An Updated Review on Probiotic Production and Applications
Abstract
:1. Introduction
2. Probiotic Strain Selection Criteria and Requirements
- Stability of phenotypes and genotypes, including plasmid stability;
- Tolerance to bile and acid, as well as survival and growth;
- The adhesion characteristics of intestinal epithelial cells;
- Antimicrobial compound production;
- Patterns of antibiotic resistance;
- Inhibition of known gut pathogens;
- Immunogenicity, spoilage organisms, or both.
2.1. Probiotics with the Best Characterization: In Health Point
2.2. Probiotic Viability: What Factors Affect It?
2.3. Physicians’ Guidance
- Evidence that the strains were tested in a randomized, controlled, or comparable human experiment and categorized based on specific host or microbial genetic characteristics in a varied population.
- In the product, the dose and viability are the same as in the human experiment.
- There is whole-genome strain characterization and precise strain designation available.
2.4. Dairy Starter Cultures of Probiotic for Manufacturing
2.5. Production and Strains Development
2.6. Nutritional Necessities of the Strain
2.7. Raw Material Production
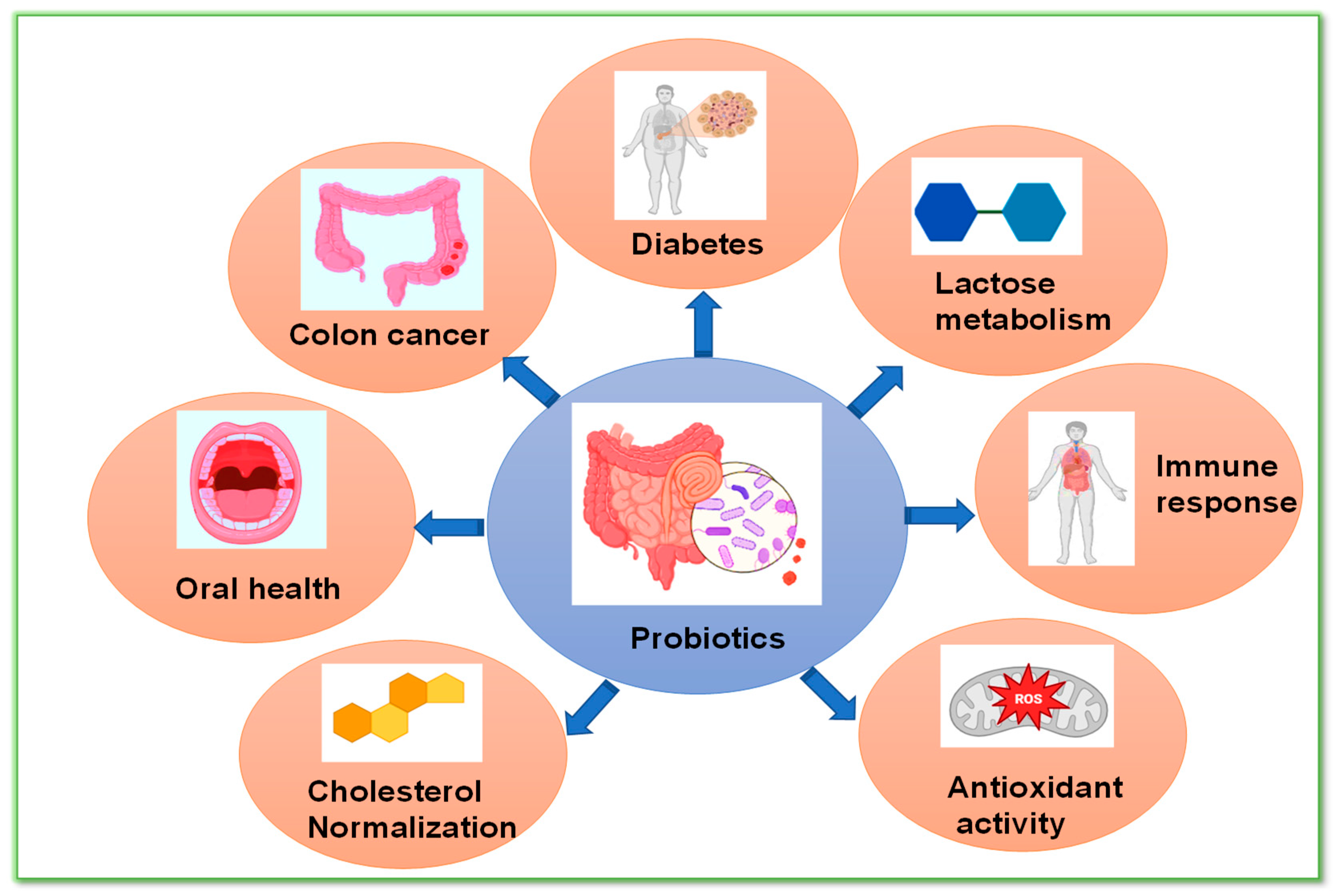
3. Applications of Probiotics
3.1. Probiotic’s Role in Colorectal Cancer
3.2. Probiotic’s Role in Type 2 Diabetes Mellitus and Obesity
3.3. Probiotic Role in Inflammatory Bowel Disease (IBD)
3.4. Probiotics on Cholesterol Metabolism
3.5. Probiotics in Lactose Metabolism
3.6. Probiotics in Immune Response
3.7. Antioxidant Activity of Probiotics
3.8. Probiotics and Oral Health
4. Summary and Future Directions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Soemarie, Y.B.; Milanda, T.; Barliana, M.I. Fermented foods as probiotics: A review. J. Adv. Pharm. Technol. Res. 2021, 12, 335. [Google Scholar]
- Zolotukhin, P.; Prazdnova, E.; Chistyakov, V. Methods to assess the antioxidative properties of probiotics. Probiotics Antimicrob. Proteins 2018, 10, 589–599. [Google Scholar] [CrossRef]
- Kim, E.N.; Kim, M.Y.; Lim, J.H.; Kim, Y.; Shin, S.J.; Park, C.W.; Kim, Y.-S.; Chang, Y.S.; Yoon, H.E.; Choi, B.S. The protective effect of resveratrol on vascular aging by modulation of the renin–angiotensin system. Atherosclerosis 2018, 270, 123–131. [Google Scholar] [CrossRef]
- Silva, D.R.; Sardi, J.d.C.O.; de Souza Pitangui, N.; Roque, S.M.; da Silva, A.C.B.; Rosalen, P.L. Probiotics as an alternative antimicrobial therapy: Current reality and future directions. J. Funct. Foods 2020, 73, 104080. [Google Scholar] [CrossRef]
- Yoha, K.; Nida, S.; Dutta, S.; Moses, J.; Anandharamakrishnan, C. Targeted delivery of probiotics: Perspectives on research and commercialization. Probiotics Antimicrob. Proteins 2022, 14, 15–48. [Google Scholar] [CrossRef] [PubMed]
- Im, E.J.; Lee, H.H.-Y.; Kim, M.; Kim, M.-K. Evaluation of Enterococcal Probiotic Usage and Review of Potential Health Benefits, Safety, and Risk of Antibiotic-Resistant Strain Emergence. Antibiotics 2023, 12, 1327. [Google Scholar] [CrossRef] [PubMed]
- Carlson, J.; Slavin, J. Health benefits of fibre, prebiotics and probiotics: A review of intestinal health and related health claims. Qual. Assur. Saf. Crops Foods 2016, 8, 539–554. [Google Scholar] [CrossRef]
- Gheorghita, R.; Anchidin-Norocel, L.; Filip, R.; Dimian, M.; Covasa, M. Applications of biopolymers for drugs and probiotics delivery. Polymers 2021, 13, 2729. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Zhang, P.; Zhang, X. Probiotics regulate gut microbiota: An effective method to improve immunity. Molecules 2021, 26, 6076. [Google Scholar] [CrossRef]
- Fadl, M.G.; Kamel, Z. Cholesterol-lowering effects and safety assessment of Lactobacillus spp. in vivo and in vitro testing for human use as probiotic from the dairy product in Egypt. J. Genet. Eng. Biotechnol. 2022, 20, 1–11. [Google Scholar] [CrossRef]
- Rashidinejad, A.; Bahrami, A.; Rehman, A.; Rezaei, A.; Babazadeh, A.; Singh, H.; Jafari, S.M. Co-encapsulation of probiotics with prebiotics and their application in functional/synbiotic dairy products. Crit. Rev. Food Sci. Nutr. 2022, 62, 2470–2494. [Google Scholar] [CrossRef]
- Varela-Pérez, A.; Romero-Chapol, O.O.; Castillo-Olmos, A.G.; García, H.S.; Suárez-Quiroz, M.L.; Singh, J.; Figueroa-Hernández, C.Y.; Viveros-Contreras, R.; Cano-Sarmiento, C. Encapsulation of Lactobacillus gasseri: Characterization, probiotic survival, in vitro evaluation and viability in apple juice. Foods 2022, 11, 740. [Google Scholar] [CrossRef]
- Kowalska, E.; Ziarno, M.; Ekielski, A.; Żelaziński, T. Materials used for the microencapsulation of probiotic bacteria in the food industry. Molecules 2022, 27, 3321. [Google Scholar] [CrossRef]
- Tan, L.L.; Ang, K.L.; Loo, S.C.J. Alginate encapsulation improves probiotics survival in carbonated sodas and beers. PLoS ONE 2023, 18, e0283745. [Google Scholar] [CrossRef]
- Terpou, A.; Papadaki, A.; Lappa, I.K.; Kachrimanidou, V.; Bosnea, L.A.; Kopsahelis, N. Probiotics in food systems: Significance and emerging strategies towards improved viability and delivery of enhanced beneficial value. Nutrients 2019, 11, 1591. [Google Scholar] [CrossRef]
- Fobofou, S.A.; Savidge, T. Microbial metabolites: Cause or consequence in gastrointestinal disease? Am. J. Physiol. Gastrointest. Liver Physiol. 2022, 322, G535–G552. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.-G.; Sakamoto, K.; Seo, S.-U.; Pickard, J.M.; Gillilland III, M.G.; Pudlo, N.A.; Hoostal, M.; Li, X.; Wang, T.D.; Feehley, T. Neonatal acquisition of Clostridia species protects against colonization by bacterial pathogens. Science 2017, 356, 315–319. [Google Scholar] [CrossRef] [PubMed]
- Feehley, T.; Plunkett, C.H.; Bao, R.; Choi Hong, S.M.; Culleen, E.; Belda-Ferre, P.; Campbell, E.; Aitoro, R.; Nocerino, R.; Paparo, L. Healthy infants harbor intestinal bacteria that protect against food allergy. Nat. Med. 2019, 25, 448–453. [Google Scholar] [CrossRef] [PubMed]
- Bajaj, J.S. Alcohol, liver disease and the gut microbiota. Nat. Rev. Gastroenterol. Hepatol. 2019, 16, 235–246. [Google Scholar] [CrossRef] [PubMed]
- Valles-Colomer, M.; Falony, G.; Darzi, Y.; Tigchelaar, E.F.; Wang, J.; Tito, R.Y.; Schiweck, C.; Kurilshikov, A.; Joossens, M.; Wijmenga, C. The neuroactive potential of the human gut microbiota in quality of life and depression. Nat. Microbiol. 2019, 4, 623–632. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Wang, T.; Li, Y.; Zhang, T.; Wang, Q.; He, J.; Wang, L.; Li, L.; Yang, N.; Fang, Y. Probiotics for the treatment of women with bacterial vaginosis: A systematic review and meta-analysis of randomized clinical trials. Eur. J. Pharmacol. 2019, 864, 172660. [Google Scholar] [CrossRef] [PubMed]
- Reid, G.; Gadir, A.A.; Dhir, R. Probiotics: Reiterating what they are and what they are not. Front. Microbiol. 2019, 10, 424. [Google Scholar] [CrossRef] [PubMed]
- Kleerebezem, M.; Binda, S.; Bron, P.A.; Gross, G.; Hill, C.; van Hylckama Vlieg, J.E.; Lebeer, S.; Satokari, R.; Ouwehand, A.C. Understanding mode of action can drive the translational pipeline towards more reliable health benefits for probiotics. Curr. Opin. Biotechnol. 2019, 56, 55–60. [Google Scholar] [CrossRef] [PubMed]
- Wandro, S.; Osborne, S.; Enriquez, C.; Bixby, C.; Arrieta, A.; Whiteson, K. The microbiome and metabolome of preterm infant stool are personalized and not driven by health outcomes, including necrotizing enterocolitis and late-onset sepsis. Msphere 2018, 3, e00104-18. [Google Scholar] [CrossRef] [PubMed]
- Popović, N.; Brdarić, E.; Đokić, J.; Dinić, M.; Veljović, K.; Golić, N.; Terzić-Vidojević, A. Yogurt produced by novel natural starter cultures improves gut epithelial barrier in vitro. Microorganisms 2020, 8, 1586. [Google Scholar] [CrossRef] [PubMed]
- Zommiti, M.; Feuilloley, M.G.; Connil, N. Update of probiotics in human world: A nonstop source of benefactions till the end of time. Microorganisms 2020, 8, 1907. [Google Scholar] [CrossRef] [PubMed]
- Kiepś, J.; Dembczyński, R. Current trends in the production of probiotic formulations. Foods 2022, 11, 2330. [Google Scholar] [CrossRef]
- Wang, G.; Chen, Y.; Xia, Y.; Song, X.; Ai, L. Characteristics of probiotic preparations and their applications. Foods 2022, 11, 2472. [Google Scholar] [CrossRef]
- Gao, H.; Li, X.; Chen, X.; Hai, D.; Wei, C.; Zhang, L.; Li, P. The Functional Roles of Lactobacillus Acidophilus in Different Physiological and Pathological Processes. J. Microbiol. Biotechnol. 2022, 32, 1226. [Google Scholar] [CrossRef]
- Chen, J.; Chen, X.; Ho, C. Recent Development of Probiotic Bifidobacteria for Treating Human Diseases. Front. Bioeng. Biotechnol. 2021, 9, 770248. [Google Scholar] [CrossRef]
- Wang, Y.; Wu, J.; Lv, M.; Shao, Z.; Hungwe, M.; Wang, J.; Bai, X.; Xie, J.; Wang, Y.; Geng, W. Metabolism characteristics of lactic acid bacteria and the expanding applications in food industry. Front. Bioeng. Biotechnol. 2021, 9, 612285. [Google Scholar] [CrossRef] [PubMed]
- Behera, S.; Ray, R.; Zdolec, N. Lactobacillus plantarum with Functional Properties: An Approach to Increase Safety and Shelf-Life of Fermented Foods. BioMed Res. Int. 2018, 2018, 9361614. [Google Scholar] [CrossRef] [PubMed]
- Davoren, M.J.; Liu, J.; Castellanos, J.; Rodríguez-Malavé, N.I.; Schiestl, R.H. A novel probiotic, Lactobacillus johnsonii 456, resists acid and can persist in the human gut beyond the initial ingestion period. Gut Microbes 2019, 10, 458–480. [Google Scholar] [CrossRef] [PubMed]
- Yilmaz, B.; Bangar, S.P.; Echegaray, N.; Suri, S.; Tomasevic, I.; Manuel Lorenzo, J.; Melekoglu, E.; Rocha, J.M.; Ozogul, F. The impacts of Lactiplantibacillus plantarum on the functional properties of fermented foods: A review of current knowledge. Microorganisms 2022, 10, 826. [Google Scholar] [CrossRef] [PubMed]
- Naibaho, J.; Jonuzi, E.; Butula, N.; Korzeniowska, M.; Föste, M.; Sinamo, K.N.; Chodaczek, G.; Yang, B. Fortification of milk-based yogurt with protein hydrolysates from brewers’ spent grain: Evaluation on microstructural properties, lactic acid bacteria profile, lactic acid forming capability and its physical behavior. Curr. Res. Food Sci. 2022, 5, 1955–1964. [Google Scholar] [CrossRef] [PubMed]
- Kamzolova, S.V.; Morgunov, I.G. Optimization of medium composition and fermentation conditions for α-ketoglutaric acid production from biodiesel waste by Yarrowia lipolytica. Appl. Microbiol. Biotechnol. 2020, 104, 7979–7989. [Google Scholar] [CrossRef]
- Zhou, Y.; Yang, X.; Li, Q.; Peng, Z.; Li, J.; Zhang, J. Optimization of fermentation conditions for surfactin production by B. subtilis YPS-32. BMC Microbiol. 2023, 23, 117. [Google Scholar] [CrossRef]
- Fenster, K.; Freeburg, B.; Hollard, C.; Wong, C.; Rønhave Laursen, R.; Ouwehand, A. The Production and Delivery of Probiotics: A Review of a Practical Approach. Microorganisms 2019, 17, 83. [Google Scholar] [CrossRef]
- Shruthi, B.R.; Achur, R.N.H.; Nayaka Boramuthi, T. Optimized solid-state fermentation medium enhances the multienzymes production from Penicillium citrinum and Aspergillus clavatus. Curr. Microbiol. 2020, 77, 2192–2206. [Google Scholar] [CrossRef]
- Wu, D.; Fu, L.; Cao, Y.; Dong, N.; Li, D. Genomic insights into antimicrobial potential and optimization of fermentation conditions of pig-derived Bacillus subtilis BS21. Front. Microbiol. 2023, 14, 1239837. [Google Scholar] [CrossRef]
- Mamun-Or-Rashid, A.N.M.; Lucy, T.T.; Pramanik, M.K. Isolation, Identification, Optimization of Baker’s Yeast from Natural Sources, Scale-Up Production Using Molasses as a Cheap Carbohydrate Source, and Evaluation for Bread Production. Appl. Microbiol. 2022, 2, 516–533. [Google Scholar] [CrossRef]
- Chotani, G.; Dodge, T.; Hsu, A.; Kumar, M.; LaDuca, R.; Trimbur, D.; Weyler, W.; Samford, K. The commercial production of chemicals using pathway engineering. Biochem. Biophys. Acta 2000, 1543, 434–455. [Google Scholar] [CrossRef] [PubMed]
- Ren, H.; Zentek, J.; Vahjen, W. Optimization of production parameters for probiotic Lactobacillus strains as feed additive. Molecules 2019, 24, 3286. [Google Scholar] [CrossRef]
- Demirgul, F.; Simsek, O.; Bozkurt, F.; Dertli, E.; Sagdic, O. Production and characterization of yeast extracts produced by Saccharomyces cerevisiae, Saccharomyces boulardii and Kluyveromyces marxianus. Prep. Biochem. Biotechnol. 2022, 52, 657–667. [Google Scholar] [CrossRef] [PubMed]
- Razali, W.A.W.; Pandhal, J. Outdoor pilot-scale cultivation and techno economic assessment of a novel omega-3 eicosapentaenoic acid over-producing Nannochloropsis oculata strain. Bioresour. Technol. Rep. 2023, 24, 101682. [Google Scholar] [CrossRef]
- Markowiak-Kopeć, P.; Śliżewska, K. The effect of probiotics on the production of short-chain fatty acids by human intestinal microbiome. Nutrients 2020, 12, 1107. [Google Scholar] [CrossRef] [PubMed]
- Merenstein, D.; Pot, B.; Leyer, G.; Ouwehand, A.C.; Preidis, G.A.; Elkins, C.A.; Hill, C.; Lewis, Z.T.; Shane, A.L.; Zmora, N. Emerging issues in probiotic safety: 2023 perspectives. Gut Microbes 2023, 15, 2185034. [Google Scholar] [CrossRef] [PubMed]
- Sivamaruthi, B.S.; Kesika, P.; Chaiyasut, C. The role of probiotics in colorectal cancer management. Evid.-Based Complement. Altern. Med. 2020, 2020, 3535982. [Google Scholar] [CrossRef]
- Shang, F.; Jiang, X.; Wang, H.; Chen, S.; Wang, X.; Liu, Y.; Guo, S.; Li, D.; Yu, W.; Zhao, Z. The inhibitory effects of probiotics on colon cancer cells: In vitro and in vivo studies. J. Gastrointest. Oncol. 2020, 11, 1224. [Google Scholar] [CrossRef]
- Peng, M.; Lee, S.-H.; Rahaman, S.O.; Biswas, D. Dietary probiotic and metabolites improve intestinal homeostasis and prevent colorectal cancer. Food Funct. 2020, 11, 10724–10735. [Google Scholar] [CrossRef]
- Ayesha, I.E.; Monson, N.R.; Klair, N.; Patel, U.; Saxena, A.; Patel, D.; Venugopal, S.; Ayesha, I.E. Probiotics and Their Role in the Management of Type 2 Diabetes Mellitus (Short-Term Versus Long-Term Effect): A Systematic Review and Meta-Analysis. Cureus 2023, 15, e46741. [Google Scholar] [CrossRef]
- Tao, Y.-W.; Gu, Y.-L.; Mao, X.-Q.; Zhang, L.; Pei, Y.-F. Effects of probiotics on type II diabetes mellitus: A meta-analysis. J. Transl. Med. 2020, 18, 30. [Google Scholar]
- Kheirkhah, A.H.; Forouzani-Moghaddam, M.J.; Afkhami-Ardekani, M. Relationship between probiotics and type 2 diabetes mellitus: A review. Iran. J. Diabetes Obes. 2023, 15, 119–128. [Google Scholar] [CrossRef]
- Toumi, R.; Samer, A.; Soufli, I.; Rafa, H.; Touil-Boukoffa, C. Role of probiotics and their metabolites in inflammatory bowel diseases (IBDs). Gastroenterol. Insights 2021, 12, 56–66. [Google Scholar]
- Pais, P.; Almeida, V.; Yılmaz, M.; Teixeira, M.C. Saccharomyces boulardii: What makes it tick as successful probiotic? J. Fungi 2020, 6, 78. [Google Scholar] [CrossRef] [PubMed]
- Ledder, O. Antibiotics in inflammatory bowel diseases: Do we know what we’re doing? Transl. Pediatr. 2019, 8, 42. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Cheng, Y.; Zhang, H.; Zhou, M.; Yu, Y.; Lin, S.; Jiang, B.; Zhao, X.; Miao, L.; Wei, C.-W. Integrated cascade nanozyme catalyzes in vivo ROS scavenging for anti-inflammatory therapy. Sci. Adv. 2020, 6, eabb2695. [Google Scholar] [CrossRef] [PubMed]
- Zhao, S.; Li, Y.; Liu, Q.; Li, S.; Cheng, Y.; Cheng, C.; Sun, Z.; Du, Y.; Butch, C.J.; Wei, H. An orally administered CeO2@ montmorillonite nanozyme targets inflammation for inflammatory bowel disease therapy. Adv. Funct. Mater. 2020, 30, 2004692. [Google Scholar] [CrossRef]
- Zhou, J.; Li, M.; Chen, Q.; Li, X.; Chen, L.; Dong, Z.; Zhu, W.; Yang, Y.; Liu, Z.; Chen, Q. Programmable probiotics modulate inflammation and gut microbiota for inflammatory bowel disease treatment after effective oral delivery. Nat. Commun. 2022, 13, 3432. [Google Scholar] [CrossRef]
- Xu, L.; Liu, B.; Huang, L.; Li, Z.; Cheng, Y.; Tian, Y.; Pan, G.; Li, H.; Xu, Y.; Wu, W.; et al. Probiotic Consortia and Their Metabolites Ameliorate the Symptoms of Inflammatory Bowel Diseases in a Colitis Mouse Model. Microbiol. Spectr. 2022, 10, e00622–e00657. [Google Scholar] [CrossRef]
- Najafi, S.; Sotoodehnejadnematalahi, F.; Amiri, M.M.; Pourshafie, M.R.; Rohani, M. Prophylactic vs. Therapeutic Effect of Probiotics on the Inflammation Mediated by the NF-κB Pathway in Inflammatory Bowel Conditions. Biomedicines 2023, 11, 1675. [Google Scholar] [CrossRef]
- Slack, R.; Macdonald, S.; Roper, J.; Jenkins, R.; Hatley, R. Emerging therapeutic opportunities for integrin inhibitors. Nat. Rev. Drug Discov. 2022, 21, 60–78. [Google Scholar] [CrossRef]
- Marzorati, M.; Bubeck, S.; Bayne, T.; Krishnan, K.; Giusto, M. Effects of combined prebiotic, probiotic, IgG and amino acid supplementation on the gut microbiome of patients with inflammatory bowel disease. Future Microbiol. 2022, 17, 1307–1324. [Google Scholar] [CrossRef]
- Chen, A.-C.; Fang, T.-J.; Ho, H.-H.; Chen, J.-F.; Kuo, Y.-W.; Huang, Y.-Y.; Tsai, S.-Y.; Wu, S.-F.; Lin, H.-C.; Yeh, Y.-T. A multi-strain probiotic blend reshaped obesity-related gut dysbiosis and improved lipid metabolism in obese children. Front. Nutr. 2022, 9, 922993. [Google Scholar] [CrossRef]
- Saarela, M.; Mättö, J.; Mattila-Sandholm, T. Safety Aspects of Lactobacillus and Bifidobacterium Species Originating from Human Oro-gastrointestinal Tract or from Probiotic Products. Microb. Ecol. Health Dis. 2002, 14, 234–241. [Google Scholar] [CrossRef]
- Momin, E.S.; Khan, A.A.; Kashyap, T.; Pervaiz, M.A.; Akram, A.; Mannan, V.; Sanusi, M.; Elshaikh, A.O.; Pervaiz Sr, M.A. The Effects of Probiotics on Cholesterol Levels in Patients with Metabolic Syndrome: A Systematic Review. Cureus 2023, 15, e37567. [Google Scholar] [CrossRef] [PubMed]
- Puttarat, N.; Kasorn, A.; Vitheejongjaroen, P.; Chantarangkul, C.; Tangwattanachuleeporn, M.; Taweechotipatr, M. Beneficial Effects of Indigenous Probiotics in High-Cholesterol Diet-Induced Hypercholesterolemic Rats. Nutrients 2023, 15, 2710. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, H.T.; Gu, M.; Werlinger, P.; Cho, J.-H.; Cheng, J.; Suh, J.-W. Lactobacillus sakei MJM60958 as a Potential Probiotic Alleviated Non-Alcoholic Fatty Liver Disease in Mice Fed a High-Fat Diet by Modulating Lipid Metabolism, Inflammation, and Gut Microbiota. Int. J. Mol. Sci. 2022, 23, 13436. [Google Scholar] [CrossRef]
- Oak, S.J.; Jha, R. The effects of probiotics in lactose intolerance: A systematic review. Crit. Rev. Food Sci. Nutr. 2019, 59, 1675–1683. [Google Scholar] [CrossRef] [PubMed]
- Iskandar, C.F.; Cailliez-Grimal, C.; Borges, F.; Revol-Junelles, A.-M. Review of lactose and galactose metabolism in lactic acid bacteria dedicated to expert genomic annotation. Trends Food Sci. Technol. 2019, 88, 121–132. [Google Scholar] [CrossRef]
- Catanzaro, R.; Sciuto, M.; Marotta, F. Lactose intolerance: An update on its pathogenesis, diagnosis, and treatment. Nutr. Res. 2021, 89, 23–34. [Google Scholar] [CrossRef] [PubMed]
- Misselwitz, B.; Butter, M.; Verbeke, K.; Fox, M.R. Update on lactose malabsorption and intolerance: Pathogenesis, diagnosis and clinical management. Gut 2019, 68, 2080–2091. [Google Scholar] [CrossRef] [PubMed]
- Hussain, N.; Li, R.; Takala, T.M.; Tariq, M.; Zaidi, A.H.; Saris, P.E. Generation of lactose-and protease-positive probiotic Lacticaseibacillus rhamnosus GG by conjugation with Lactococcus lactis NCDO 712. Appl. Environ. Microbiol. 2021, 87, e02920–e02957. [Google Scholar] [CrossRef] [PubMed]
- Plaza-Vinuesa, L.; Sánchez-Arroyo, A.; Moreno, F.J.; de Las Rivas, B.; Muñoz, R. Dual 6Pβ-Galactosidase/6Pβ-Glucosidase GH1 family for Lactose Metabolism in the probiotic bacterium lactiplantibacillus plantarum WCFS1. J. Agric. Food Chem. 2023, 71, 10693–10700. [Google Scholar] [CrossRef] [PubMed]
- Dang, Y.; Sun, Y.; Zhou, Y.; Men, X.; Wang, B.; Li, B.; Ren, Y. Effects of probiotics on growth, the toll-like receptor mediated immune response and susceptibility to Aeromonas salmonicida infection in rainbow trout Oncorhynchus mykiss. Aquaculture 2022, 561, 738668. [Google Scholar] [CrossRef]
- Zhu, L.; Kong, Y.; Chang, X.; Feng, J.; Wang, X.; Hou, L.; Zhao, X.; Pei, C.; Kong, X. Effects of two fish-derived probiotics on growth performance, innate immune response, intestinal health, and disease resistance of Procambarus clarkii. Aquaculture 2023, 562, 738765. [Google Scholar] [CrossRef]
- Liu, Y.; Wang, J.; Wu, C. Modulation of gut microbiota and immune system by probiotics, pre-biotics, and post-biotics. Front. Nutr. 2022, 8, 634897. [Google Scholar] [CrossRef]
- Li, A.; Wang, Y.; Li, Z.; Qamar, H.; Mehmood, K.; Zhang, L.; Liu, J.; Zhang, H.; Li, J. Probiotics isolated from yaks improves the growth performance, antioxidant activity, and cytokines related to immunity and inflammation in mice. Microb Cell Fact 2019, 18, 112. [Google Scholar] [CrossRef]
- Hoffmann, A.; Kleniewska, P.; Pawliczak, R. Antioxidative activity of probiotics. Arch. Med. Sci. 2021, 17, 792–804. [Google Scholar] [CrossRef] [PubMed]
- Muniandy, P.; Shori, A.B.; Baba, A.S. Influence of green, white and black tea addition on the antioxidant activity of probiotic yogurt during refrigerated storage. Food Packag. Shelf Life 2016, 8, 1–8. [Google Scholar] [CrossRef]
- Shori, A.B.; Baba, A.S. Comparative antioxidant activity, proteolysis and in vitro α-amylase and α-glucosidase inhibition of Allium sativum-yogurts made from cow and camel milk. J. Saudi Chem. Soc. 2014, 18, 456–463. [Google Scholar] [CrossRef]
- Shori, A.B.; Aljohani, G.S.; Al-zahrani, A.J.; Al-sulbi, O.S.; Baba, A.S. Viability of probiotics and antioxidant activity of cashew milk-based yogurt fermented with selected strains of probiotic Lactobacillus spp. LWT 2022, 153, 112482. [Google Scholar] [CrossRef]
- Li, B.; Zhang, T.; Dai, Y.; Jiang, G.; Peng, Y.; Wang, J.; Song, Y.; Ding, Z. Effects of probiotics on antioxidant activity, flavor compounds and sensory evaluation of Rosa roxburghii Tratt. LWT 2023, 179, 114664. [Google Scholar] [CrossRef]
- Jain, N.; Dutt, U.; Radenkov, I.; Jain, S. WHO’s global oral health status report 2022: Actions, discussion and implementation. Oral Dis. 2023, 30, 73–79. [Google Scholar] [CrossRef] [PubMed]
- Kekkonen, R.A.; Lummela, N.; Karjalainen, H.; Latvala, S.; Tynkkynen, S.; Jarvenpaa, S.; Kautiainen, H.; Julkunen, I.; Vapaatalo, H.; Korpela, R. Probiotic intervention has strain-specific anti-inflammatory effects in healthy adults. World J. Gastroenterol. 2008, 14, 2029–2036. [Google Scholar] [CrossRef] [PubMed]
- Paineau, D.; Carcano, D.; Leyer, G.; Darquy, S.; Alyanakian, M.A.; Simoneau, G.; Bergmann, J.F.; Brassart, D.; Bornet, F.; Ouwehand, A.C. Effects of seven potential probiotic strains on specific immune responses in healthy adults: A double-blind, randomized, controlled trial. FEMS Immunol. Med. Microbiol. 2008, 53, 107–113. [Google Scholar] [CrossRef] [PubMed]
- Haukioja, A.; Yli-Knuuttila, H.; Loimaranta, V.; Kari, K.; Ouwehand, A.C.; Meurman, J.H.; Tenovuo, J. Oral adhesion and survival of probiotic and other lactobacilli and bifidobacteria in vitro. Oral. Microbiol. Immunol. 2006, 21, 326–332. [Google Scholar] [CrossRef]
- Haukioja, A.; Loimaranta, V.; Tenovuo, J. Probiotic bacteria affect the composition of salivary pellicle and streptococcal adhesion in vitro. Oral. Microbiol. Immunol. 2008, 23, 336–343. [Google Scholar] [CrossRef]
- Haukioja, A. Probiotics and oral health. Eur. J. Dent. 2010, 4, 348–355. [Google Scholar] [CrossRef]
- Chen, W.; Ren, J.; Li, J.; Peng, S.; Zhang, C.; Lin, Y. Effects of probiotics on the oral health of patients undergoing orthodontic treatment: A systematic review and meta-analysis. Eur. J. Orthod. 2023, 45, 599–611. [Google Scholar] [CrossRef]
- Radhamanalan, G.; Dharumadurai, D. Prevalence of dental caries and knowledge of probiotics according to the Oral Health Monitoring Survey (OHMS) in Tamil Nadu, India. Preprint 2023. [Google Scholar] [CrossRef]
- Drago, L. Probiotics and colon cancer. Microorganisms 2019, 7, 66. [Google Scholar] [CrossRef] [PubMed]
- Mathipa, M.G.; Thantsha, M.S. Probiotic engineering: Towards development of robust probiotic strains with enhanced functional properties and for targeted control of enteric pathogens. Gut Pathog. 2017, 9, 1–17. [Google Scholar] [CrossRef] [PubMed]
- Beck, L.C.; Masi, A.C.; Young, G.R.; Vatanen, T.; Lamb, C.A.; Smith, R.; Coxhead, J.; Butler, A.; Marsland, B.J.; Embleton, N.D. Strain-specific impacts of probiotics are a significant driver of gut microbiome development in very preterm infants. Nat. Microbiol. 2022, 7, 1525–1535. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Venkatesh, G.P.; Kuruvalli, G.; Syed, K.; Reddy, V.D. An Updated Review on Probiotic Production and Applications. Gastroenterol. Insights 2024, 15, 221-236. https://doi.org/10.3390/gastroent15010016
Venkatesh GP, Kuruvalli G, Syed K, Reddy VD. An Updated Review on Probiotic Production and Applications. Gastroenterology Insights. 2024; 15(1):221-236. https://doi.org/10.3390/gastroent15010016
Chicago/Turabian StyleVenkatesh, Guru Prasad, Gouthami Kuruvalli, Khajamohiddin Syed, and Vaddi Damodara Reddy. 2024. "An Updated Review on Probiotic Production and Applications" Gastroenterology Insights 15, no. 1: 221-236. https://doi.org/10.3390/gastroent15010016







