Increased Susceptibility to Pilocarpine-Induced Status Epilepticus and Reduced Latency in TRPC1/4 Double Knockout Mice
Abstract
:1. Introduction
2. Materials and Methods
3. Results
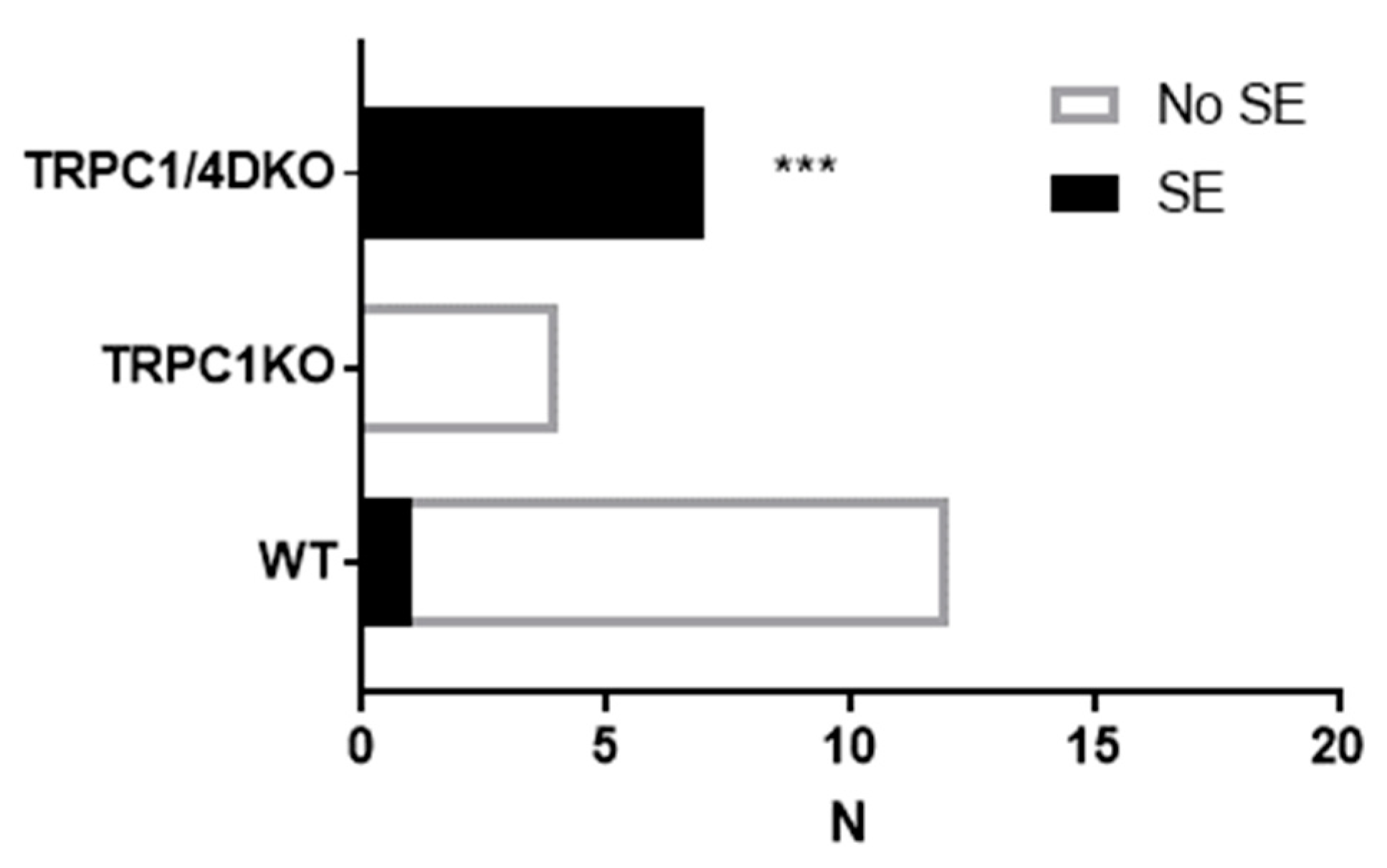
3.1. TRPC1/4 DKO Mice Exhbit Increased SE Susceptibility
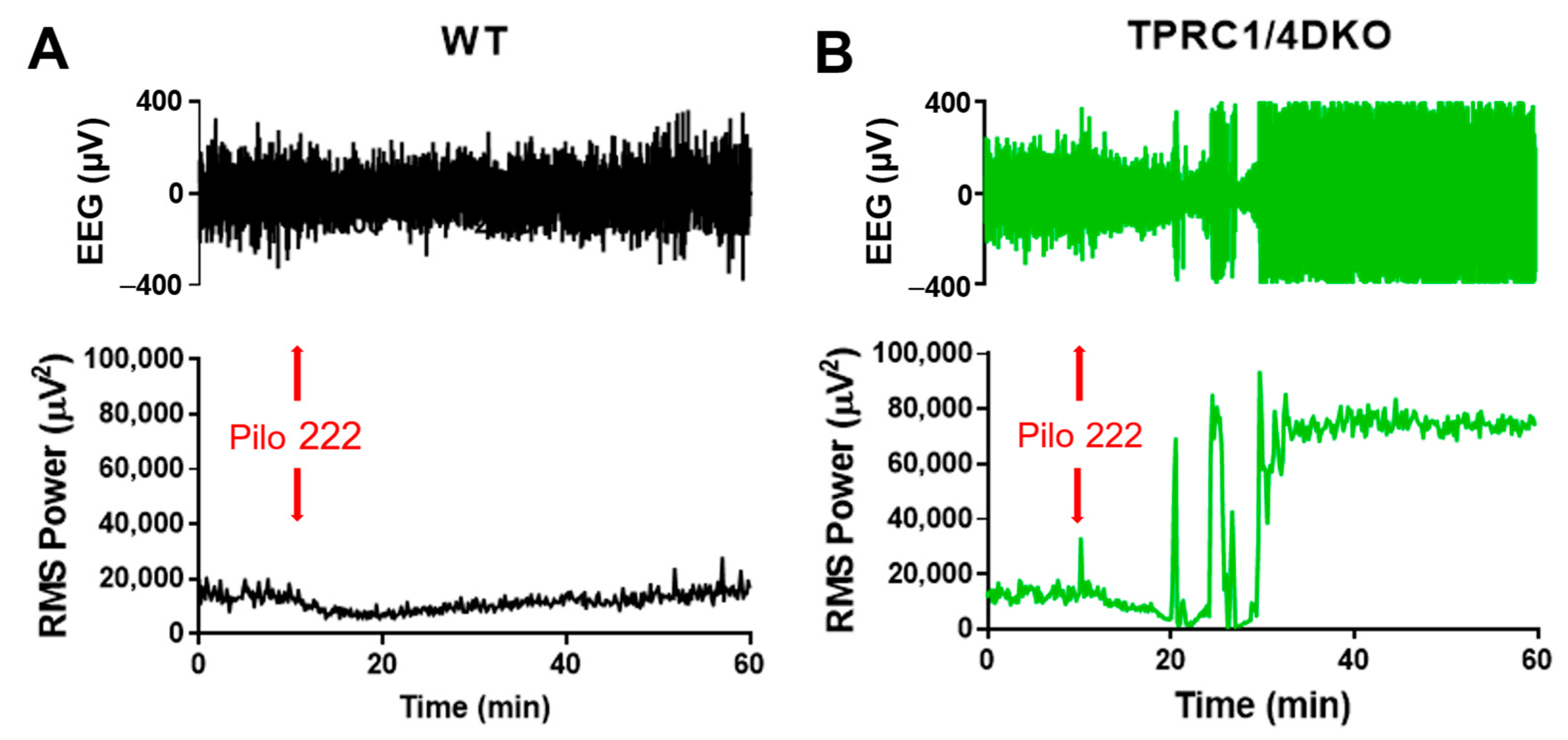
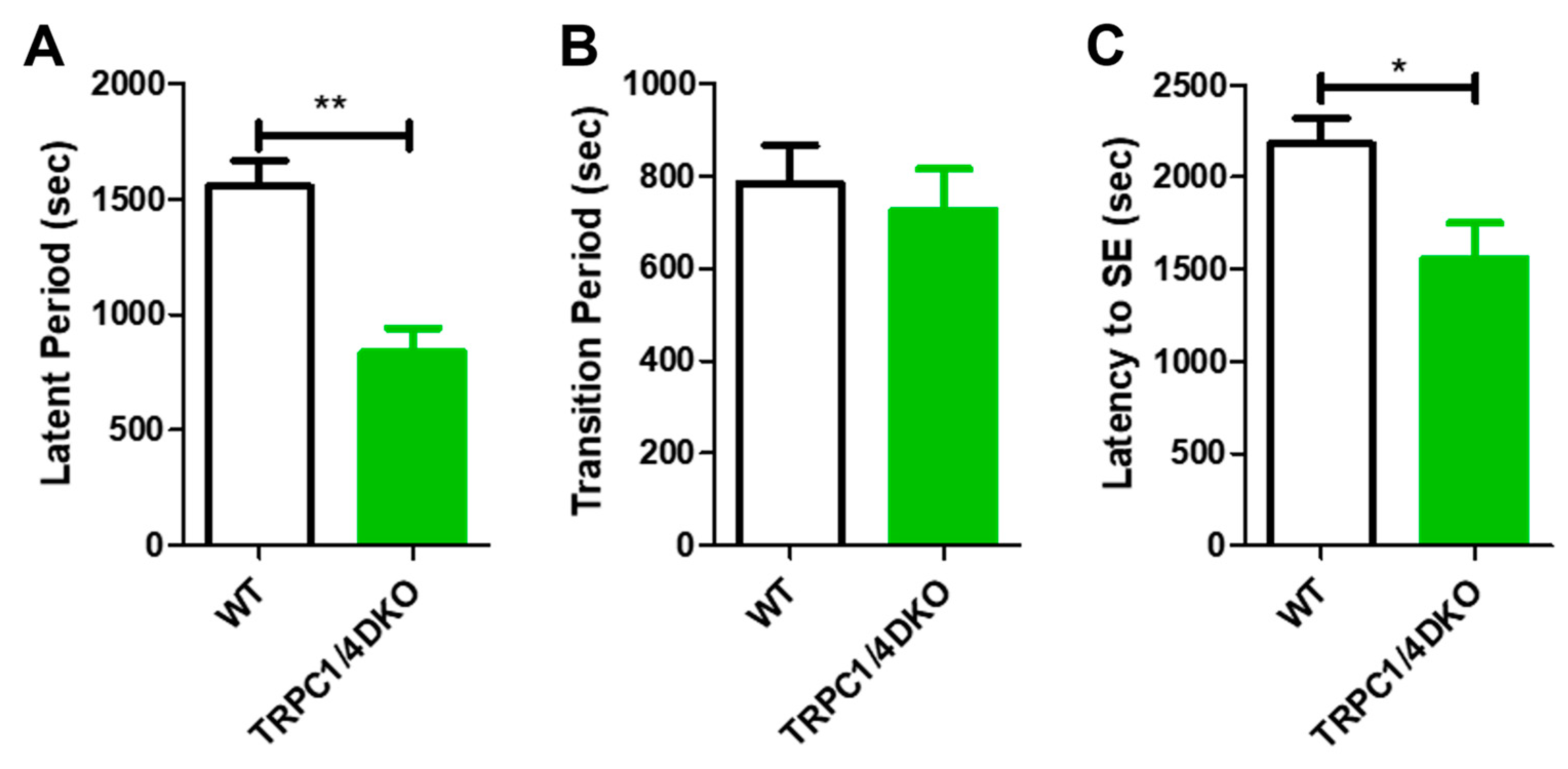
3.2. TRPC1/4 DKO Mice Exhibit Early Cortical Seizures and Reduced SE Latency
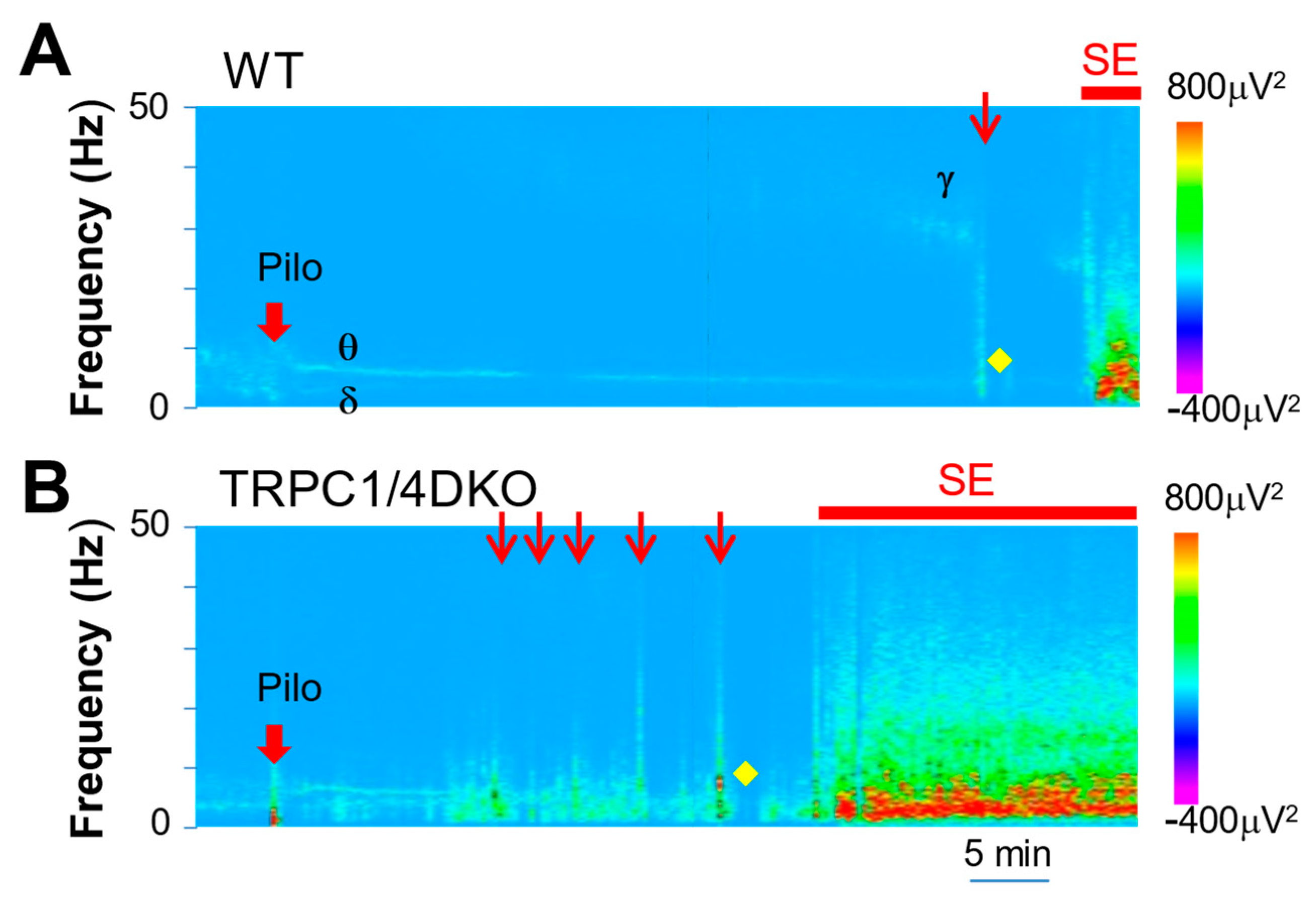
3.3. Spectral Analysis of SE Progression in WT and TRPC1/4 DKO Mice
3.4. SE intensities Were Comparable in WT and TRPC1/4 DKO Mice
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A
References
- Lowenstein, D.H.; Alldredge, B.K. Status Epilepticus. N. Engl. J. Med. 1998, 338, 970–976. [Google Scholar] [CrossRef]
- DeLorenzo, R.J.; Hauser, W.A.; Towne, A.R.; Boggs, J.G.; Pellock, J.M.; Penberthy, L.; Garnett, L.; Fortner, C.A.; Ko, D. A Prospective, Population-Based Epidemiologic Study of Status Epilepticus in Richmond, Virginia. Neurology 1996, 46, 1029–1035. [Google Scholar] [CrossRef] [PubMed]
- Brophy, G.M.; Bell, R.; Claassen, J.; Alldredge, B.; Bleck, T.P.; Glauser, T.; Laroche, S.M.; Riviello, J.J.; Shutter, L.; Sperling, M.R.; et al. Guidelines for the Evaluation and Management of Status Epilepticus. Neurocrit. Care 2012, 17, 3–23. [Google Scholar] [CrossRef] [PubMed]
- Glauser, T.; Shinnar, S.; Gloss, D.; Alldredge, B.; Arya, R.; Bainbridge, J.; Bare, M.; Bleck, T.; Dodson, W.E.; Garrity, L.; et al. Evidence-Based Guideline: Treatment of Convulsive Status Epilepticus in Children and Adults: Report of the Guideline Committee of the American Epilepsy Society. Epilepsy Curr. 2016, 16, 48–61. [Google Scholar] [CrossRef] [PubMed]
- Rossetti, A.O.; Lowenstein, D.H. Management of Refractory Status Epilepticus in Adults: Still More Questions than Answers. Lancet Neurol. 2011, 10, 922–930. [Google Scholar] [CrossRef] [PubMed]
- Löscher, W. Mechanisms of Drug Resistance in Status Epilepticus. Epilepsia 2007, 48 (Suppl. S8), 74–77. [Google Scholar] [CrossRef] [PubMed]
- Pereira De Vasconcelos, A.; Mazarati, A.M.; Wasterlain, C.G.; Nehlig, A. Self-Sustaining Status Epilepticus after a Brief Electrical Stimulation of the Perforant Path: A 2-Deoxyglucose Study. Brain Res. 1999, 838, 110–118. [Google Scholar] [CrossRef]
- Chen, J.W.Y.; Naylor, D.E.; Wasterlain, C.G. Advances in the Pathophysiology of Status Epilepticus. Acta Neurol. Scand. 2007, 115, 7–15. [Google Scholar] [CrossRef] [PubMed]
- Turski, W.A.; Cavalheiro, E.A.; Bortolotto, Z.A.; Mello, L.M.; Schwarz, M.; Turski, L. Seizures Produced by Pilocarpine in Mice: A Behavioral, Electroencephalographic and Morphological Analysis. Brain Res. 1984, 321, 237–253. [Google Scholar] [CrossRef]
- Turski, L.; Ikonomidou, C.; Turski, W.A.; Bortolotto, Z.A.; Cavalheiro, E.A. Review: Cholinergic Mechanisms and Epileptogenesis. The Seizures Induced by Pilocarpine: A Novel Experimental Model of Intractable Epilepsy. Synapse 1989, 3, 154–171. [Google Scholar] [CrossRef]
- Cavalheiro, E.A.; Santos, N.F.; Priel, M.R. The Pilocarpine Model of Epilepsy in Mice. Epilepsia 1996, 37, 1015–1019. [Google Scholar] [CrossRef] [PubMed]
- Mello, L.E.A.M.; Cavalheiro, E.A.; Tan, A.M.; Kupfer, W.R.; Pretorius, J.K.; Babb, T.L.; Finch, D.M.; Genton, P.; Portera-Sanchez, A. Circuit Mechanisms of Seizures in the Pilocarpine Model of Chronic Epilepsy: Cell Loss and Mossy Fiber Sprouting. Epilepsia 1993, 34, 985–995. [Google Scholar] [CrossRef]
- Treiman, D.M.; Walton, N.Y.; Kendrick, C. A Progressive Sequence of Electroencephalographic Changes during Generalized Convulsive Status Epilepticus. Epilepsy Res. 1990, 5, 49–60. [Google Scholar] [CrossRef] [PubMed]
- Handforth, A.; Ackermann, R.F. Hierarchy of Seizure States in the Electrogenic Limbic Status Epilepticus Model: Behavioral and Electrographic Observations of Initial States and Temporal Progression. Epilepsia 1992, 33, 589–600. [Google Scholar] [CrossRef]
- Phelan, K.D.; Shwe, U.T.; Williams, D.K.; Greenfield, L.J.; Zheng, F. Pilocarpine-Induced Status Epilepticus in Mice: A Comparison of Spectral Analysis of Electroencephalogram and Behavioral Grading Using the Racine Scale. Epilepsy Res. 2015, 117, 90–96. [Google Scholar] [CrossRef]
- Clapham, D.E.; Runnels, L.W.; Strübing, C. The TRP Ion Channel Family. Nat. Rev. Neurosci. 2001, 2, 387–396. [Google Scholar] [CrossRef] [PubMed]
- Moran, M.M.; Xu, H.; Clapham, D.E. TRP Ion Channels in the Nervous System. Curr. Opin. Neurobiol. 2004, 14, 362–369. [Google Scholar] [CrossRef] [PubMed]
- Pedersen, S.F.; Owsianik, G.; Nilius, B. TRP Channels: An Overview. Cell Calcium 2005, 38, 233–252. [Google Scholar] [CrossRef]
- Birnbaumer, L. The TRPC Class of Ion Channels: A Critical Review of Their Roles in Slow, Sustained Increases in Intracellular Ca2+ Concentrations. Annu. Rev. Pharmacol. Toxicol. 2009, 49, 395–426. [Google Scholar] [CrossRef]
- Wang, H.; Cheng, X.; Tian, J.; Xiao, Y.; Tian, T.; Xu, F.; Hong, X.; Zhu, M.X. TRPC Channels: Structure, Function, Regulation and Recent Advances in Small Molecular Probes. Pharmacol. Ther. 2020, 209, 107497. [Google Scholar] [CrossRef]
- Phelan, K.D.; Shwe, U.T.; Abramowitz, J.; Birnbaumer, L.; Zheng, F. Critical Role of Canonical Transient Receptor Potential Channel 7 in Initiation of Seizures. Proc. Natl. Acad. Sci. USA 2014, 111, 11533–11538. [Google Scholar] [CrossRef]
- Phelan, K.D.; Shwe, U.T.; Cozart, M.A.; Wu, H.; Mock, M.M.; Abramowitz, J.; Birnbaumer, L.; Zheng, F. TRPC3 Channels Play a Critical Role in the Theta Component of Pilocarpine-Induced Status Epilepticus in Mice. Epilepsia 2017, 58, 247–254. [Google Scholar] [CrossRef]
- Zeng, C.; Zhou, P.; Jiang, T.; Yuan, C.; Ma, Y.; Feng, L.; Liu, R.; Tang, W.; Long, X.; Xiao, B.; et al. Upregulation and Diverse Roles of TRPC3 and TRPC6 in Synaptic Reorganization of the Mossy Fiber Pathway in Temporal Lobe Epilepsy. Mol. Neurobiol. 2015, 52, 562–572. [Google Scholar] [CrossRef]
- Zhang, J.; Li, Z. TRPC4 Ion Channel Regulations by Small-Molecular Inhibitors and Calmodulin. Cell Calcium 2021, 95, 102361. [Google Scholar] [CrossRef]
- Strübing, C.; Krapivinsky, G.; Krapivinsky, L.; Clapham, D.E. Formation of Novel TRPC Channels by Complex Subunit Interactions in Embryonic Brain. J. Biol. Chem. 2003, 278, 39014–39019. [Google Scholar] [CrossRef]
- Duan, J.; Li, J.; Zeng, B.; Chen, G.-L.; Peng, X.; Zhang, Y.; Wang, J.; Clapham, D.E.; Li, Z.; Zhang, J. Structure of the Mouse TRPC4 Ion Channel. Nat. Commun. 2018, 9, 3102. [Google Scholar] [CrossRef]
- Schaefer, M.; Plant, T.D.; Obukhov, A.G.; Hofmann, T.; Gudermann, T.; Schultz, G. Receptor-Mediated Regulation of the Nonselective Cation Channels TRPC4 and TRPC5. J. Biol. Chem. 2000, 275, 17517–17526. [Google Scholar] [CrossRef] [PubMed]
- Storch, U.; Forst, A.-L.L.; Philipp, M.; Gudermann, T.; Mederos, Y.; Schnitzler, M. Transient Receptor Potential Channel 1 (TRPC1) Reduces Calcium Permeability in Heteromeric Channel Complexes. J. Biol. Chem. 2012, 287, 3530–3540. [Google Scholar] [CrossRef] [PubMed]
- Ko, J.; Myeong, J.; Shin, Y.-C.; So, I. Differential PI(4,5)P2 Sensitivities of TRPC4, C5 Homomeric and TRPC1/4, C1/5 Heteromeric Channels. Sci. Rep. 2019, 9, 1849. [Google Scholar] [CrossRef] [PubMed]
- Kollewe, A.; Schwarz, Y.; Oleinikov, K.; Raza, A.; Haupt, A.; Wartenberg, P.; Wyatt, A.; Boehm, U.; Ectors, F.; Bildl, W.; et al. Subunit Composition, Molecular Environment, and Activation of Native TRPC Channels Encoded by Their Interactomes. Neuron 2022, 110, 4162–4175. [Google Scholar] [CrossRef] [PubMed]
- Phelan, K.D.; Mock, M.M.; Kretz, O.; Shwe, U.T.; Kozhemyakin, M.; Greenfield, L.J.; Dietrich, A.; Birnbaumer, L.; Freichel, M.; Flockerzi, V.; et al. Heteromeric Canonical Transient Receptor Potential 1 and 4 Channels Play a Critical Role in Epileptiform Burst Firing and Seizure-Induced Neurodegeneration. Mol. Pharmacol. 2012, 81, 384–392. [Google Scholar] [CrossRef]
- Phelan, K.D.; Shwe, U.T.; Abramowitz, J.; Wu, H.; Rhee, S.W.; Howell, M.D.; Gottschall, P.E.; Freichel, M.; Flockerzi, V.; Birnbaumer, L.; et al. Canonical Transient Receptor Channel 5 (TRPC5) and TRPC1/4 Contribute to Seizure and Excitotoxicity by Distinct Cellular Mechanisms. Mol. Pharmacol. 2013, 83, 429–438. [Google Scholar] [CrossRef]
- Tian, J.; Thakur, D.P.; Lu, Y.; Zhu, Y.; Freichel, M.; Flockerzi, V.; Zhu, M.X. Dual Depolarization Responses Generated within the Same Lateral Septal Neurons by TRPC4-Containing Channels. Pflugers Arch. 2014, 466, 1301–1316. [Google Scholar] [CrossRef] [PubMed]
- Shao, L.-R.; Dudek, F.E. Changes in MIPSCs and SIPSCs after Kainate Treatment: Evidence for Loss of Inhibitory Input to Dentate Granule Cells and Possible Compensatory Responses. J. Neurophysiol. 2005, 94, 952–960. [Google Scholar] [CrossRef] [PubMed]
- McBain, C.J.; Dingledine, R. Heterogeneity of Synaptic Glutamate Receptors on CA3 Stratum Radiatum Interneurones of Rat Hippocampus. J. Physiol. 1993, 462, 373–392. [Google Scholar] [CrossRef] [PubMed]
- Mulle, C.; Sailer, A.; Pérez-Otaño, I.; Dickinson-Anson, H.; Castillo, P.E.; Bureau, I.; Maron, C.; Gage, F.H.; Mann, J.R.; Bettler, B.; et al. Altered Synaptic Physiology and Reduced Susceptibility to Kainate-Induced Seizures in GluR6-Deficient Mice. Nature 1998, 392, 601–605. [Google Scholar] [CrossRef] [PubMed]
- Löscher, W. Critical Review of Current Animal Models of Seizures and Epilepsy Used in the Discovery and Development of New Antiepileptic Drugs. Seizure 2011, 20, 359–368. [Google Scholar] [CrossRef]
- Lüttjohann, A.; Fabene, P.F.; van Luijtelaar, G. A Revised Racine’s Scale for PTZ-Induced Seizures in Rats. Physiol. Behav. 2009, 98, 579–586. [Google Scholar] [CrossRef] [PubMed]
- Frohlich, J.; Toker, D.; Monti, M.M. Consciousness among Delta Waves: A Paradox? Brain 2021, 144, 2257–2277. [Google Scholar] [CrossRef]
- Dichter, M.A.; Ayala, G.F. Cellular Mechanisms of Epilepsy: A Status Report. Science 1987, 237, 157–164. [Google Scholar] [CrossRef]
- Oddie, S.D.; Bland, B.H. Hippocampal Formation Theta Activity and Movement Selection. Neurosci. Biobehav. Rev. 1998, 22, 221–231. [Google Scholar] [CrossRef]
- Vandecasteele, M.; Varga, V.; Berényi, A.; Papp, E.; Barthó, P.; Venance, L.; Freund, T.F.; Buzsáki, G. Optogenetic Activation of Septal Cholinergic Neurons Suppresses Sharp Wave Ripples and Enhances Theta Oscillations in the Hippocampus. Proc. Natl. Acad. Sci. USA 2014, 111, 13535–13540. [Google Scholar] [CrossRef]
- Leranth, C.; Deller, T.; Buzsáki, G. Intraseptal Connections Redefined: Lack of a Lateral Septum to Medial Septum Path. Brain Res. 1992, 583, 1–11. [Google Scholar] [CrossRef]
- Bains, J.S.; Longacher, J.M.; Staley, K.J. Reciprocal Interactions between CA3 Network Activity and Strength of Recurrent Collateral Synapses. Nat. Neurosci. 1999, 2, 720–726. [Google Scholar] [CrossRef]
- Stoop, R.; Conquet, F.; Zuber, B.; Voronin, L.L.; Pralong, E. Activation of Metabotropic Glutamate 5 and NMDA Receptors Underlies the Induction of Persistent Bursting and Associated Long-Lasting Changes in CA3 Recurrent Connections. J. Neurosci. 2003, 23, 5634–5644. [Google Scholar] [CrossRef]
- Bon, R.S.; Wright, D.J.; Beech, D.J.; Sukumar, P. Pharmacology of TRPC Channels and Its Potential in Cardiovascular and Metabolic Medicine. Annu. Rev. Pharmacol. Toxicol. 2022, 62, 427–446. [Google Scholar] [CrossRef]
- Bon, R.S.; Beech, D.J. In Pursuit of Small Molecule Chemistry for Calcium-Permeable Non-Selective TRPC Channels -- Mirage or Pot of Gold? Br. J. Pharmacol. 2013, 170, 459–474. [Google Scholar] [CrossRef]
- Minard, A.; Bauer, C.C.; Wright, D.J.; Rubaiy, H.N.; Muraki, K.; Beech, D.J.; Bon, R.S. Remarkable Progress with Small-Molecule Modulation of TRPC1/4/5 Channels: Implications for Understanding the Channels in Health and Disease. Cells 2018, 7, 52. [Google Scholar] [CrossRef] [PubMed]
- Muraki, K.; Ohnishi, K.; Takezawa, A.; Suzuki, H.; Hatano, N.; Muraki, Y.; Hamzah, N.; Foster, R.; Waldmann, H.; Nussbaumer, P.; et al. Na+ Entry through Heteromeric TRPC4/C1 Channels Mediates (-)Englerin A-Induced Cytotoxicity in Synovial Sarcoma Cells. Sci. Rep. 2017, 7, 16988. [Google Scholar] [CrossRef] [PubMed]
- Beck, A.; Speicher, T.; Stoerger, C.; Sell, T.; Dettmer, V.; Jusoh, S.A.; Abdulmughni, A.; Cavalié, A.; Philipp, S.E.; Zhu, M.X.; et al. Conserved Gating Elements in TRPC4 and TRPC5 Channels. J. Biol. Chem. 2013, 288, 19471–19483. [Google Scholar] [CrossRef] [PubMed]
- Schaefer, M.; Plant, T.D.; Stresow, N.; Albrecht, N.; Schultz, G. Functional Differences between TRPC4 Splice Variants. J. Biol. Chem. 2002, 277, 3752–3759. [Google Scholar] [CrossRef] [PubMed]
- Phelan, K.D.; Shwe, U.T.; Zheng, F. Pharmacological Differences between Native Homomeric Transient Receptor Potential Canonical Type 4 Channels and Heteromeric Transient Receptor Potential Canonical Type 1/4 Channels in Lateral Septal Neurons. Pharmaceuticals 2023, 16, 1291. [Google Scholar] [CrossRef] [PubMed]

| Frequency Range | WT (n = 5) | TRPC1/4 DKO (n = 6) |
|---|---|---|
| Full (0–1000 Hz) | 917,781.1 ± 225,984.3 | 966,520.4 ± 231,360.0 |
| Delta | 71,909.6 ± 7527.5 | 69,378.0 ± 23,807.7 |
| Theta | 62,473.5 ± 14,462.0 | 62,483.5 ± 16,099.3 |
| Alpha | 90,492.3 ± 20,899.5 | 100,915.5 ± 27,197.3 |
| Beta | 181,098.8 ± 42,418.7 | 202,542.0 ± 59,527.1 |
| Gamma | 58,089.1 ± 14,935.9 | 70,425.1 ± 21,369.4 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zheng, F.; Phelan, K.D.; Shwe, U.T. Increased Susceptibility to Pilocarpine-Induced Status Epilepticus and Reduced Latency in TRPC1/4 Double Knockout Mice. Neurol. Int. 2023, 15, 1469-1479. https://doi.org/10.3390/neurolint15040095
Zheng F, Phelan KD, Shwe UT. Increased Susceptibility to Pilocarpine-Induced Status Epilepticus and Reduced Latency in TRPC1/4 Double Knockout Mice. Neurology International. 2023; 15(4):1469-1479. https://doi.org/10.3390/neurolint15040095
Chicago/Turabian StyleZheng, Fang, Kevin D. Phelan, and U Thaung Shwe. 2023. "Increased Susceptibility to Pilocarpine-Induced Status Epilepticus and Reduced Latency in TRPC1/4 Double Knockout Mice" Neurology International 15, no. 4: 1469-1479. https://doi.org/10.3390/neurolint15040095





