Development of Liposomal Ciprofloxacin to Treat Lung Infections
Abstract
:1. Introduction
1.1. Rationale for Inhaled Antibiotics
1.2. Opportunities to Treat Lung Infections
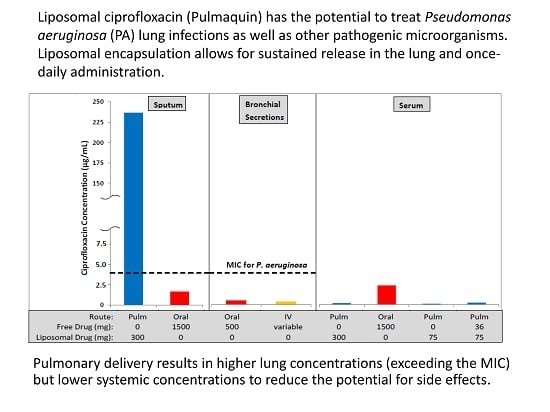
1.3. Rationale for Using a Liposomal Formulation of Ciprofloxacin
2. Pharmaceutical Development of a Liposomal Formulation of Ciprofloxacin
2.1. Evaluation and Selection of the Composition of Liposomal Ciprofloxacin
2.2. Stability of Liposomal Ciprofloxacin to the Nebulization Process
2.3. In Vitro Characterization and Stability of Liposomal Ciprofloxacin
3. Preclinical Development of Liposomal Ciprofloxacin
3.1. Pharmacology
3.2. Efficacy against Pseudomonas aeruginosa: In Vitro Studies
3.3. Efficacy against Pseudomonas aeruginosa: In Vivo Studies
3.4. Efficacy against Biodefense Pathogens
3.4.1. Pneumonic (Respiratory/Inhalational) Tularemia—Lipoquin Efficacy against F. tularensis
3.4.2. Q Fever–Lipoquin Efficacy against C. burnetii
4. Clinical Development of Liposomal Ciprofloxacin
5. Next Generation Formulations of Liposomal Ciprofloxacin
6. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
Abbreviations
| AUC | Area under the curve |
| BIC | biofilm inhibitory concentration |
| BID | twice daily |
| CF | cystic fibrosis |
| CFTR | cystic fibrosis transmembrane conductance regulator |
| CFU | colony forming unit |
| Cmax | maximum concentration |
| CFI | ciprofloxacin for inhalation (Lipoquin, liposomal ciprofloxacin) |
| CH | cholesterol |
| CIP | ciprofloxacin |
| COPD | Chronic Obstructive Pulmonary Disease |
| Cryo-TEM | cryogenic transmission electron microscope |
| D25 | diameter for which 25% of the particles have a smaller size |
| D50 | diameter for which 50% of the particles have a smaller size |
| D75 | diameter for which 75% of the particles have a smaller size |
| D90 | diameter for which 90% of the particles have a smaller size |
| DOX | Doxycycline |
| DLS | dynamic light scattering |
| EPC | egg phosphatidylcholine |
| ESM | egg sphingomyelin |
| FCI | free ciprofloxacin for inhalation (unencapsulated ciprofloxacin) |
| FEV1 | forced expiratory volume in one second |
| GLP | good laboratory practices |
| GMP | good manufacturing processes |
| GSD | geometric standard deviation |
| HSPC | hydrogenated soy phosphatidylcholine |
| IV | intravenous |
| IVR | In Vitro Release |
| LB | lysogeny broth |
| LPS | lipopolysaccharide |
| MDI | metered dose inhaler |
| MIC | minimum inhibitory concentration |
| mITT | modified intention to treat |
| NA | not applicable |
| NCFB | non-cystic fibrosis bronchiectasis |
| NTM | non-tuberculous mycobacteria |
| OD | optical density |
| ORBIT | Once-daily Respiratory Bronchiectasis Inhalation Treatment |
| PA | Pseudomonas aeruginosa |
| PBS | phosphate buffered saline |
| PC | phosphatidylcholine |
| PEP | post-exposure prophylaxis |
| POPC | 1-palmitoyl-2-oleoylphosphatidylcholine |
| Pulm | pulmonary |
| QoL | Quality of Life |
| SAE | serious adverse event |
| VMD | volume mean diameter |
References
- Cipolla, D.; Chan, H.-K. Inhaled Antibiotics to Treat Lung Infection. Pharm. Patent Analyst. 2013, 2, 647–663. [Google Scholar] [CrossRef] [PubMed]
- Weers, J. Inhaled antimicrobial therapy - barriers to effective treatment. Adv. Drug Deliv. Rev. 2015, 85, 24–43. [Google Scholar] [CrossRef] [PubMed]
- Wright, D.H.; Brown, G.H.; Peterson, M.L.; Rotschafer, J.C. Application of fluoroquinolone pharmacodynamics. J Antimicrob. Chemo. 2000, 46, 669–683. [Google Scholar] [CrossRef]
- Wargo, K.A.; Edwards, J.D. Aminoglycoside-Induced Nephrotoxicity. J. Pharm. Pract. 2014, 27, 573–577. [Google Scholar] [CrossRef] [PubMed]
- Fink, M.P.; Snydman, D.R.; Niederman, M.S.; Leeper, K.V., Jr.; Johnson, R.H.; Heard, S.O.; Wunderink, R.G.; Caldwell, J.W.; Schentag, J.J.; Siami, G.A.; et al. Treatment of severe pneumonia in hospitalized patients: results of a multicenter, randomized, double-blind trial comparing intravenous ciprofloxacin with imipenem-cilastatin. Antimicrob. Agents Chemother. 1994, 38, 547–557. [Google Scholar] [CrossRef] [PubMed]
- Peloquin, C.A.; Cumbo, T.J.; Nix, D.E.; Sands, M.F.; Schentag, J.J. Evaluation of intravenous ciprofloxacin in patients with nosocomial lower respiratory tract infections. Impact of plasma concentrations, organism, minimum inhibitory concentration, and clinical condition on bacterial eradication. Arch. Intern Med. 1989, 149, 2269–2273. [Google Scholar] [CrossRef] [PubMed]
- Firsov, A.A.; Vostrov, S.N.; Shevchenko, A.A.; Zinner, S.H.; Cornaglia, G.; Portnoy, Y.A. MIC-based interspecies prediction of the antimicrobial effects of ciprofloxacin on bacteria of different susceptibilities in an in vitro dynamic model. Antimicrob. Agents Chemother. 1998, 42, 2848–2852. [Google Scholar] [PubMed]
- Forrest, A.; Nix, D.E.; Ballow, C.H.; Goss, T.F.; Birmingham, M.C.; Schentag, J.J. Pharmacodynamics of intravenous ciprofloxacin in seriously ill patients. Antimicrob. Agents Chemother. 1993, 37, 1073–1081. [Google Scholar] [CrossRef] [PubMed]
- Bruinenberg, P.; Blanchard, J.D.; Cipolla, D.C.; Dayton, F.; Mudumba, S.; Gonda, I. Inhaled liposomal ciprofloxacin: Once a day management of respiratory infections. In Proceedings of Respiratory Drug Delivery 2010; Dalby, R.N., Byron, P.R., Peart, J., Suman, J.D., Farr, S.J., Young, P.M., Eds.; Davis Healthcare International Publishing: River Grove, IL, USA, 2010; pp. 73–81. [Google Scholar]
- Bruinenberg, P.; Serisier, D.; Cipolla, D.; Blanchard, J. Safety, Tolerability, Pharmacokinetics and Antimicrobial Activity of Inhaled Liposomal Ciprofloxacin Formulations in Humans. Pediatr. Pulmonol. 2010, 45, 354. [Google Scholar]
- Rotrovato, C.A.; Deeter, R.G. Respiratory Tract Penetration of Quinolone Antimicrobials: A Case in Study. Pharmacotherapy 1991, 11, 38–49. [Google Scholar]
- Moskowitz, S.M.; Foster, J.M.; Emerson, J.; Burns, J.L. Clinically Feasible Biofilm Susceptibility Assay for Isolates of Pseudomonas aeruginosa from Patients with Cystic Fibrosis. J. Clin. Microbiol. 2004, 42, 1915–1922. [Google Scholar] [CrossRef] [PubMed]
- Chalmers, J.D.; Aliberti, S.; Blasi, F. Management of bronchiectasis in adults. Eur. Respir. J. 2015, 45, 1446–1462. [Google Scholar] [CrossRef] [PubMed]
- Parameswaran, I.; Sethi, S. Pseudomonas infection in chronic obstructive pulmonary disease. Future Microbiol. 2012, 7, 1129–1132. [Google Scholar] [CrossRef] [PubMed]
- Finch, S.; McDonnell, M.J.; Abo-Leyah, H.; Aliberti, S.; Chalmers, J.D. A Comprehensive Analysis of the Impact of Pseudomonas aeruginosa Colonization on Prognosis in Adult Bronchiectasis. Ann. Am. Thorac. Soc. 2015, 2, 1602–1611. [Google Scholar]
- Bilton, D.; Henig, N.; Morrissey, B.; Gotfried, M. Addition of inhaled tobramycin to ciprofloxacin for acute exacerbations of Pseudomonas aeruginosa infection in adult bronchiectasis. Chest 2006, 130, 1503–1510. [Google Scholar] [CrossRef] [PubMed]
- Barker, A.F.; O’Donnell, A.E.; Flume, P.; Thompson, P.J.; Ruzi, J.D.; de Gracia, J.; Boersma, W.G.; De Soyza, A.; Shao, L.; Zhang, J.; et al. Aztreonam for inhalation solution in patients with non-cystic fibrosis bronchiectasis (AIR-BX1 and AIR-BX2): Two randomised double-blind, placebo-controlled phase 3 trials. Lancet Respir. Med. 2014, 2, 738–749. [Google Scholar] [CrossRef]
- Haworth, C.S.; Foweraker, J.E.; Wilkinson, P.; Kenyon, R.F.; Bilton, D. Inhaled colistin in patients with bronchiectasis and chronic Pseudomonas aeruginosa infection. Am. J. Respir. Crit. Care Med. 2014, 189, 975–982. [Google Scholar] [CrossRef] [PubMed]
- Grimwood, K.; Bell, S.C.; Chang, A.B. Antimicrobial treatment of non-cystic fibrosis bronchiectasis. Expert Rev. Anti-Infect. Ther. 2014, 12, 1277–1296. [Google Scholar] [CrossRef] [PubMed]
- Chalmers, J.D.; Smith, M.P.; McHugh, B.J.; Doherty, C.; Govan, J.R.; Hill, A.T. Short- and long-term antibiotic treatment reduces airway and systemic inflammation in non-cystic fibrosis bronchiectasis. Am. J. Respir. Crit. Care Med. 2012, 186, 657–665. [Google Scholar] [CrossRef] [PubMed]
- Serisier, D.J.; Bilton, D.; De Soyza, A.; Thompson, P.J.; Kolbe, J.; Greville, H.W.; Cipolla, D.; Bruinenberg, P.; Gonda, I. Inhaled, dual release liposomal ciprofloxacin in non-cystic fibrosis bronchiectasis (ORBIT-2): A randomised, double-blind, placebo-controlled trial. Thorax 2013, 68, 812–817. [Google Scholar] [CrossRef] [PubMed]
- Adjemian, J.; Olivier, K.N.; Seitz, A.E.; Holland, S.M.; Prevots, D.R. Prevalence of nontuberculosis mycobacterial lung disease in US Medicare beneficiaries. Am. J. Respir. Crit. Care Med. 2012, 185, 881–886. [Google Scholar] [CrossRef] [PubMed]
- Olivier, K.N.; Gupta, R.; Daley, C.L.; Winthrop, K.L.; Ruoss, S.; Addrizzo-Harris, D.J.; Flume, P.; Dorgan, D.; Salathe, M.A.; Brown-Elliott, B.A.; et al. A Randomized, Double-Blind, Placebo-Controlled Study of Liposomal Amikacin for Inhalation in Patients with Recalcitrant Nontuberculous Mycobacterial Lung Disease. In Presented at ATS Conference, San Diego, CA, USA, 20 May 2014; #50985. Insmed Publications & Presentations. Available online: http://www.insmed.com/publications-presentations/ (accessed on 31 December 2015).
- Ruoss, S.; Eagle, G.; McGinnis, J.P., II; Micioni, L.; Daley, C.L.; Winthrop, K.L.; Addrizzo-Harris, D.J.; Flume, P.; Dorgan, D.; Salathe, M.; et al. Analysis of Functional Exercise Capacity (via the Six-Minute Walk Test [6MWT]) and Negative Sputum Culture for Nontuberculous Mycobacteria (NTM) in Patients With NTM Lung Infection Refractory to Guideline-Based Therapy Treated With Liposomal Amikacin for Inhalation (LAI). In Presented at ATS Conference, Denver, CO, USA, 20 May 2015; #612. Insmed Publications & Presentations. Available online: http://www.insmed.com/publications-presentations/ (accessed on 31 December 2015).
- Rose, S.J.; Neville, M.E.; Gupta, R.; Bermudez, L.E. Delivery of Aerosolized Liposomal Amikacin as a Novel Approach for the Treatment of Nontuberculous Mycobacteria in an Experimental Model of Pulmonary Infection. PLoS ONE 2014, 9, e108703. [Google Scholar] [CrossRef] [PubMed]
- Blanchard, J.; Danelishvili, L.; Gonda, I.; Bermudez, L. Liposomal Ciprofloxacin Preparation is Active against Mycobacterium Avium Subsp Hominissuis and Mycobacterium Abscessus in Macrophages and in Biofilm. In Presented at ATS Conference, San Diego, CA, USA, 20 May 2014; #57372. Available online: http://investor.aradigm.com/releasedetail.cfm?ReleaseID=840192 (accessed on 31 December 2015).
- Blanchard, J.; Danelishvili, L.; Gonda, I.; Bermudez, L. Treatment of lung infection caused by Mycobacterium abscessus in Beige mice with pulmonary delivery of liposomally encapsulated ciprofloxacin is associated with significant reduction of bacterial load. In Presented at Interscience Conference on Antimicrobial Agents and Chemotherapy (ICAAC); 2015. Poster #B-536. Available online: http://investor.aradigm.com/releasedetail.cfm?ReleaseID=930955 (accessed on 31 December 2015). [Google Scholar]
- Bermudez, L.E.; Blanchard, J.D.; Hauck, L.; Gonda, I. Treatment of Mycobacterium avium subsp hominissuis (MAH) lung infection with liposome-encapsulated ciprofloxacin resulted in significant decrease in bacterial load in the lung. Am J Respir Crit Care Med. 2015, 191, A6293. Available online: http://investor.aradigm.com/releasedetail.cfm?releaseid=912485 (accessed on 31 December 2015). [Google Scholar]
- Meers, P.; Neville, M.; Malinin, V.; Scotto, A.W.; Sardaryan, G.; Kurumunda, R.; Mackinson, C.; James, G.; Fisher, S.; Perkins, W.R. Biofilm penetration, triggered release and in vivo activity of inhaled liposomal amikacin in chronic Pseudomonas aeruginosa lung infections. J. Antimicrob. Chemo. 2008, 61, 859–868. [Google Scholar] [CrossRef] [PubMed]
- Cipolla, D.; Gonda, I.; Chan, H.-K. Liposomal Formulations for Inhalation. Ther. Deliv. 2013, 4, 1047–1072. [Google Scholar] [CrossRef] [PubMed]
- Cipolla, D.; Shekunov, B.; Blanchard, J.; Hickey, T. Lipid-based carriers for pulmonary products: Preclinical development and case studies in Humans. Adv. Drug Deliv. Rev. 2014, 75, 53–80. [Google Scholar] [CrossRef] [PubMed]
- Cipolla, D.; Redelmeier, T.; Eastman, S.; Bruinenberg, P.; Gonda, I. Liposomes, Niosomes and Proniosomes - a Critical Update of Their (Commercial) Development as Inhaled Products. In Proceedings of Respiratory Drug Delivery Europe 2011; Dalby, R.N., Byron, P.R., Peart, J., Suman, J.D., Farr, S.J., Young, P.M., Eds.; Davis Healthcare International Publishing: River Grove, IL, USA, 2011; pp. 41–54. [Google Scholar]
- Yim, D.; Blanchard, J.D.; Mudumba, S.; Eastman, S.; Manda, K.; Redelmeier, T.; Farr, S. The Development of Inhaled Liposome-Encapsulated Ciprofloxacin to Treat Cystic Fibrosis. In Proceedings of Respiratory Drug Delivery 2006; Dalby, R.N., Byron, P.R., Peart, J., Suman, J.D., Farr, S.J., Eds.; Davis Healthcare International Publishing: River Grove, IL, USA, 2006; pp. 425–428. [Google Scholar]
- Webb, M.S.; Boman, N.L.; Wiseman, D.J.; Saxon, D.; Sutton, K.; Wong, K.F.; Logan, P.; Hope, M.J. Antibacterial Efficacy Against an in Vivo Salmonella Typhimurium Infection Model and Pharmacokinetics of a Liposomal Ciprofloxacin Formulation. Antimicrob. Agents Chemother. 1998, 42, 45–52. [Google Scholar] [PubMed]
- Barriere, S.L.; Kaatz, G.W.; Schaberg, D.R.; Fekety, R. Altered pharmacologic disposition of ciprofloxacin and vancomycin after single and multiple doses in rabbits. Antimicrob. Agents Chemother. 1987, 31, 1075–1078. [Google Scholar] [CrossRef] [PubMed]
- Blanchard, J. Pulmonary Drug Delivery as a First Response to Bioterrorism. In Proceedings of Respiratory Drug Delivery 2006; Dalby, R.N., Byron, P.R., Peart, J., Suman, J.D., Farr, S.J., Eds.; Davis Healthcare International Publishing: River Grove, IL, USA, 2006; pp. 73–82. [Google Scholar]
- Wong, J.P.; Yang, H.; Blasetti, K.L.; Schnell, G.; Conley, J.; Schofield, L.N. Liposome Delivery of Ciprofloxacin against Intracellular Francisella tularensis Infection. J. Control. Release 2003, 92, 265–273. [Google Scholar] [CrossRef]
- Ong, H.X.; Traini, D.; Cipolla, D.; Gonda, I.; Bebawy, M.; Agus, H.; Young, P.Y. Liposomal Nanoparticles Control the Uptake of Ciprofloxacin Across Respiratory Epithelia. Pharm. Res. 2012, 29, 3335–3346. [Google Scholar] [CrossRef] [PubMed]
- Ong, H.X.; Benaouda, F.; Traini, D.; Cipolla, D.; Gonda, I.; Forbes, B.; Young, P.M. In Vitro and ex Vivo Methods Predict the Enhanced Lung Residence Time of Liposomal Ciprofloxacin Formulations for Nebulisation. Eur. J. Pharm. Biopharm. 2014, 86, 83–89. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Zhang, Y.; Wurtz, W.; Lee, J.K.; Malinin, V.S.; Durwas-Krishnan, S.; Meers, P.; Perkins, W.R. Characterization of nebulized liposomal amikacin (Arikace) as a function of droplet size. J. Aerosol. Med. Pulm. Drug Deliv. 2008, 21, 245–254. [Google Scholar] [CrossRef] [PubMed]
- Cipolla, D.C.; Dayton, F.; Fulzele, S.; Gabatan, E.; Mudumba, S.; Yim, D.; Wu, H.; Zwolinski, R. Inhaled Liposomal Ciprofloxacin: In Vitro Properties and Aerosol Performance. In Proceedings of Respiratory Drug Delivery 2010; Dalby, R.N., Byron, P.R., Peart, J., Suman, J.D., Farr, S.J., Young, P.M., Eds.; Davis Healthcare International Publishing: River Grove, IL, USA, 2010; pp. 409–414. [Google Scholar]
- Cipolla, D.; Wu, H.; Chan, J.; Chan, H.-K.; Gonda, I. Liposomal Ciprofloxacin for Inhalation Retains Integrity Following Nebulization. In Proceedings of Respiratory Drug Delivery Europe 2013; Dalby, R.N., Byron, P.R., Peart, J., Suman, J.D., Farr, S.J., Young, P.M., Eds.; Davis Healthcare International Publishing: River Grove, IL, USA, 2013; pp. 237–242. [Google Scholar]
- Cipolla, D.; Wu, H.; Eastman, S.; Redelmeier, T.; Gonda, I.; Chan, H.-K. Development and characterization of an in vitro release assay for liposomal ciprofloxacin for inhalation. J. Pharm. Sci. 2014, 103, 314–327. [Google Scholar] [CrossRef]
- Finlay, W.H.; Wong, J.P. Regional lung deposition of nebulized liposome-encapsulated ciprofloxacin. Int. J. Pharm. 1998, 167, 121–127. [Google Scholar] [CrossRef]
- Grit, M.; Crommelin, D.J.A. Chemical stability of liposomes: Implications for their physical stability. Chem. Phys. Lipids. 1993, 64, 3–18. [Google Scholar] [CrossRef]
- Cipolla, D.; Gonda, I.; Serisier, D.; Bruinenberg, P. Dual Release Ciprofloxacin for Inhalation (DRCFI) Improves Time to First Exacerbation in Bronchiectasis. J. Aerosol. Med. Pulm. Drug Deliv. 2011, 24, A-27. [Google Scholar]
- Almgren, M.; Edwards, K.; Karlsson, G. Cryo transmission electron microscopy of liposomes and related structures. Colloids Surf. A 2000, 174, 3–21. [Google Scholar] [CrossRef]
- FDA. Draft Guidance for Industry. Liposome Drug Products: Chemistry, Manufacturing, and Controls; Human Pharmacokinetics and Bioavailability; and Labeling Documentation October 2015. Available online: http://www.fda.gov/downloads/drugs/guidancecomplianceregulatoryinformation/guidances/ucm070570.pdf (accessed on 18 February 2016).
- ICH Quality Guidelines. Available online: http://www.ich.org/products/guidelines/quality/article/quality-guidelines.html.
- Hamblin, K.A.; Armstrong, S.J.; Barnes, K.B.; Davies, C.; Wong, J.P.; Blanchard, J.D.; Harding, S.V.; Simpson, A.J.; Atkins, H.S. Liposome encapsulation of ciprofloxacin improves protection against highly virulent Francisella tularensis strain Schu S4. Antimicrob. Agents Chemother. 2014, 58, 3053–3059. [Google Scholar] [CrossRef] [PubMed]
- Hamblin, K.A.; Blanchard, J.D.; Davis, C.; Harding, S.V.; Simpson, A.J.H. Efficacy of inhaled liposome-encapsulated ciprofloxacin against Yersinia pestis. J. Aer. Med. Pulm. Drug Deliv. 2013, 26, A-16. [Google Scholar]
- Norville, I.H.; Hatch, G.J.; Bewley, K.R.; Atkinson, D.J.; Hamblin, K.A.; Blanchard, J.D.; Armstrong, S.J.; Pitman, J.K.; Rayner, E.; Hall, G.; et al. Efficacy of liposome-encapsulated ciprofloxacin in a murine model of Q fever. Antimicrob. Agents Chemother. 2014, 58, 5510–5518. [Google Scholar] [CrossRef] [PubMed]
- Sheth, V.R.; van Heeckeren, R.C.; Wilson, A.G.; van Heeckeren, A.M.; Pagel, M.D. Monitoring infection and inflammation in murine models of cystic fibrosis with magnetic resonance imaging. J. Magn. Reson. Imaging. 2008, 28, 527–532. [Google Scholar] [CrossRef] [PubMed]
- Dennis, D.T.; Inglesby, T.V.; Henderson, D.A.; Bartlett, J.G.; Ascher, M.S.; Eitzen, E.; Fine, A.D.; Friedlander, A.M.; Hauer, J.; Layton, M.; et al. Working Group on Civilian Biodefense. Tularemia as a biological weapon: Medical and public health management. JAMA 2001, 285, 2763–2773. [Google Scholar] [CrossRef] [PubMed]
- Perez-Castrillon, J.L.; Bachiller-Luque, P.; Martin-Luquero, M.; Mena-Martin, F.J.; Herreros, V. Tularemia epidemic in northwestern Spain: Clinical description and therapeutic response. Clin. Infect. Dis. 2001, 33, 573–576. [Google Scholar] [CrossRef] [PubMed]
- Chocarro, A.; Gonzalez, A.; Garcia, I. Treatment of tularemia with ciprofloxacin. Clin. Infect. Dis. 2000, 31, 623. [Google Scholar] [CrossRef] [PubMed]
- Hepburn, M.J.; Simpson, A.J.H. Tularemia: Current diagnosis and treatment options. Expert Rev. Anti-Infect. Ther. 2008, 6, 231–240. [Google Scholar] [CrossRef] [PubMed]
- Steward, J.; Piercy, T.; Lever, M.S.; Simpson, A.J.H.; Brooks, T.J.G. Treatment of murine pneumonic Francisella tularensis infection with gatifloxacin, moxifloxacin or ciprofloxacin. Int. J. Antimicrob. Agents. 2006, 27, 439–443. [Google Scholar] [CrossRef] [PubMed]
- Conley, J.; Yang, H.; Wilson, T.; Blasetti, K.; Di Ninno, V.; Schnell, G.; Wong, J.P. Aerosol delivery of liposome-encapsulated ciprofloxacin: Aerosol characterization and efficacy against Francisella tularensis infection in mice. Antimicrob. Agents Chemother. 1997, 41, 1288–1292. [Google Scholar] [PubMed]
- Di Ninno, V.L.; Cherwanogrodzky, J.W.; Wong, J.P. Liposome-encapsulated ciprofloxacin is effective in the protection and treatment of BALB/c mice against Francisella tularensis. J. Infect. Dis. 1993, 168, 793–794. [Google Scholar] [CrossRef] [PubMed]
- Hamblin, K.A.; Wong, J.P.; Blanchard, J.D.; Atkins, H.S. The Potential of Liposome-Encapsulated Ciprofloxacin as a Tularemia Therapy. Front. Cell Infect. Microbiol. 2014, 4, 79. [Google Scholar] [CrossRef] [PubMed]
- Brain, J.D.; Knudson, D.E.; Sorokin, S.P.; Davis, M.A. Pulmonary distribution of particles given by intratracheal instillation or by aerosol inhalation. Environ. Res. 1976, 11, 13–33. [Google Scholar] [CrossRef]
- FDA. Draft Guidance for Industry. Animal models - essential elements to address efficacy under the Animal Rule. Available online: http://www.fda.gov/downloads/drugs/guidancecomplianceregulatoryinformation/guidances/ucm399217.pdf (accessed on 18 February 2016).
- Maurin, M.; Raoult, D.Q. Fever. Clin Microbiol Rev. 1999, 12, 518–553. [Google Scholar] [PubMed]
- Rolain, J.M.; Boulos, A.; Mallet, M.N.; Raoult, D. Correlation between ratio of serum doxycycline concentration to MIC and rapid decline of antibody levels during treatment of Q fever endocarditis. Antimicrob. Agents Chemother. 2005, 49, 2673–2676. [Google Scholar] [CrossRef] [PubMed]
- Fenollar, F.; Fournier, P.E.; Carrieri, M.P.; Habib, G.; Messana, T.; Raoult, D. Risks factors and prevention of Q fever endocarditis. Clin. Infect. Dis. 2001, 33, 312–316. [Google Scholar] [CrossRef] [PubMed]
- Anderson, A.; Bijlmer, H.; Fournier, P.E.; Graves, S.; Hartzell, J.; Kersh, G.J.; Limonard, G.; Marrie, T.J.; Massung, R.F.; McQuiston, J.H.; et al. Diagnosis and management of Q fever--United States, 2013: Recommendations from CDC and the Q Fever Working Group. MMWR Recomm. Rep. 2013, 62(RR-03), 1–30. [Google Scholar] [PubMed]
- CDC Q fever. Available online: http://www.cdc.gov/qfever/index.html (accessed on 18 February 2016).
- Raoult, D.; Houpikian, P.; Dupont, H.; Riss, J.; Arditi-Djiane, J.J.; Brouqui, P. Treatment of Q fever endocarditis: Comparison of 2 regimens containing doxycycline and ofloxacin or hydroxychloroquine. Arch. Intern. Med. 1999, 159, 167–173. [Google Scholar] [CrossRef] [PubMed]
- Maurin, M.; Benoliel, A.M.; Bongrand, P.; Raoult, D. Phagolysosomal alkalinization and the bactericidal effect of antibiotics: the Coxiella burnetii paradigm. J Infect Dis. 1992, 166, 1097–1102. [Google Scholar] [CrossRef] [PubMed]
- Rouli, L.; Rolain, J.M.; El Filali, A.; Robert, C.; Raoult, D. Genome sequence of Coxiella burnetii 109, a doxycycline-resistant clinical isolate. J. Bacteriol. 2012, 194, 6939. [Google Scholar] [CrossRef] [PubMed]
- Gikas, A.; Spyridaki, I.; Psaroulaki, A.; Kofterithis, D.; Tselentis, Y. In vitro susceptibility of Coxiella burnetii to trovafloxacin in comparison with susceptibilities to pefloxacin, ciprofloxacin, ofloxacin, doxycycline, and clarithromycin. Antimicrob. Agents Chemother. 1998, 42, 2747–2748. [Google Scholar] [PubMed]
- Rubin, B.K. Aerosolized antibiotics for non-cystic fibrosis bronchiectasis. J. Aer. Med. Pulm. Drug Deliv. 2008, 21, 71–76. [Google Scholar] [CrossRef] [PubMed]
- Darweesh, R.S.; Sakagami, M. In Vitro Inhibitory Activities of Liposomal Ciprofloxacin Against Lipopolysaccharide (LPS)-Induced IL-8 Release from the Calu-3 Cells. In Proceedings of Respiratory Drug Delivery 2014; Dalby, R.N., Byron, P.R., Peart, J., Suman, J.D., Farr, S.J., Young, P.M., Eds.; Davis Healthcare International Publishing: River Grove, IL, USA, 2014; pp. 747–750. [Google Scholar]
- Bruinenberg, P.; Serisier, D.; Cipolla, D.; Blanchard, J. Safety, Tolerability, Pharmacokinetics and Antimicrobial Activity of Inhaled Liposomal Ciprofloxacin Formulations in Humans. North American Cystic Fibrosis Conference. Pediatr. Pulmonol. 2010, 45 (S33), 354. [Google Scholar]
- Bruinenberg, P.; Blanchard, J.; Cipolla, D.; Serisier, D. Safety, Tolerability and Pharmacokinetics of Novel Liposomal Ciprofloxacin Formulations for Inhalation in Healthy Volunteers and in Non-Cystic Bronchiectasis Patients. ATS International Conference 2010. [Google Scholar] [CrossRef]
- Aradigm press release on 13 October 2015. Available online: http://investor.aradigm.com/releasedetail.cfm?ReleaseID=936283 (accessed on 18 February 2016).
- Cipolla, D.; Chan, H.-K.; Schuster, J.; Farina, D. Personalized Medicine: Development of Inhalation Systems Tailored to the Individual. Ther. Deliv. 2010, 1, 667–682. [Google Scholar] [CrossRef] [PubMed]
- Cipolla, D.; Wu, H.; Eastman, S.; Redelmeier, T.; Gonda, I.; Chan, H.-K. Modifying the Release Properties of Liposomes toward Personalized Medicine. J. Pharm. Sci. 2014, 103, 1851–1862. [Google Scholar] [CrossRef] [PubMed]
- Cipolla, D.; Wu, H.; Salentenig, S.; Boyd, B.; Rades, T.; Vanhecke, D.; Petri-Fink, A.; Rothen-Rutishauser, B.; Eastman, S.; Redelmeier, T.; et al. Formation of Drug Nanocrystals under Nanoconfinement Afforded by Liposomes. RSC Adv. 2016, 6, 6223–6233. [Google Scholar] [CrossRef]
- Cipolla, D.; Wu, H.; Gonda, I.; Chan, H.-K. Aerosol performance and long term stability of surfactant-associated liposomal ciprofloxacin formulations with modified encapsulation and release properties. AAPS PharmSciTech. 2014, 15, 1218–1227. [Google Scholar] [CrossRef] [PubMed]
- Cipolla, D.; Wu, H.; Gonda, I.; Chan, H.-K. Aerosol Performance and Stability of Liposomes Containing Ciprofloxacin Nanocrystals. J. Aer. Med. Pulm. Drug Deliv. 2015, 28, 411–422. [Google Scholar] [CrossRef] [PubMed]
- Schreier, H. Liposome Aerosols. J. Liposome Res. 1992, 2, 145–184. [Google Scholar] [CrossRef]




















| Storage Condition | Time Point (Months) | Nebulizer | Collected Aerosol | Mass Balance (%) | ||
|---|---|---|---|---|---|---|
| Recovery ± SD (%) | Encapsulation ± SD (%) | Emitted Dose ± SD (%) | Encapsulation ± SD (%) | |||
| Release Data | Initial | 25.6 ± 1.9 | 98.8 ± 0.20 | 67.5 ± 2.8 | 96.8 ± 0.20 | 93.1 |
| 5 °C | 3 | 23.0 ± 3.9 | 98.9 ± 0.14 | 70.7 ± 1.0 | 96.6 ± 0.10 | 93.7 |
| 6 | 20.9 ± 0.6 | 99.0 ± 0.04 | 74.0 ± 1.7 | 98.1 ± 0.16 | 94.9 | |
| 9 | 26.5 ± 1.0 | 99.2 ± 0.01 | 65.9 ± 0.9 | 98.7 ± 0.02 | 91.5 | |
| 12 | 23.8 ± 1.2 | 98.9 ± 0.08 | 66.0 ± 1.9 | 97.7 ± 0.17 | 89.8 | |
| 18 | 23.8 ± 0.7 | 99.0 ± 0.09 | 71.8 ± 2.1 | 98.6 ± 0.21 | 95.6 | |
| 24 | 24.5 ± 0.8 | 99.2 ± 0.06 | 66.0 ± 1.2 | 97.6 ± 0.02 | 90.5 | |
| 25 °C | 3 | 25.0 ± 2.5 | 99.1 ± 0.05 | 69.4 ± 1.4 | 96.7 ± 0.21 | 94.4 |
| 6 | 21.9 ± 3.0 | 98.7 ± 0.16 | 72. 6± 1.4 | 96.9 ± 0.49 | 93.3 | |
| Lot Number | Control | Nebulizer | Collected Aerosol | Mass Balance (%) | |||||
|---|---|---|---|---|---|---|---|---|---|
| Encapsulation ± SD (%) | Mean Size (nm) | Recovery ± SD (%) | Encapsulation ± SD (%) | Mean Size (nm) | Emitted Dose ± SD (%) | Encapsulation ± SD (%) | Mean Size (nm) | ||
| Lot 4 | 99.6 ± 0.1 | 89.4 | 41.8 ± 1.6 | 99.4 ± 0.2 | 92.7 | 53.3 ± 1.1 | 96.0 ± 0.1 | 89.9 | 95.1 |
| Lot 5 | 99.6 ± 0.1 | 90.6 | 39.8 ± 2.3 | 99.5 ± 0.0 | 90.7 | 56.0 ± 0.6 | 96.4 ± 0.2 | 91.7 | 95.8 |
| Lot 6 | 99.5 ± 0.1 | 86.2 | 39.5 ± 0.2 | 99.4 ± 0.1 | 85.6 | 54.6 ± 1.9 | 97.0 ± 0.4 | 85.7 | 94.1 |
| Lot 7 | 99.6 ± 0.0 | 90.4 | 44.0 ± 0.2 | 99.6 ± 0.0 | 96.1 | 51.9 ± 0.9 | 96.9 ± 0.9 | 93.0 | 95.9 |
| Lot 8 | 97.0 ± 2.5 | 74.7 | 34.1 ± 2.3 | 98.5 ± 1.1 | 83.6 | 60.5 ± 1.3 | 96.1 ± 2.3 | 83.8 | 94.6 |
| Lot 9 | 99.6 ± 0.1 | 89.3 | 42.0 ± 2.5 | 99.4 ± 0.2 | 88.2 | 53.9 ± 1.3 | 96.5 ± 0.9 | 91.3 | 95.9 |
| Lot 10 | 99.6 ± 0.0 | 83.8 | 42.0 ± 0.3 | 99.6 ± 0.0 | 86.2 | 52.3 ± 1.1 | 97.1 ± 0.4 | 88.8 | 94.3 |
| Lot 11 | 99.5 ± 0.1 | 86.8 | 40.3 ± 4.6 | 99.6 ± 0.0 | 88.0 | 52.3 ± 1.9 | 97.0 ± 0.1 | not tested | 92.6 |
| Lot 12 | 98.8 ± 1.0 | 91.5 | 40.6 ± 1.6 | 99.6 ± 0.1 | 90.5 | 55.4 ± 1.7 | 97.2 ± 0.1 | not tested | 96.0 |
| Mean | 99.2 ± 0.9 | 87.0 ± 5.2 | 40.5 ± 2.8 | 99.4 ± 0.4 | 89.1 ± 3.9 | 54.5 ± 2.7 | 96.7 ± 0.5 | 89.2 ± 3.3 | 94.9 ± 1.1 |
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons by Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cipolla, D.; Blanchard, J.; Gonda, I. Development of Liposomal Ciprofloxacin to Treat Lung Infections. Pharmaceutics 2016, 8, 6. https://doi.org/10.3390/pharmaceutics8010006
Cipolla D, Blanchard J, Gonda I. Development of Liposomal Ciprofloxacin to Treat Lung Infections. Pharmaceutics. 2016; 8(1):6. https://doi.org/10.3390/pharmaceutics8010006
Chicago/Turabian StyleCipolla, David, Jim Blanchard, and Igor Gonda. 2016. "Development of Liposomal Ciprofloxacin to Treat Lung Infections" Pharmaceutics 8, no. 1: 6. https://doi.org/10.3390/pharmaceutics8010006
APA StyleCipolla, D., Blanchard, J., & Gonda, I. (2016). Development of Liposomal Ciprofloxacin to Treat Lung Infections. Pharmaceutics, 8(1), 6. https://doi.org/10.3390/pharmaceutics8010006