Mathematical Model to Predict Skin Concentration after Topical Application of Drugs
Abstract
:1. Introduction
2. Theoretical
2.1. One-Layered Diffusion Model
2.1.1. Determination of Membrane Concentration
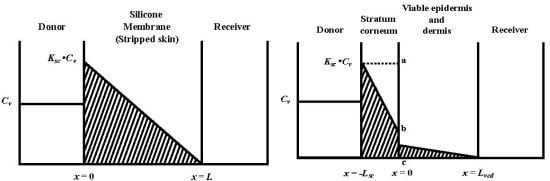
 , under the assumption that the membrane is a homogeneous single layer, where C is the penetrant concentration in the membrane at position, x, and time, t [25,26,27]. When a sink condition is assumed on the receiver side of the membrane, i.e., x = L, a set of initial conditions (C = 0 at t = 0 and 0 < x < L) and boundary conditions (C = KCv at x = 0 and C = 0 at x = L, where K is the partition coefficient of the penetrant from the vehicle to membrane and Cv is the penetrant concentration in the vehicle) are obtained (see Figure 1a).
, under the assumption that the membrane is a homogeneous single layer, where C is the penetrant concentration in the membrane at position, x, and time, t [25,26,27]. When a sink condition is assumed on the receiver side of the membrane, i.e., x = L, a set of initial conditions (C = 0 at t = 0 and 0 < x < L) and boundary conditions (C = KCv at x = 0 and C = 0 at x = L, where K is the partition coefficient of the penetrant from the vehicle to membrane and Cv is the penetrant concentration in the vehicle) are obtained (see Figure 1a).




2.1.2. Determination of Membrane Permeation

 .
.2.2. Two-Layered Diffusion Model
2.2.1. Determination of Membrane Concentration Using Two-Layered Diffusion Model
 , where Ksc and Kved are partition coefficients of the penetrant from the vehicle to stratum corneum and to the viable epidermis and dermis, and Dsc and Dved are diffusion coefficients in the stratum corneum and viable epidermis and dermis, respectively.
, where Ksc and Kved are partition coefficients of the penetrant from the vehicle to stratum corneum and to the viable epidermis and dermis, and Dsc and Dved are diffusion coefficients in the stratum corneum and viable epidermis and dermis, respectively.







2.2.2. Determination of Membrane Permeation Using Two-Layered Diffusion Model


2.3. Calculation of Permeation Parameters
2.4. Calculation of Theoretical Membrane Concentration of Parabens
3. Materials and Methods
3.1. Reagents and Materials
3.2. Experimental Animals
3.3. Membrane Permeation Experiments of Parabens

3.4. Determination of Extraction Ratio of Parabens
3.4.1. Silicone Membrane
3.4.2. Hairless Rat Skin
3.5. Determination of Paraben Concentration in Silicone Membrane and Hairless Rat Skin
3.5.1. Silicone Membrane
3.5.2. Hairless Rat Skin
3.6. Determination Methods of Parabens
4. Results and Discussions
4.1. Partition Coefficient and Concentration of Parabens into and in the Silicone Membrane


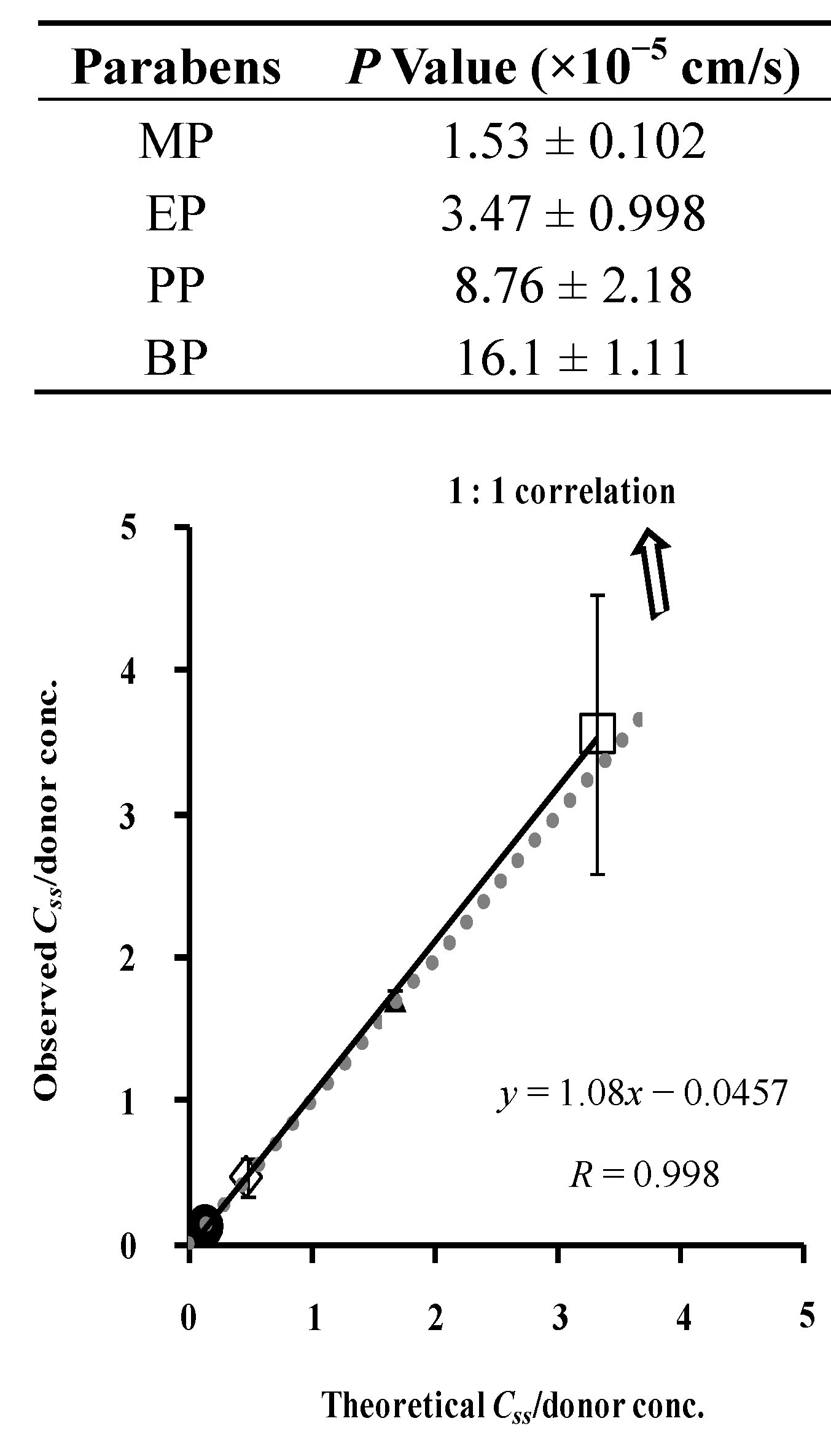
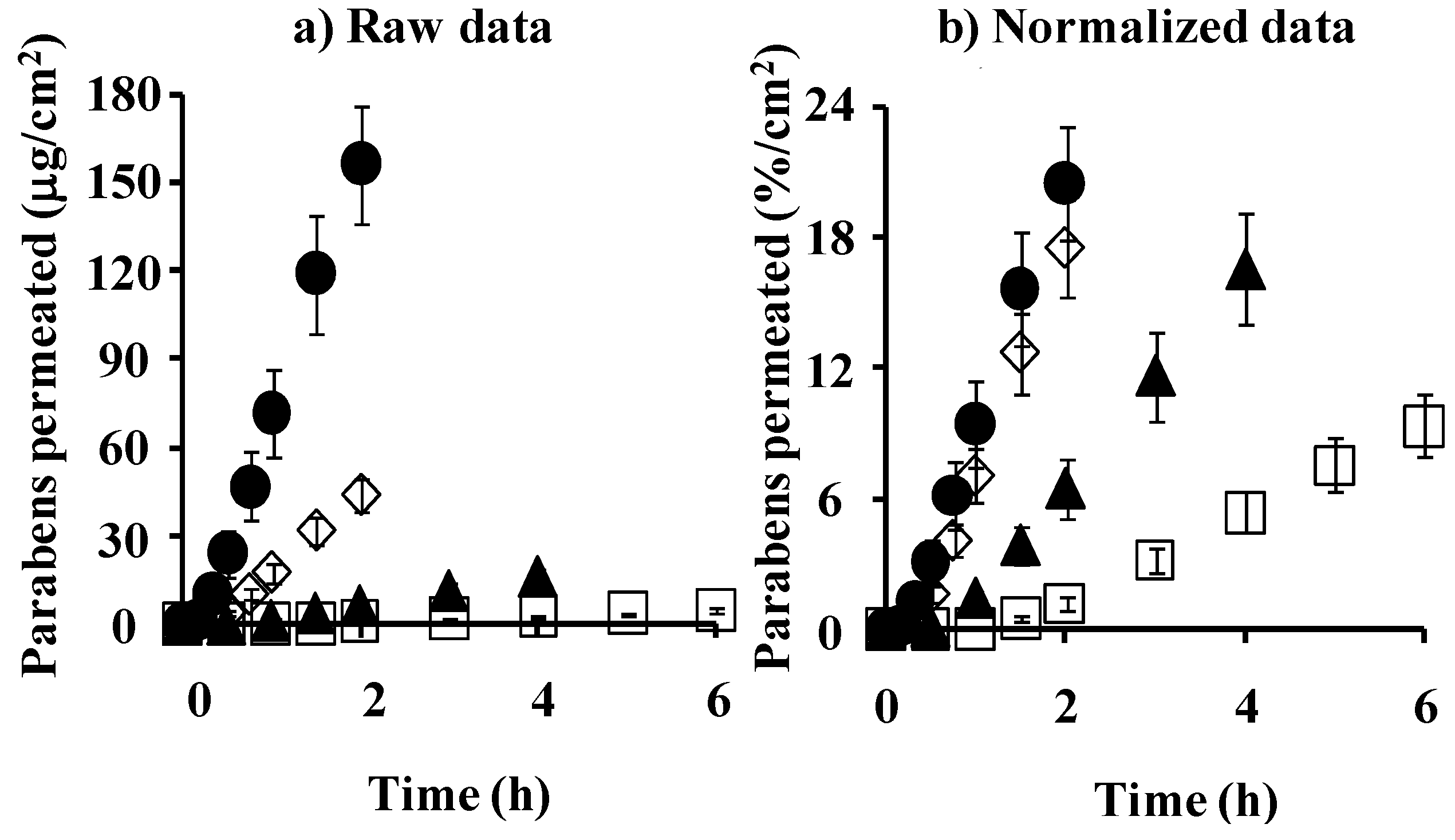
4.2. Membrane Permeation and Concentration: Comparison between Silicone Membrane and Animal Skin




4.3. Simulation of Skin Concentration of Parabens


5. Conclusions
Conflicts of Interest
References
- Knepp, V.M.; Hadgraft, J.; Guy, R.H. Transdermal drug delivery: Problems and possibilities. Crit. Rev. Ther. Drug Carr. Syst. 1987, 4, 13–37. [Google Scholar]
- Kano, S.; Sugibayashi, K. Kinetic analysis on the skin disposition of cytotoxicity as an index of skin irritation produced by cetylpyridinium chloride: Comparison of in vitro data using a three-dimensional cultured human skin model with in vivo results in hairless mice. Pharm. Res. 2006, 23, 329–335. [Google Scholar] [CrossRef]
- Huffman, D.H.; Crow, J.W.; Pentikäinen, P.; Azarnoff, D.L. Association between clinical cardiac status, laboratory parameters, and digoxin usage. Am. Heart J. 1976, 91, 28–34. [Google Scholar] [CrossRef]
- Sheiner, L.B.; Rosenberg, B.; Marathe, W. Estimation of population characteristics of pharmacokinetic parameters from routine clinical data. J. Pharmacokin. Biopharm. 1977, 5, 445–479. [Google Scholar] [CrossRef]
- Vozeh, S.; Hillman, R.; Wandell, M.; Ludden, T.; Sheiner, L. Computer-assisted drug assay interpretation based on Bayesian estimation of individual pharmacokinetics: Application to lidocaine. Ther. Drug Monit. 1985, 7, 66–73. [Google Scholar] [CrossRef]
- Sato, K.; Oda, T.; Sugibayashi, K.; Morimoto, Y. Estimation of blood concentration of drugs after topical application from in vitro skin permeation data. I. Prediction by convolution and confirmation by deconvolution. Chem. Pharm. Bull. 1988, 36, 2232–2238. [Google Scholar] [CrossRef]
- Sato, K.; Oda, T.; Sugibayashi, K.; Morimoto, Y. Estimation of blood concentration of drugs after topical application from in vitro skin permeation data. II. Approach by using diffusion model and compartment model. Chem. Pharm. Bull. 1988, 36, 2624–2632. [Google Scholar] [CrossRef]
- Hatanaka, T.; Katayama, K.; Koizumi, T.; Sugibayashi, K.; Morimoto, Y. In vitro–in vivo correlation of percutaneous absorption: Isosorbide dinitrate and morphine hydrochloride. Biol. Pharm. Bull. 1994, 17, 826–830. [Google Scholar] [CrossRef]
- Kiistala, U. Suction blister device for separation of viable epidermis from dermis. J. Invest. Dermatol. 1968, 50, 129–137. [Google Scholar]
- Surber, C.; Wilhelm, K.P.; Bermann, D.; Maibach, H.I. In vivo skin penetration of acitretin in volunteers using three sampling techniques. Pharm. Res. 1993, 10, 1291–1294. [Google Scholar] [CrossRef]
- Surber, C.; Wilhelm, K.P.; Hori, M.; Maibach, H.I.; Guy, R.H. Optimization of topical therapy: Partitioning of drugs into stratum corneum. Pharm. Res. 1990, 7, 1320–1324. [Google Scholar] [CrossRef]
- Schaefer, H.; Stüttgen, G.; Zesch, A.; Schalla, W.; Gazith, J. Quantitative determination of percutaneous absorption of radiolabeled drugs in vitro and in vivo by human skin. Curr. Probl. Dermatol. 1978, 7, 80–94. [Google Scholar]
- Pershing, L.K.; Silver, B.S.; Krueger, G.G.; Shah, V.P.; Skelley, J.P. Feasibility of measuring the bioavailability of topical betamethasone dipropionate in commercial formulations using drug content in skin and a skin blanching bioassay. Pharm. Res. 1992, 9, 45–51. [Google Scholar] [CrossRef]
- Rougier, A.; Dupuis, D.; Lotte, C.; Roguet, R.; Schaefer, H. In vivo correlation between stratum corneum reservoir function and percutaneous absorption. J. Invest. Dermatol. 1983, 81, 275–278. [Google Scholar]
- N’Dri-Stempfer, B.; Navidi, W.C.; Guy, R.H.; Bunge, A.L. Improved bioequivalence assessment of topical dermatological drug products using dermatopharmacokinetics. Pharm. Res. 2009, 26, 316–328. [Google Scholar] [CrossRef]
- Anissimov, Y.G.; Roberts, M.S. Modelling dermal drug distribution after topical application in human. Pharm Res. 2011, 28, 2119–2129. [Google Scholar] [CrossRef]
- Lodén, M.; Ungerth, L.; Serup, J. Changes in European legislation make it timely to introduce a transparent market surveillance system for cosmetics. Acta Derm. Venereol. 2007, 87, 485–492. [Google Scholar] [CrossRef]
- Toyoda, H. Regulation of the animal experiments and testing in EU. Envir. Mutagen. Res. 2005, 27, 125–128. [Google Scholar] [CrossRef]
- Kolar, R. Animal experimentation. Sci. Eng. Ethics 2006, 12, 111–122. [Google Scholar]
- Spielmann, H. Animal use in the safety evaluation of chemicals: Harmonization and emerging needs. ILAR J. 2002, 43, S11–S17. [Google Scholar]
- Leveque, N.; Raghavan, S.L.; Lane, M.E.; Hadgraft, J. Use of a molecular form technique for the penetration of supersaturated solutions of salicylic acid across silicone membranes and human skin in vitro. Int. J. Pharm. 2006, 318, 49–54. [Google Scholar] [CrossRef]
- Ottaviani, G.; Martel, S.; Carrupt, P. Parallel artificial membrane permeability assay: A new membrane for the fast prediction of passive human skin permeability. J. Med. Chem. 2006, 49, 3948–3954. [Google Scholar] [CrossRef]
- Hatanaka, T.; Inuma, M.; Sugibayashi, K.; Morimoto, Y. Prediction of skin permeability of drugs. I. Comparison with artificial membrane. Chem. Pharm. Bull. 1990, 38, 3452–3459. [Google Scholar] [CrossRef]
- Geinoz, S.; Rey, S.; Boss, G.; Bunge, A.L.; Guy, R.H.; Carrupt, P.A.; Reist, M.; Testa, B. Quantitative structure: Permeation relationships for solute transport across silicone membranes. Pharm. Res. 2002, 19, 1622–1629. [Google Scholar] [CrossRef]
- Herkenne, C.; Naik, A.; Kalia, Y.N.; Hadgraft, J.; Guy, R.H. Ibuprofen transport into and through skin from topical formulations: In vitro–in vivo comparison. J. Investig. Dermatol. 2007, 127, 135–142. [Google Scholar] [CrossRef]
- Hada, N.; Hasegawa, T.; Takahashi, H.; Ishibashi, T.; Sugibayashi, K. Cultured skin loaded with tetracycline HCl and chloramphenicol as dermal delivery system: Mathematical evaluation of the cultured skin containing antibiotics. J. Control. Release 2005, 108, 341–350. [Google Scholar] [CrossRef]
- Scheuplein, R.J.; Blank, I.H. Mechanism of percutaneous absorption. IV. Penetration of nonelectrolytes (alcohols) from aqueous solutions and from pure liquids. J. Investig. Dermatol. 1973, 60, 286–296. [Google Scholar]
- Hasegawa, T.; Kim, S.; Tsuchida, M.; Isshiki, Y.; Kondo, S.; Sugibayashi, K. Decrease in skin permeation and antibacterial effect of parabens by a polymeric additive, poly(2-methacryloyloxyethyl phosphorylcholine-cobutylmetacrylate). Chem. Pharm. Bull. 2005, 53, 271–276. [Google Scholar] [CrossRef]
- Scheuplein, R.J. Mechanism of percutaneous absorption: Transient diffusion and the relative importance of various routes of skin penetration. J. Investig. Dermatol. 1967, 48, 79–88. [Google Scholar]
- Watanabe, T.; Hasegawa, T.; Takahashi, H.; Ishibashi, T.; Sugibayashi, K. Utility of three-dimensional cultured human skin model as a tool to evaluate skin permeation drugs. Altern. Anim. Test. Exp. 2001, 8, 1–14. [Google Scholar]
- Ghanem, A.H.; Mahmoud, H.; Higuchi, W.I.; Liu, P. The effects of ethanol on the transport of lipophilic and polar permeants across hairless mouse skin: Methods/validation of a novel approach. Int. J. Pharm. 1992, 78, 137–156. [Google Scholar] [CrossRef]
© 2013 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Todo, H.; Oshizaka, T.; Kadhum, W.R.; Sugibayashi, K. Mathematical Model to Predict Skin Concentration after Topical Application of Drugs. Pharmaceutics 2013, 5, 634-651. https://doi.org/10.3390/pharmaceutics5040634
Todo H, Oshizaka T, Kadhum WR, Sugibayashi K. Mathematical Model to Predict Skin Concentration after Topical Application of Drugs. Pharmaceutics. 2013; 5(4):634-651. https://doi.org/10.3390/pharmaceutics5040634
Chicago/Turabian StyleTodo, Hiroaki, Takeshi Oshizaka, Wesam R. Kadhum, and Kenji Sugibayashi. 2013. "Mathematical Model to Predict Skin Concentration after Topical Application of Drugs" Pharmaceutics 5, no. 4: 634-651. https://doi.org/10.3390/pharmaceutics5040634