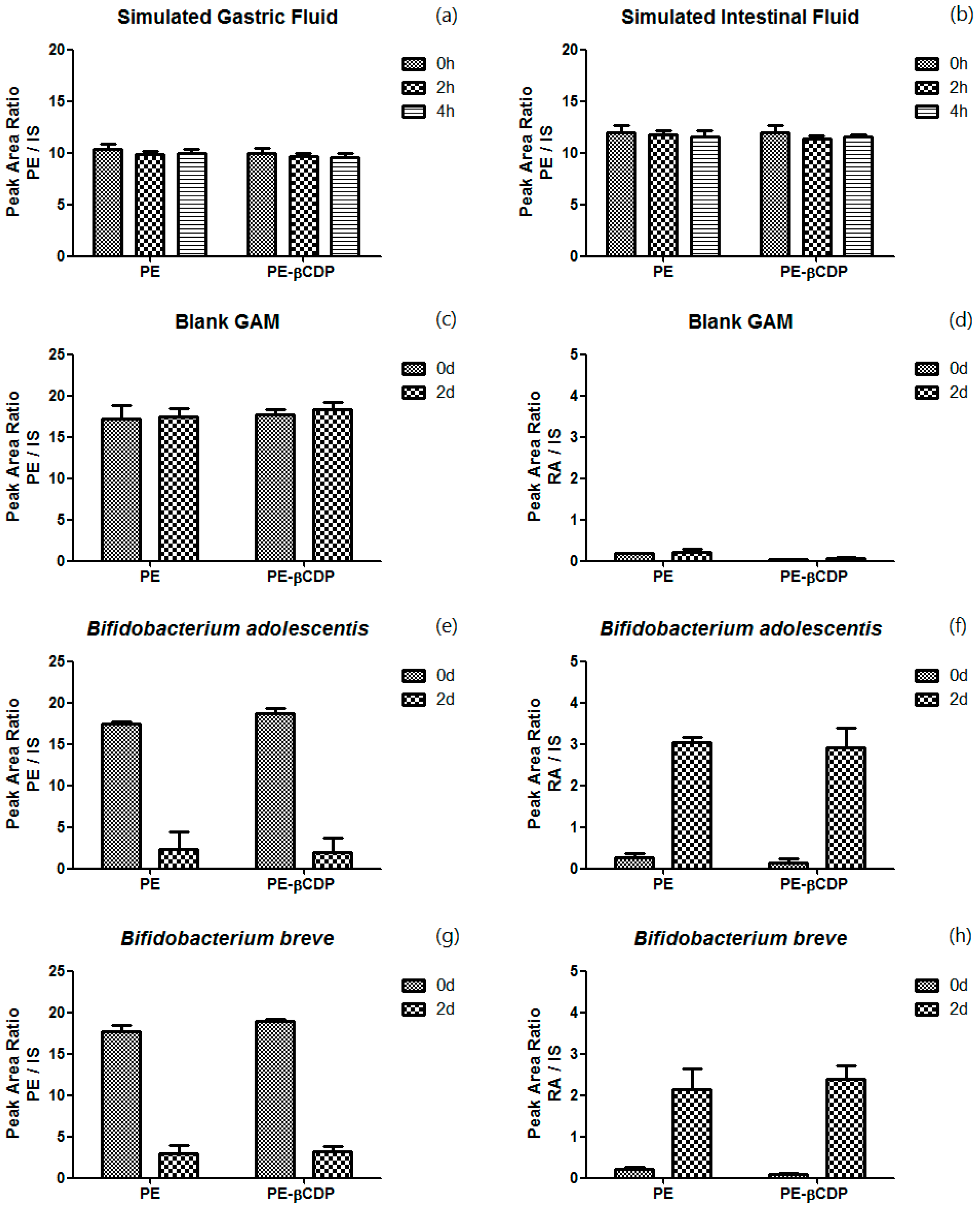
3.1. In Vitro Stability Study of Pedunculoside and Pedunculoside–βCDP in the Gastrointestinal Environment
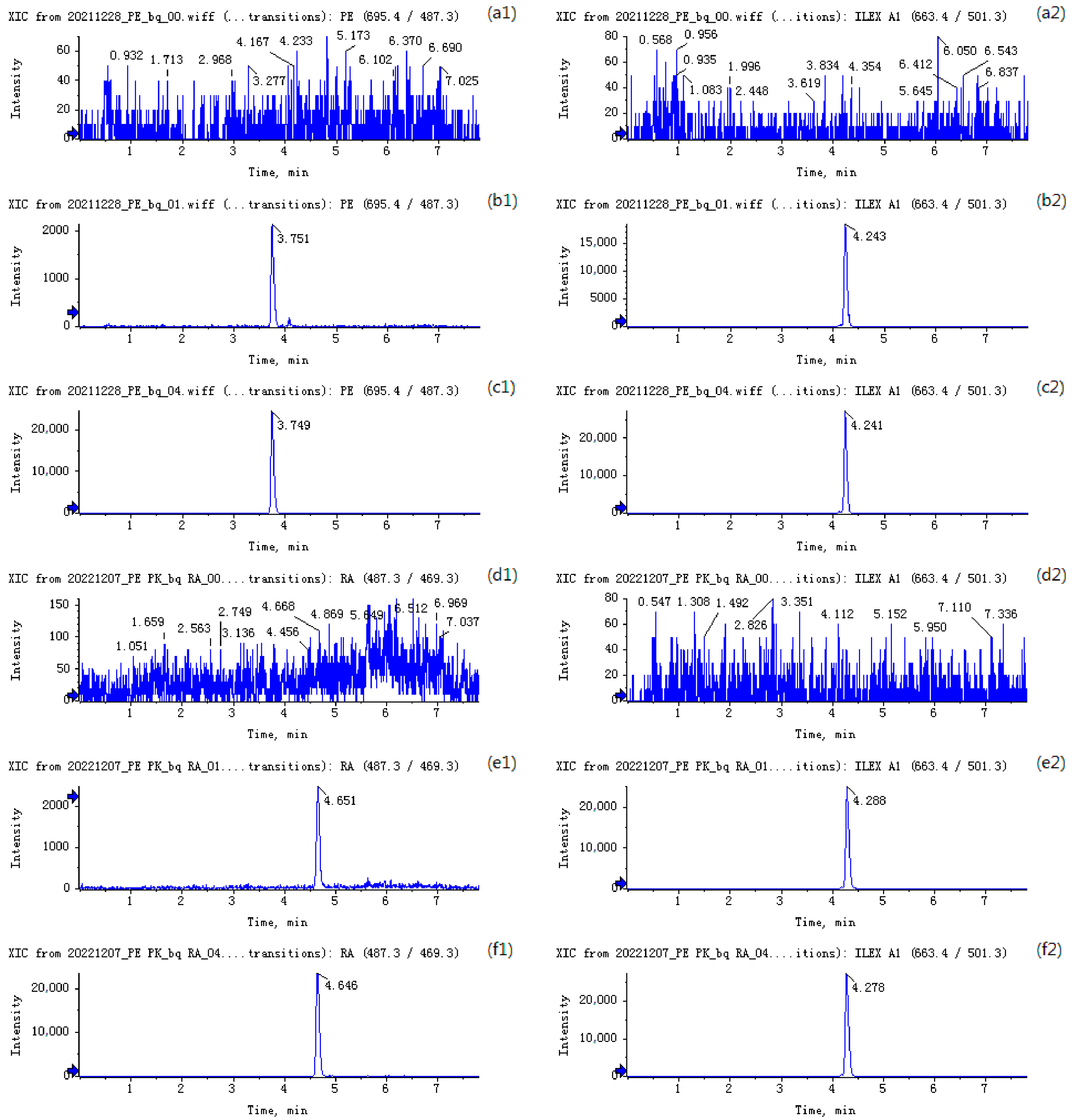
3.2. Simultaneous Determination of Pedunculoside and Rotundic Acid in Rat Plasma—Method Validation
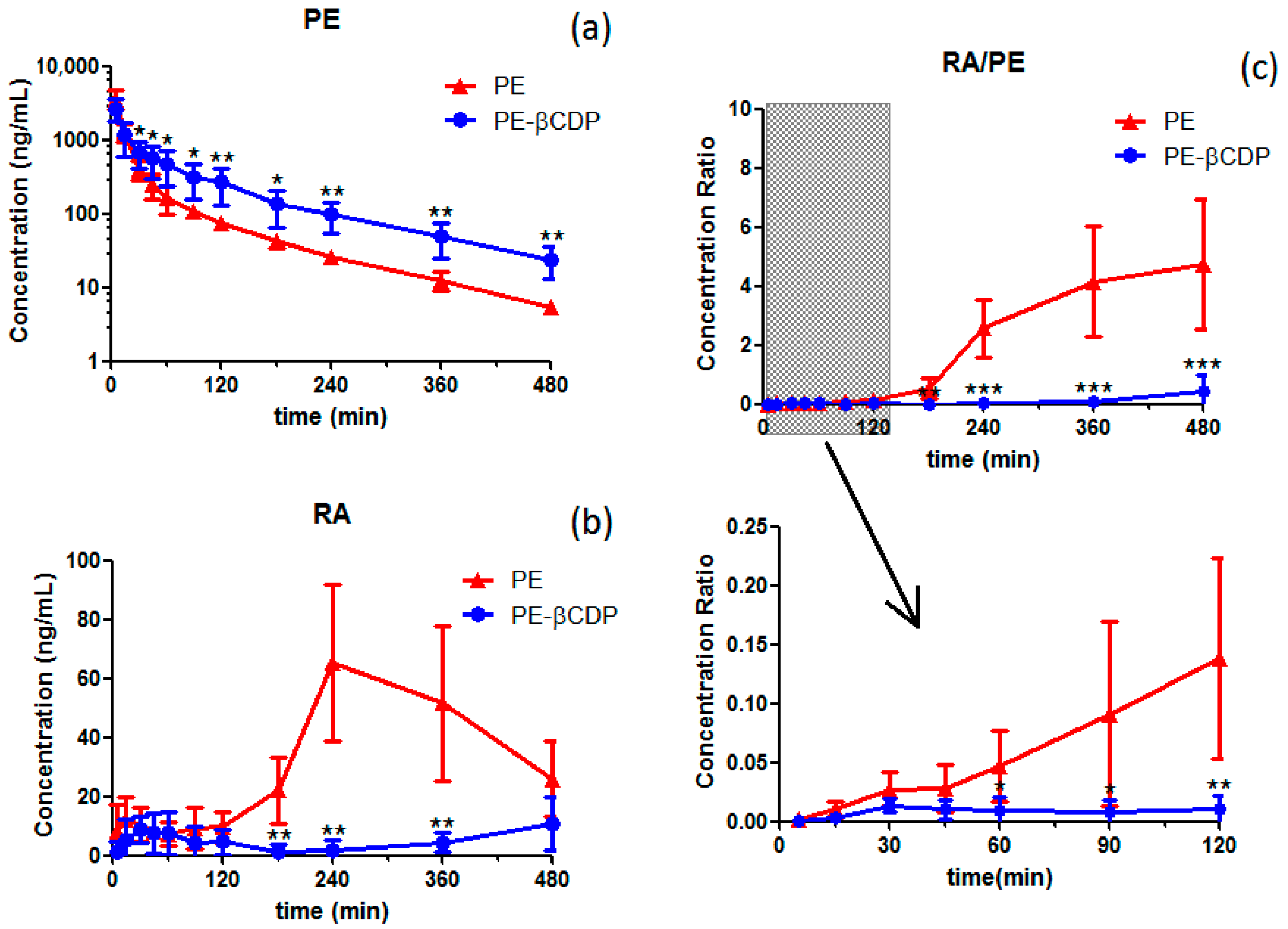
3.3. Pharmacokinetic Behavior Study of Pedunculoside after Pedunculoside or Pedunculoside–βCDP Was Intravenously Injected to Rats
4. Discussion
4.1. In Vitro Stability Study of Pedunculoside and Pedunculoside–βCDP in the Gastrointestinal Environment
4.2. Simultaneous Determination of Pedunculoside and Rotundic Acid in Rat Plasma—Method Validation
4.3. Pharmacokinetic Behavior Study of Pedunculoside after Pedunculoside or Pedunculoside–βCDP Was Intravenously Injected to Rats
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Yi, F.; Zhao, X.-L.; Peng, Y.; Xiao, P.-G. Genus Ilex L.: Phytochemistry, Ethnopharmacology, and Pharmacology. Chin. Herb. Med. 2016, 8, 209–230. [Google Scholar]
- Yang, B.; Li, H.; Ruan, Q.-F.; Xue, Y.-Y.; Cao, D.; Zhou, X.-H.; Jiang, S.-Q.; Yi, T.; Jin, J.; Zhao, Z.-X. A facile and selective approach to the qualitative and quantitative analysis of triterpenoids and phenylpropanoids by UPLC/Q-TOF-MS/MS for the quality control of Ilex rotunda. J. Pharm. Biomed. Anal. 2018, 157, 44–58. [Google Scholar] [CrossRef]
- Jiang, S.-Q.; Cui, H.; Wu, P.; Liu, Z.-Q.; Zhao, Z.-X. Botany, traditional uses, phytochemistry, pharmacology and toxicology of Ilex pubescens Hook et Arn. J. Ethnopharmacol. 2019, 245, 112147. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.-X.; Zhang, Y.-H. Study on the effect of the presence of pedunculoside on myocardial ischemia in rats. Smart Healthc. 2017, 3, 53–55. [Google Scholar]
- Yang, A.-P.; Zhang, Q.-C.; Song, Y.; Yang, M.; Wang, J.-Y.; Wang, C.; Hu, L.-H.; Wang, X.-C. Study on protective effect of pedunculoside on acute myocardial ischemia/reperfusion injury in rats. Chin. Pharmacol. Bull. 2021, 37, 484–489. [Google Scholar]
- Rong, Y.; Shen, Y.-J.; Wang, J.; Zhang, Y.-Q.; Fan, Y.; Sun, Y.; Sun, W.-D. The effects of pedunculoside on the liver injury in mice and the gastrocnemius damage in rat. J. Yangzhou Univ. 2016, 37, 24–29. [Google Scholar]
- Liu, C.; Shen, Y.-J.; Tu, Q.-B.; Zhao, Y.-R.; Guo, H.; Wang, J.; Zhang, L.; Shi, H.-W.; Sun, Y. Pedunculoside, a novel triterpene saponin extracted from Ilex rotunda, ameliorates high-fat diet induced hyperlipidemia in rats. Biomed. Pharmacother. 2018, 101, 608–616. [Google Scholar] [CrossRef]
- Ma, X.; Chen, G.; Wang, J.; Xu, J.; Zhao, F.; Hu, M.; Xu, Z.; Yang, B.; Guo, J.; Sun, S.; et al. Pedunculoside attenuates pathological phenotypes of fibroblast-like synoviocytes and protects against collagen-induced arthritis. Scand. J. Rheumatol. 2019, 48, 383–392. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Cheng, B.; Song, N.; Wu, Y.-L.; Li, Y.-W. Effect of pedunculoside on MiR-29a/TET3 and STAT3 in mice with colitis-associated colorectal cancer (CAC). Pharmacol. Clin. Chin. Materia Med. 2019, 35, 39–42. [Google Scholar]
- Liu, K.-J.; Li, G.-F.; Guo, W.-J.; Zhang, J. The protective effect and mechanism of pedunculoside on DSS (dextran sulfate sodium) induced ulcerative colitis in mice. Int. Immunopharmacol. 2020, 88, 107017. [Google Scholar] [CrossRef]
- Zhao, W.-O.; Pang, L.; Xu, D.-H.; Zhang, N. LC-MS/MS determination and pharmacokinetic study of pedunculoside in rat plasma after oral administration of pedunculoside and Ilex rotunda extract. Molecules 2015, 20, 9084–9098. [Google Scholar] [CrossRef] [PubMed]
- Yang, A.-P.; Dong, J.-J.; Zhao, H.-M.; Zhang, Q.-C.; Zhu, X.-Y.; Gao, L.-N.; Ding, N.; Li, C.-H.; Peng, R.; Lu, T.-L.; et al. Quantification and pharmacokinetics study of pedunculoside in rats by using UPLC-MS/MS. Curr. Pharm. Anal. 2021, 17, 731–737. [Google Scholar] [CrossRef]
- Cao, D.; Fan, Z.; Zhu, J.-P.; Yang, B.; Zhou, L.; Jin, J.; Zhao, Z.-X. Effect of rat intestinal bacteria on metabolism of pedunculoside in vitro. China Pharm. 2016, 19, 621–624. [Google Scholar]
- Wu, L.; Kang, A.; Shan, C.-X.; Chai, C.; Zhou, Z.; Lin, Y.-J.; Bian, Y.-Y. LC-Q-TOF/MS-oriented systemic metabolism study of pedunculoside with in vitro and in vivo biotransformation. J. Pharm. Biomed. Anal. 2019, 175, 112762. [Google Scholar] [CrossRef] [PubMed]
- Yang, B.; Li, H.; Ruan, Q.-F.; Xuan, S.-X.; Chen, X.-J.; Cui, H.; Liu, Z.-Q.; Jin, J.; Zhao, Z.-X. Effects of gut microbiota and ingredient-ingredient interaction on the pharmacokinetic properties of rotundic acid and pedunculoside. Planta Med. 2019, 85, 729–737. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.; Zhang, W.; Yang, H.; Sun, W.-D.; Gong, X.-D.; Zhao, J.-X.; Sun, Y.; Diao, G.-W. A water-soluble inclusion complex of pedunculoside with the polymer β-cyclodextrin: A novel anti-inflammation agent with low toxicity. PLoS ONE 2014, 9, e101761. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Gong, X.-D.; Cai, Y.; Zhang, C.-L.; Yu, X.; Fan, J.; Diao, G.-W. Investigation of water-soluble inclusion complex of hypericin with β-cyclodextrin polymer. Carbohydr. Polym. 2013, 95, 366–370. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.; Zhang, W.; Wang, Q.; Sun, Y.; Diao, G.-W. The water-soluble inclusion complex of ilexgenin A with β-cyclodextrin polymer-a novel lipid-lowering drug candidate. Org. Biomol. Chem. 2013, 11, 4993–4999. [Google Scholar] [CrossRef]
- Zhang, W.; Xiao, P.; Lin, L.-W.; Guo, F.; Wang, Q.-Y.; Piao, Y.-Z.; Diao, G.-W. Study of a water-soluble supramolecular complex of curcumin and β-cyclodextrin polymer with electrochemical property and potential anti-cancer activity. Chin. Chem. Lett. 2022, 33, 4043–4047. [Google Scholar] [CrossRef]
- Chen, W.-J.; Zheng, X.-W.; Lao, W.-X.; Wang, H.-X.; Chen, S.-F.; Liu, C.-Y.; Chen, Z.-S.; Bai, Y.-S.; Zhang, H.; Zhan, X.-S.; et al. Enhancement of the solubility and oral bioavailability of altrenogest through complexation with hydroxypropyl-β-cyclodextrin. Eur. J. Pharm. Sci. 2024, 194, 106691. [Google Scholar] [CrossRef]
- Ding, Y.-L.; Cui, W.-T.; Zhang, Z.-Y.; Ma, Y.-Z.; Ding, C.; Lin, Y.-K.; Xu, Z. Solubility and Pharmacokinetic Profile Improvement of Griseofulvin through Supercritical Carbon Dioxide-Assisted Complexation with HP-γ-Cyclodextrin. Molecules 2023, 28, 7360. [Google Scholar] [CrossRef] [PubMed]
- Zhao, W.-Z.; Ju, W.-Z.; Zang, Y.-X.; Sun, B.-T.; Tan, H.-S. Pharmacokinetics of pedunculosid in rat. Chin. J. New Drugs Clin. Rem. 2016, 35, 46–49. [Google Scholar]
- Liu, H.; Guo, S.-L.; Wei, S.-J.; Liu, J.-Y.; Tian, B.-R. Pharmacokinetics and pharmacodynamics of cyclodextrin-based oral drug delivery formulations for disease therapy. Carbohydr. Polym. 2024, 329, 121763. [Google Scholar] [CrossRef] [PubMed]
- Sarabia-Vallejo, Á.; Caja MD, M.; Olives, A.-I.; Martín, M.-A.; Menéndez, J.-C. Cyclodextrin Inclusion Complexes for Improved Drug Bioavailability and Activity: Synthetic and Analytical Aspects. Pharmaceutics 2023, 15, 2345. [Google Scholar] [CrossRef]
- Zhang, W.; Zheng, Z.-Q.; Lin, L.-W.; Zhang, X.; Bae, M.; Lee, J.; Xie, J.; Diao, G.-W.; Im, H.-J.; Piao, Y.-Z.; et al. Ultrafast Synthesis of Graphene-Embedded Cyclodextrin-Metal-Organic Framework for Supramolecular Selective Absorbency and Supercapacitor Performance. Adv. Sci. 2023, 10, 2304062. [Google Scholar] [CrossRef]
| Spiked Concentration (ng/mL) | Intra-Day (D1, n = 6) | Intra-Day (D2, n = 6) | Intra-Day (D3, n = 6) | Inter-Day (n = 3) | |||||
|---|---|---|---|---|---|---|---|---|---|
| Measured Concentration (ng/mL) | RSD | Measured Concentration (ng/mL) | RSD | Measured Concentration (ng/mL) | RSD | Measured Concentration (ng/mL) | RSD | ||
| 5 | 4.95 ± 0.38 | 7.71% | 5.37 ± 0.36 | 6.72% | 5.26 ± 0.27 | 5.12% | 5.19 ± 0.22 | 4.26% | |
| Pedunculoside | 50 | 53.15 ± 3.11 | 5.85% | 54.47 ± 2.32 | 4.27% | 54.35 ± 1.27 | 2.34% | 53.99 ± 0.73 | 1.35% |
| 500 | 516.83 ± 17.33 | 3.35% | 519.20 ± 27.02 | 5.20% | 522.34 ± 16.89 | 3.23% | 519.46 ± 2.76 | 0.53% | |
| 10 | 9.29 ± 0.73 | 7.86% | 11.08 ± 0.50 | 4.48% | 10.28 ± 0.92 | 8.99% | 10.22 ± 0.89 | 8.75% | |
| Rotundic acid | 100 | 110.78 ± 4.51 | 4.07% | 104.09 ± 6.66 | 6.40% | 107.54 ± 3.53 | 3.28% | 107.47 ± 3.35 | 3.11% |
| 500 | 475.81 ± 23.51 | 4.94% | 542.05 ± 26.07 | 4.81% | 486.70 ± 25.20 | 5.18% | 501.52 ± 35.52 | 7.08% | |
| Spiked Concentration (ng/mL) | Matrix Effect (%) | Recovery (%) | Stability (%) | |||||
|---|---|---|---|---|---|---|---|---|
| 24 h in Autosampler | 4 h at Room Temperature | 3 Freeze–Thaw Cycles | Frozen Storage for One Week | |||||
| Mean | 107.70 | 98.57 | 96.97 | 92.30 | 99.66 | 95.57 | ||
| 5 | SD | 7.89 | 9.03 | 7.80 | 4.15 | 8.70 | 3.04 | |
| RSD | 7.32 | 9.16 | 8.05 | 4.49 | 8.73 | 3.18 | ||
| Mean | 102.53 | 104.25 | 106.76 | 106.22 | 100.97 | 98.01 | ||
| Pedunculoside | 50 | SD | 6.28 | 2.35 | 3.62 | 4.30 | 4.60 | 2.81 |
| RSD | 6.13 | 2.25 | 3.40 | 4.05 | 4.55 | 2.86 | ||
| Mean | 96.42 | 103.56 | 103.17 | 96.34 | 100.80 | 97.30 | ||
| 500 | SD | 4.61 | 5.01 | 1.96 | 3.07 | 4.37 | 2.49 | |
| RSD | 4.78 | 4.84 | 1.90 | 3.18 | 4.34 | 2.56 | ||
| Mean | 107.70 | 111.10 | 108.09 | 102.09 | 99.09 | 100.17 | ||
| 10 | SD | 9.82 | 8.55 | 6.95 | 7.24 | 11.12 | 2.91 | |
| RSD | 9.12 | 7.70 | 6.43 | 7.10 | 11.22 | 2.90 | ||
| Mean | 104.54 | 98.25 | 105.08 | 99.85 | 98.29 | 92.85 | ||
| Rotundic acid | 50 | SD | 3.24 | 2.71 | 9.29 | 5.34 | 6.80 | 3.41 |
| RSD | 3.10 | 2.76 | 8.85 | 5.35 | 6.92 | 3.67 | ||
| Mean | 108.70 | 100.15 | 96.09 | 95.03 | 94.84 | 103.39 | ||
| 500 | SD | 4.55 | 4.12 | 3.13 | 4.42 | 5.97 | 5.69 | |
| RSD | 4.19 | 4.12 | 3.26 | 4.65 | 6.29 | 5.50 | ||
| Parameter | Parameter Given from the Software | Unit | Pedunculoside | Pedunculoside–βCDP |
|---|---|---|---|---|
| k | Lambda_z | 1/min | 0.007126 ± 0.0012735 | 0.0057647 ± 0.0015335 |
| t1/2 | HL_Lambda_z | min | 99.968 ± 18.284 | 129.641 ± 44.217 |
| C480min | Clast | ng/mL | 5.488 ± 1.037 | 24.113 ± 11.415 ** |
| AUC0→t | AUClast | min·ng/mL | 81107.642 ± 12835.326 | 116598.701 ± 47591.249 |
| AUC0→∞ | AUCINF_obs | min·ng/mL | 81914.111 ± 12666.038 | 121065.16 ± 48490.659 |
| AUCt→∞/AUC0→∞ | AUC_%Extrap_obs | % | 1.031 ± 0.492 | 3.993 ± 2.011 |
| CL | Cl_obs | ml/min/kg | 62.194 ± 9.033 | 49.214 ± 24.335 |
| MRT | MRTINF_obs | min | 48.598 ± 8.362 | 112.004 ± 12.419 *** |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wu, L.; Li, D.; Wang, P.; Dong, L.; Zhang, W.; Xu, J.; Jin, X. In Vitro Stability and Pharmacokinetic Study of Pedunculoside and Its Beta-CD Polymer Inclusion Complex. Pharmaceutics 2024, 16, 591. https://doi.org/10.3390/pharmaceutics16050591
Wu L, Li D, Wang P, Dong L, Zhang W, Xu J, Jin X. In Vitro Stability and Pharmacokinetic Study of Pedunculoside and Its Beta-CD Polymer Inclusion Complex. Pharmaceutics. 2024; 16(5):591. https://doi.org/10.3390/pharmaceutics16050591
Chicago/Turabian StyleWu, Liang, Danfeng Li, Peijing Wang, Linling Dong, Wang Zhang, Jianjun Xu, and Xiaoliang Jin. 2024. "In Vitro Stability and Pharmacokinetic Study of Pedunculoside and Its Beta-CD Polymer Inclusion Complex" Pharmaceutics 16, no. 5: 591. https://doi.org/10.3390/pharmaceutics16050591





