The Potential for Genotoxicity, Mutagenicity and Endocrine Disruption in Triclosan and Triclocarban Assessed through a Combination of In Vitro Methods
Abstract
:1. Introduction
2. Materials and Methods
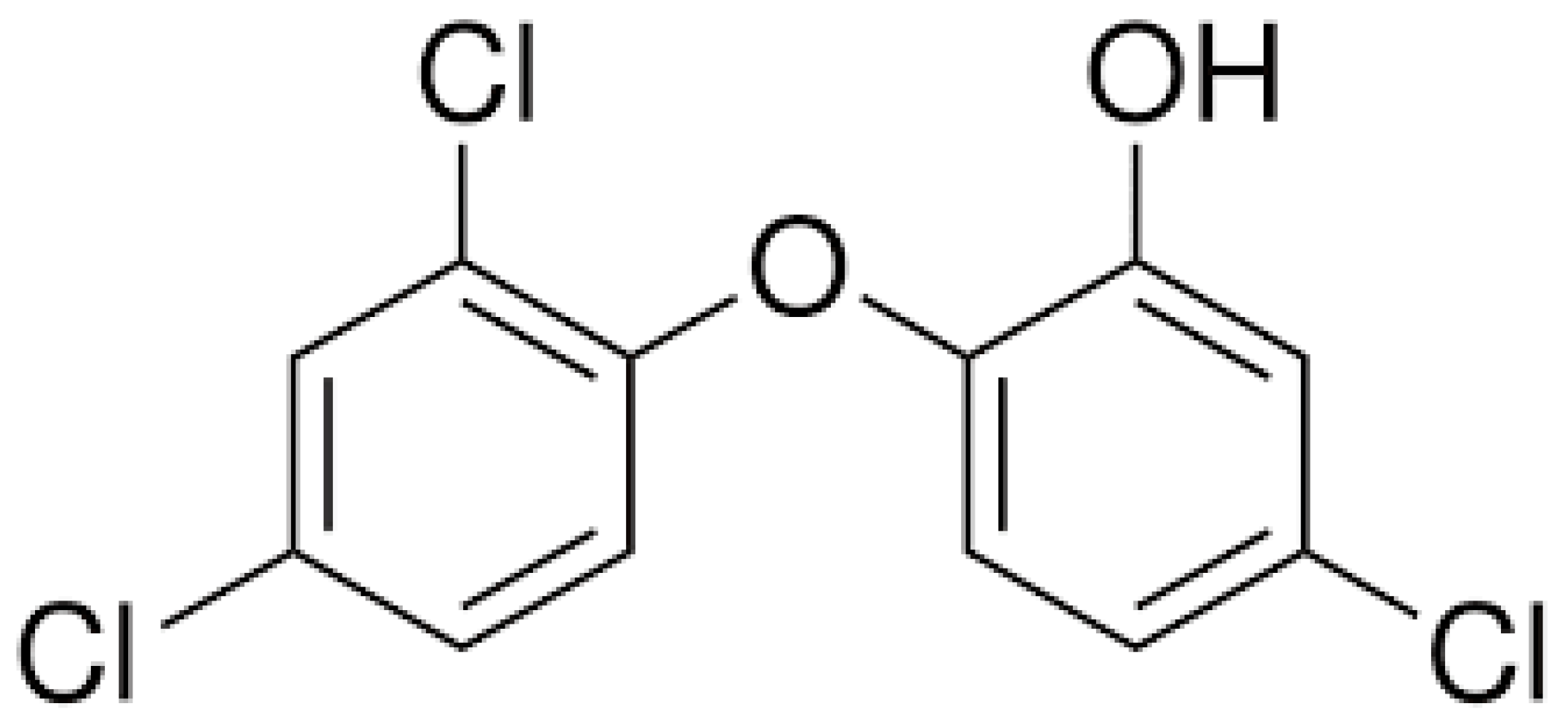
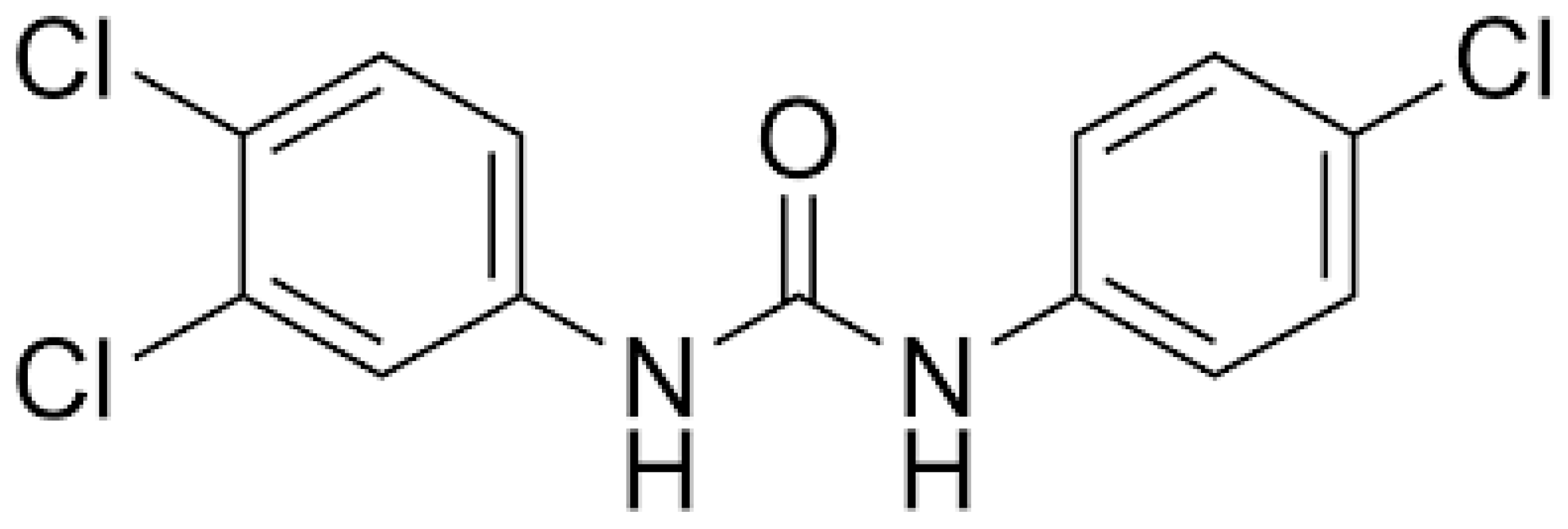
2.1. Chemicals
2.2. Metabolic Activation
2.3. Methods
2.3.1. Bacterial Reverse Mutation Test (Ames MPF™ Test)
2.3.2. In Vitro Mammalian Chromosome Aberration Test
2.3.3. In Vitro Comet Assay
2.3.4. XenoScreen® YES/YAS Assay
3. Results
3.1. Bacterial Reverse Mutation Test (Ames MPF™ Test)
3.2. In Vitro Mammalian Chromosome Aberration Test (CA)
3.3. In Vitro Comet Assay
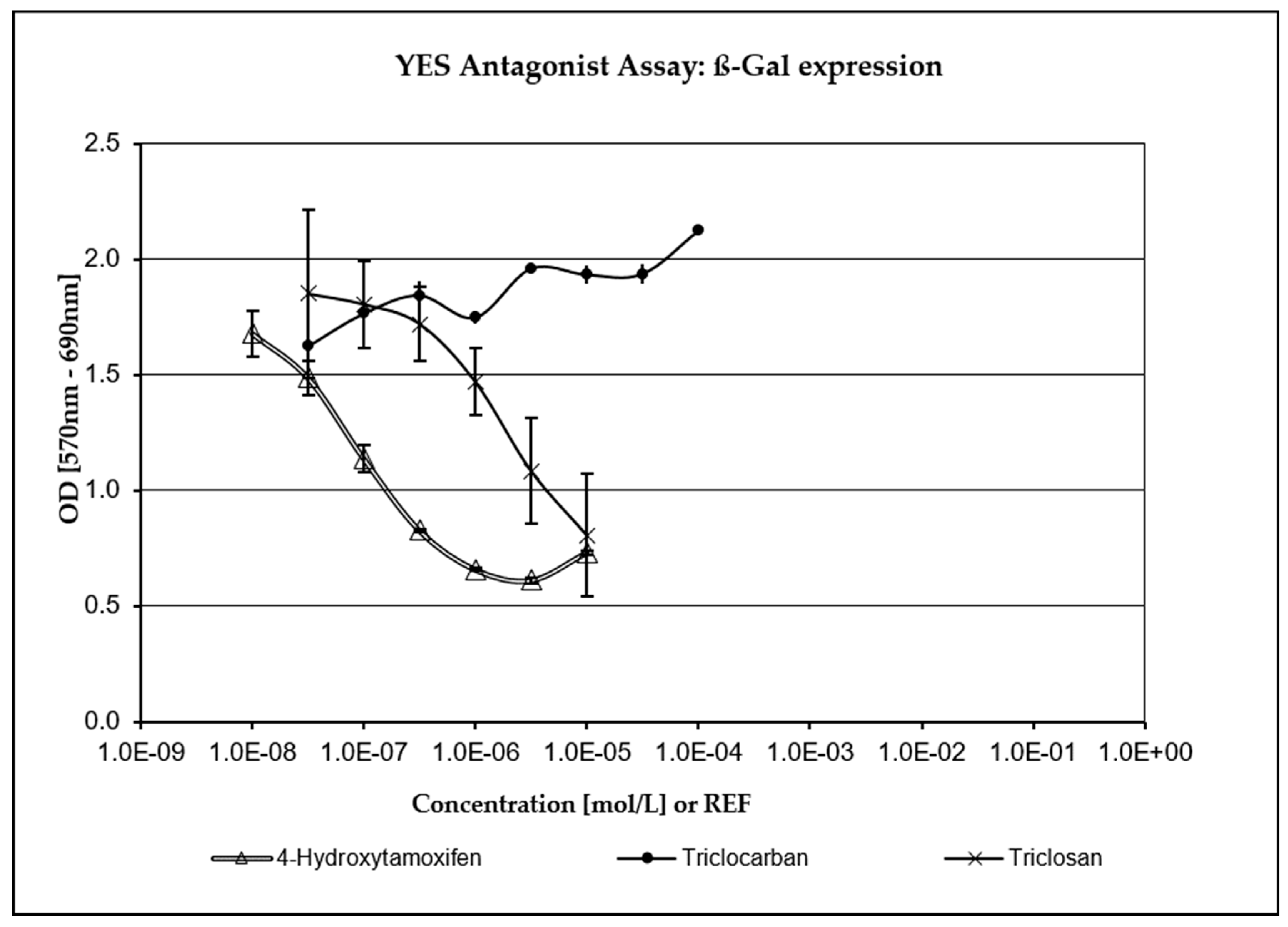
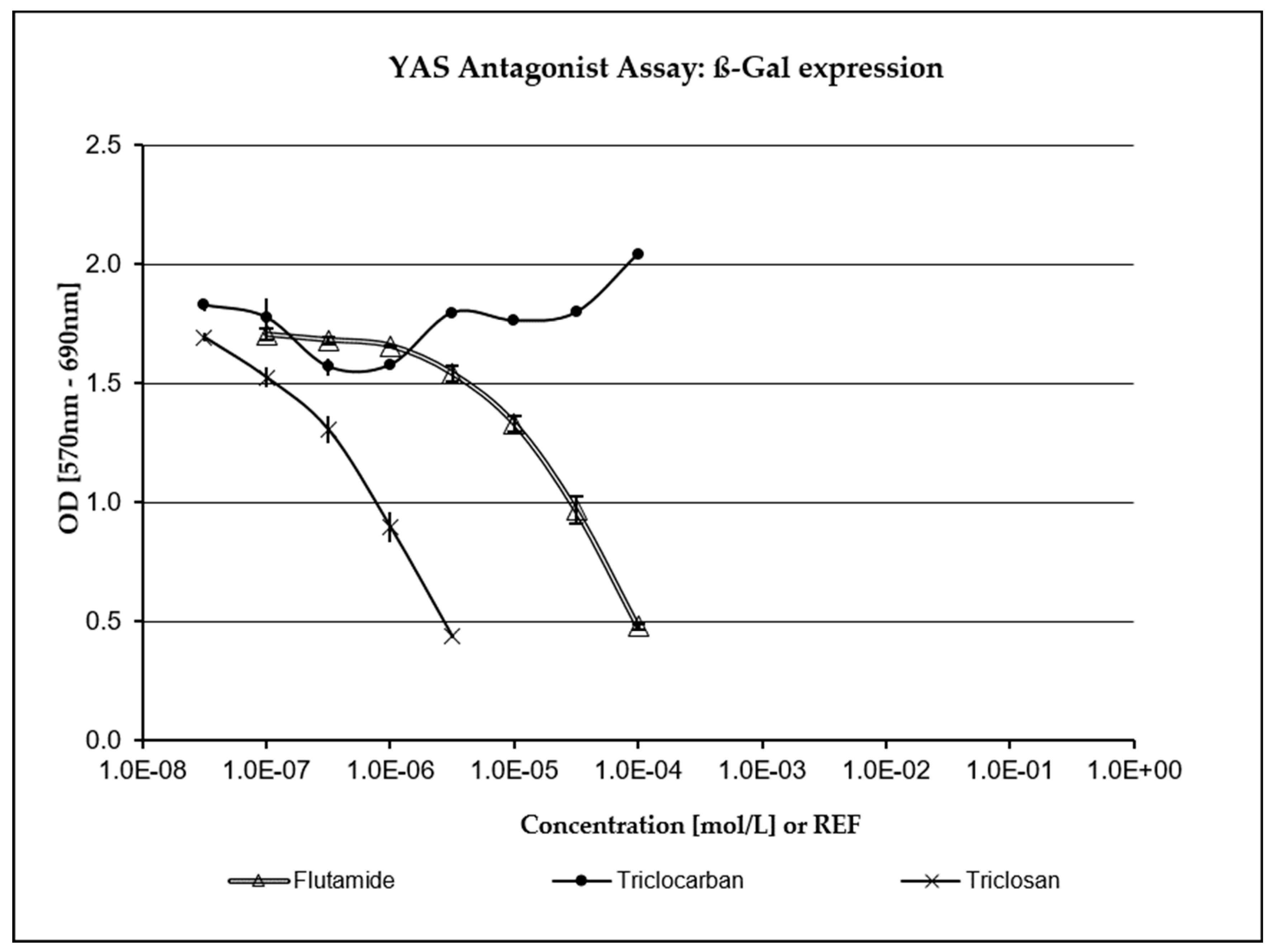
3.4. XenoScreen® YES/YAS Assay
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Iacopetta, D.; Catalano, A.; Ceramella, J.; Saturnino, C.; Salvagno, L.; Ielo, I.; Drommi, D.; Scali, E.; Plutino, M.R.; Rosace, G.; et al. The Different Facets of Triclocarban: A Review. Molecules 2021, 26, 2811. [Google Scholar] [CrossRef] [PubMed]
- SCCS (Scientific Committee on Consumer Safety). Request for a Scientific Advice on the Safety of Triclocarban (CAS No. 101-20-2, EC No. 202-924-1) and Triclosan (CAS No. 3380-34-5, EC No. 222-182-2) as Substances with Potential Endocrine Disrupting Properties Used in Cosmetic Products, Preliminary Version of 15–16 March 2022, final version of 24–25 October 2022, SCCS/1643/22. 2022. Available online: https://health.ec.europa.eu/system/files/2023-08/sccs_o_265.pdf (accessed on 13 November 2023).
- Shrestha, P.; Zhang, Y.; Chen, W.J.; Wong, T.Y. Triclosan: Antimicrobial mechanisms, antibiotics interactions, clinical applications, and human health. J. Environ. Sci. Health C Toxicol. Carcinog. 2020, 38, 245–268. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Li, J.; An, Y.; Wang, D.; Zhao, J.; Zhan, M.; Xu, W.; Lu, L.; Gao, Y. Concentrations of bisphenols, benzophenone-type ultraviolet filters, triclosan, and triclocarban in the paired urine and blood samples from young adults: Partitioning between urine and blood. Chemosphere 2022, 288, 132563. [Google Scholar] [CrossRef] [PubMed]
- Pycke, G.F.B.; Geer, A.L.; Dalloul, M.; Abulafia, O.; Jenck, M.A.; Halden, U.R. Human fetal exposure to triclosan and triclocarban in an urban population from Brooklyn, New York. Environ. Sci. Technol. 2014, 48, 8831–8838. [Google Scholar] [CrossRef] [PubMed]
- Wei, L.; Qiao, P.; Shi, Y.; Ruan, Y.; Yin, J.; Wu, Q.; Shao, B. Triclosan/triclocarban levels in maternal and umbilical blood samples and their association with fetal malformation. Clin. Chim. Acta 2017, 466, 133–137. [Google Scholar] [CrossRef] [PubMed]
- Toms, L.M.L.; Allmyr, M.; Mueller, J.F.; Adolfsson-Erici, M.; McLachlan, M.; Murby, J.; Harden, F.A. Triclosan in individual human milk samples from Australia. Chemosphere 2011, 85, 1682–1686. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.H.; Kim, D.; Moon, S.M.; Yang, E.J. Associations of lifestyle factors with phthalate metabolites, bisphenol A, parabens, and triclosan concentrations in breast milk of Korean mothers. Chemosphere 2020, 249, 126149. [Google Scholar] [CrossRef]
- Asimakopoulos, A.G.; Thomaidis, N.S.; Kannan, K. Widespread occurrence of bisphenol A diglycidyl ethers, p-hydroxybenzoic acid esters (parabens), benzophenone type-UV filters, triclosan, and triclocarban in human urine from Athens, Greece. Sci. Total Environ. 2014, 470–471, 1243–1249. [Google Scholar] [CrossRef]
- Xue, J.; Wu, Q.; Sakthivel, S.; Pavithran, V.P.; Vasukutty, R.J.; Kannan, K. Urinary levels of endocrine-disrupting chemicals, including bisphenols, bisphenol A diglycidyl ethers, benzophenones, parabens, and triclosan in obese and non-obese Indian children. Environ. Res. 2015, 137, 120–128. [Google Scholar] [CrossRef]
- Iyer, P.A.; Xue, J.; Honda, M.; Robinson, M.; Kumosami, A.T.; Abulnaja, K.; Kannan, K. Urinary levels of triclosan and triclocarban in several Asian countries, Greece and the USA: Association with oxidative stress. Environ. Res. 2018, 106, 91–96. [Google Scholar] [CrossRef]
- Li, W.; Zhang, W.; Chang, M.; Ren, J.; Xie, W.; Chen, H.; Zhang, Z.; Zhuang, X.; Shen, G.; Li, H. Metabonomics reveals that triclocarban affects liver metabolism by affecting glucose metabolism, β-oxidation of fatty acids, and the TCA cycle in male mice. Toxicol. Lett. 2018, 299, 76–85. [Google Scholar] [CrossRef] [PubMed]
- Tian, X.; Huang, K.; Liu, Y.; Jiang, K.; Liu, R.; Cui, J.; Wang, F.; Yu, Y.; Zhang, H.; Lin, M.; et al. Distribution of phthalate metabolites, benzophenone-type ultraviolet filters, parabens, triclosan and triclocarban in paired human hair, nail and urine samples. Environ. Pollut. 2023, 333, 122083. [Google Scholar] [CrossRef] [PubMed]
- Yin, J.; Wei, L.; Shi, Y.; Zhang, J.; Wu, Q.; Shao, B. Chinese population exposure to triclosan and triclocarban as measured via human urine and nails. Environ. Geochem. Health 2016, 38, 1125–1135. [Google Scholar] [CrossRef] [PubMed]
- Armstrong, D.L.; Lozano, N.; Rice, C.P.; Ramirez, M.; Torrents, A. Degradation of triclosan and triclocarban and formation of transformation products in activated sludge using benchtop bioreactors. Environ. Res. 2018, 161, 17–25. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Meng, X.Z.; Bergman, A.; Halden, R.U. Nationwide reconnaissance of five parabens, triclosan, triclocarban and its transformation products in sewage sludge from China. J. Hazard. Mater. 2019, 365, 502–510. [Google Scholar] [CrossRef] [PubMed]
- Meador, J.P.; Yeh, A.; Young, G.; Gallagher, E.P. Contaminants of emerging concern in a large temperate estuary. Environ. Pollut. 2016, 213, 254–267. [Google Scholar] [CrossRef] [PubMed]
- Gomes, M.F.; de Paula, V.D.C.S.; Martins, L.R.R.; Garcia, J.R.E.; Yamamoto, F.Y.; de Freitas, A.M. Sublethal effects of triclosan and triclocarban at environmental concentrations in silver catfish (Rhamdia quelen) embryos. Chemosphere 2021, 263, 127985. [Google Scholar] [CrossRef]
- Lozano, N.; Rice, C.P.; Ramirez, M.; Torrents, A. Fate of triclocarban in agricultural soils after biosolid applications. Environ. Sci. Pollut. Res. 2018, 25, 222–232. [Google Scholar] [CrossRef]
- Vimalkumar, K.; Seethappan, S.; Pugazhendhi, A. Fate of Triclocarban (TCC) in aquatic and terrestrial systems and human exposure. Chemosphere 2019, 230, 201–209. [Google Scholar] [CrossRef]
- Yang, H.; Sanidad, K.Z.; Wang, W.; Xie, M.; Gu, M.; Cao, X.; Xiao, H.; Zhang, G. Triclocarban exposure exaggerates colitis and colon tumorigenesis: Roles of gut microbiota involved. Gut Microbes 2020, 12, 1690364. [Google Scholar] [CrossRef]
- Wu, Y.; Beland, F.A.; Fang, J.L. Effect of triclosan, triclocarban, 2,2′,4,4′-tetrabromodiphenyl ether, and bisphenol A on the iodide uptake, thyroid peroxidase activity, and expression of genes involved in thyroid hormone synthesis. Toxicol. Vitr. 2016, 32, 310–319. [Google Scholar] [CrossRef] [PubMed]
- Rochester, J.R.; Bolden, A.L.; Pelch, K.E.; Kwiatkowski, C.F. Potential developmental and reproductive impacts of triclocarban: A scoping review. J. Toxicol. 2017, 2017, 9679738. [Google Scholar] [CrossRef] [PubMed]
- Aker, A.M.; Ferguson, K.K.; Rosario, Z.Y.; Mukherjee, B.; Alshawabkeh, A.N.; Cordero, J.F.; Meeker, J.D. The associations between prenatal exposure to triclocarban, phenols and parabens with gestational age and birth weight in northern Puerto Rico. Environ. Res. 2019, 169, 41–51. [Google Scholar] [CrossRef] [PubMed]
- Cao, L.Y.; Xu, Y.H.; He, S.; Ren, X.M.; Yang, Y.; Luo, S.; Xie, X.D.; Luo, L. Antimicrobial triclocarban exhibits higher agonistic activity on estrogen-related receptor γ than triclosan at human exposure levels: A novel estrogenic disruption mechanism. Environ. Sci. Technol. Lett. 2020, 7, 434–439. [Google Scholar] [CrossRef]
- Costa, N.O.; Forcato, S.; Cavichioli, A.M.; Pereira, M.R.F.; Gerardin, D.C.C. In utero and lactational exposure to triclocarban: Age-associated changes in reproductive parameters of male rat offspring. Toxicol. Appl. Pharmacol. 2020, 401, 115077. [Google Scholar] [CrossRef]
- Xie, M.; Zhang, H.; Wang, W.; Sherman, H.L.; Minter, L.M.; Cai, Z.; Zhang, G. Triclocarban exposure exaggerates spontaneous colonic inflammation in Il-10−/− mice. Toxicol. Sci. 2020, 174, 92–99. [Google Scholar] [CrossRef]
- Sanidad, K.Z.; Wang, G.; Panigrahy, A.; Zhang, G. Triclosan and triclocarban as potential risk factors of colitis and colon cancer: Roles of gut microbiota involved. Sci. Total Environ. 2022, 842, 156776. [Google Scholar] [CrossRef]
- Giuliano, C.A.; Rybak, M.J. Efficacy of triclosan as an antimicrobial hand soap and its potential impact on antimicrobial resistance: A focused review. Pharmacotherapy 2015, 35, 328–336. [Google Scholar] [CrossRef]
- Hartmann, E.M.; Hickey, R.; Hsu, T.; Betancourt Roman, C.M.; Chen, J.; Schwager, R.; Kline, J.; Brown, G.Z.; Halden, R.U.; Huttenhower, C.; et al. Antimicrobial chemicals are associated with elevated antibiotic resistance genes in the indoor dust microbiome. Environ. Sci. Technol. 2016, 50, 9807–9815. [Google Scholar] [CrossRef]
- Zhang, D.; Lu, S. A holistic review on triclosan and triclocarban exposure: Epidemiological outcomes, antibiotic resistance, and health risk assessment. Sci. Total Environ. 2023, 872, 162114. [Google Scholar] [CrossRef]
- Westfall, C.; Flores-Mireles, A.L.; Robinson, J.I.; Lynch, A.J.L.; Hultgren, S.; Henderso, J.P.; Levin, P.A. The widely used antimicrobial Triclosan induces high levels of antibiotic tolerance in vitro and reduces antibiotic efficacy up to 100-fold in vivo. Antimicrob. Agents Chemother. 2019, 63, 02312–02318. [Google Scholar] [CrossRef] [PubMed]
- European Union. Regulation EC No. 1223/2009 of the European Parliament and of the Council of 30 November 2009 on Cosmetic Products (Recast) (Text with EEA Relevance). Off. J. Eur. Union 2009, 342, 59–209. Available online: https://health.ec.europa.eu/system/files/2016-11/cosmetic_1223_2009_regulation_en_0.pdf (accessed on 13 November 2023).
- FDA (U.S. Food and Drug Administration). Safety and Effectiveness of Consumer Antiseptics. Topical Antimicrobial Drug Products for Over-the-Counter Human Use. Final Rule. Fed. Reg. 2016, 81, 61106–61130. Available online: https://www.federalregister.gov/documents/2016/09/06/2016-21337/safety-and-effectiveness-of-consumer-antiseptics-topical-antimicrobial-drug-products-for (accessed on 13 November 2023).
- Cartus, A.; Schrenk, D. Current methods in risk assessment of genotoxic chemicals. Food Chem. Toxicol. 2016, 106, 574–582. [Google Scholar] [CrossRef]
- OECD Test No. 471: Bacterial Reverse Mutation Test, OECD Guidelines for the Testing of Chemicals, Section 4, OECD Publishing, Paris. 2020. Available online: https://www.oecd-ilibrary.org/environment/test-no-471-bacterial-reverse-mutation-test_9789264071247-en (accessed on 13 November 2023).
- Xenometrix. Ames MPFTM Penta 1 Microplate Format Mutagenicity Assay. Instructions for Use; Version 2.01; Xenometrix: Allschwil, Switzerland, 2019; pp. 1–39. [Google Scholar]
- OECD Test No. 473: In Vitro Mammalian Chromosomal Aberration Test, OECD Guidelines for the Testing of Chemicals, Section 4, OECD Publishing, Paris. 2016. Available online: https://www.oecd-ilibrary.org/environment/test-no-473-in-vitro-mammalian-chromosomal-aberration-test_9789264264649-en (accessed on 13 November 2023).
- OECD Test No. 489: In Vivo Mammalian Alkaline Comet Assay, OECD Guidelines for the Testing of Chemicals, Section 4, OECD Publishing, Paris. 2016. Available online: https://www.oecd-ilibrary.org/environment/test-no-489-in-vivo-mammalian-alkaline-comet-assay_9789264264885-en (accessed on 13 November 2023).
- Jiravova, J.; Tomankova, K.; Harvanova, M.; Malina, L.; Malohlava, J.; Luhova, L.; Panacek, A.; Manisova, B.; Kolarova, H. The effect of silver nanoparticles and silver ions on mammalian and plant cells in vitro. Food Chem. Toxicol. 2016, 96, 50–61. [Google Scholar] [CrossRef] [PubMed]
- Xenometrix AG. XenoScreen YES/YAS Instructions for Use; Version 3.08; Xenometrix: Allschwil, Switzerland, 2017; pp. 1–22. [Google Scholar]
- European Chemical Agency ECHA: Triclosan Dossier. Available online: https://echa.europa.eu/cs/registration-dossier/-/registered-dossier/12675/7/7/1 (accessed on 13 November 2023).
- European Chemical Agency ECHA: Triclocarban Dossier. Available online: https://echa.europa.eu/cs/registration-dossier/-/registered-dossier/12075/7/7/2 (accessed on 13 November 2023).
- SCCP (Scientific Committee on Consumer Products). Opinion on Triclosan, 21 January 2009, SCCP/1192/08. Available online: https://ec.europa.eu/health/ph_risk/committees/04_sccp/docs/sccp_o_166.pdf (accessed on 13 November 2023).
- Sun, D.; Zhao, T.; Wang, T.; Wu, M.; Zhang, Z. Genotoxicity assessment of triclocarban by comet and micronucleus assays and Ames test. Environ. Sci. Pollut. Res. 2020, 27, 7430–7438. [Google Scholar] [CrossRef] [PubMed]
- Sun, D.; Zhao, T.; Li, X.; Zhang, Z. Evaluation of DNA and chromosomal damage in two human HaCaT and L02 cells treated with varying triclosan concentrations. J. Toxicol. Environ. Health A 2019, 82, 473–482. [Google Scholar] [CrossRef] [PubMed]
- Heidemann, A. Chromosome aberration assay in Chinese Hamster V79 cells in vitro with FAT 80′ 023/Q. Cytotest Cell Res. 1990; CCR project 179100. [Google Scholar]
- SCCS (Scientific Committee on Consumer Safety). Opinion on Triclosan, ADDENDUM to the SCCP Opinion on Triclosan (SCCP/1192/08) from January 2009, 22 March 2011, SCCS/1414/11. Available online: https://ec.europa.eu/health/scientific_committees/consumer_safety/docs/sccs_o_054.pdf (accessed on 13 November 2023).
- Sharma, S.; Dar, O.I.; Andotra, M.; Sharma, S.; Bhagat, A.; Thakur, S.; Kesavan, A.K.; Kaur, A. Cellular, molecular and genomic alterations in the hatchlings of Labeo rohita after exposure to Triclosan. Front. Environ. Sci. 2022, 10, 992435. [Google Scholar] [CrossRef]
- US Environmental Protection Agency. 5-Chloro-2-(2,4-dichlorophenoxy)phenol (Triclosan): Toxicology Chapter for the Reregistration Eligibility Decision (RED) Document; US Environmental Protection Agency, Office of Prevention, Pesticides and Toxic Substances: Washington, DC, USA, 2008. Available online: www.regulations.gov/#!searchResults;rpp=10;po=10;s=EPA-HQ-OPP-2007-0513 (accessed on 13 November 2023).
- Government of Canada. Screening Assessment Urea, N-(4-chlorophenyl)-N’-(3,4-dichlorophenyl)-(Triclocarban). Chemical Abstracts Service Registry Number 101-20-2. Environment and Climate Change Canada. Health Canada, March 2023, Cat. No.: En84-317/2022E-PDF. 2023. Available online: https://publications.gc.ca/collections/collection_2023/eccc/En84-317-2022-eng.pdf (accessed on 13 November 2023).
- Soap and Detergent Association. In vitro Mammalian Chromosome Aberration Test. Report no. 2002-01-TCC. 2002. Available online: https://www.cleaninginstitute.org/sites/default/files/research-pdfs/Triclocarban_in_vitro_mammalian_chromosome_aberration_test.pdf (accessed on 13 November 2023).
- Kim, S.; Chen, J.; Cheng, T.; Gindulyte, A.; He, J.; He, S.; Li, Q.; Shoemaker, B.A.; Thiessen, P.A.; Yu, B.; et al. PubChem 2019 update: Improved access to chemical data. Nucleic Acids Res. 2019, 47, 1102–1109. [Google Scholar] [CrossRef]
- Martínez-Paz, P.; Morales, M.; Martínez-Guitarte, J.L.; Morcillo, G. Genotoxic effects of environmental endocrine disruptors on the aquatic insect Chironomus riparius evaluated using the comet assay. Mutat. Res. Genet. Toxicol. Environ. Mutagen. 2013, 758, 41–47. [Google Scholar] [CrossRef]
- Silva, A.R.; Cardoso, D.N.; Cruz, A.; Lourenço, J.; Mendo, S.; Soares, A.M.; Loureiro, S. Ecotoxicity and genotoxicity of a binary combination of triclosan and carbendazim to Daphnia magna. Ecotoxicol. Environ. Saf. 2015, 115, 279–290. [Google Scholar] [CrossRef] [PubMed]
- Gao, L.; Yuan, T.; Cheng, P.; Bai, Q.; Zhou, C.; Ao, J.; Wang, W.; Zhang, H. Effects of triclosan and triclocarban on the growth inhibition, cell viability, genotoxicity and multixenobiotic resistance responses of Tetrahymena thermophila. Chemosphere 2015, 139, 434–440. [Google Scholar] [CrossRef] [PubMed]
- OECD Report of the JaCVAM Initiative International Pre-Validation and Validation Studies of the In Vivo Rodent Alkaline Comet Assay for the Detection of Genotoxic Carcinogens, Series on Testing and Assessment, Nos. 195 and 196. OECD Publishing: Paris, France, 2014; Available online: https://www.oecd.org/env/ehs/testing/Come%20assay%20revised%20pre-validation%20report%202013.pdf (accessed on 13 November 2023).
- Burlinson, B.; Tice, R.R.; Speit, G.; Agurell, E.; Brendler-Schwaab, S.Y.; Collins, A.R.; Escobar, P.; Honma, M.; Kumaravel, T.S.; Nakajima, M.; et al. In Vivo Comet Assay Workgroup, part of the Fourth International Workgroup on Genotoxicity Testing. Fourth International Workgroup on Genotoxicity testing: Results of the in vivo Comet assay workgroup. Mutat. Res. 2007, 627, 31–35. [Google Scholar] [CrossRef] [PubMed]
- Paul, T.; Shukla, S.P.; Kumar, K.; Poojary, N.; Kumar, S. Effect of temperature on triclosan toxicity in Pangasianodon hypophthalmus (Sauvage, 1878): Hematology, biochemistry and genotoxicity evaluation. Sci. Total Environ. 2019, 668, 104–114. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.S.; Oh, Y.; Lee, J.S.; Kim, H.S. Acute toxicity, oxidative stress, and apoptosis due to short-term triclosan exposure and multi- and transgenerational effects on in vivo endpoints, antioxidant defense, and DNA damage response in the freshwater water flea Daphnia magna. Sci. Total Environ. 2023, 864, 160925. [Google Scholar] [CrossRef] [PubMed]
- Wang, F.; Xu, R.; Zheng, F.; Liu, H. Effects of triclosan on acute toxicity, genetic toxicity and oxidative stress in goldfish (Carassius auratus). Exp. Anim. 2018, 67, 219–227. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.; Lu, Y.; Zhang, D.; Wang, Y.; Zhou, X.; Xu, H.; Mei, Y. Toxic assessment of Triclosan and Triclocarban on Artemia salina. Bull. Environ. Contam. Toxicol. 2015, 95, 728–733. [Google Scholar] [CrossRef]
- Ma, Y.; Chen, C.; Wang, J.B.; Cheng, J.L.; Shen, S.; Chen, X.; Huo, J.S. Triclosan-induced oxidative stress injury and apoptosis by regulating the PI3K/Akt/Caspase-3 signaling pathway in human renal glomerular endothelial cells. Biomed. Environ. Sci. 2022, 35, 547–551. [Google Scholar] [CrossRef]
- Zhong, R.; He, H.; Jin, M.; Lu, Z.; Deng, Y.; Liu, C.; Shen, N.; Li, J.; Wang, H.; Ying, P.; et al. Genome-wide gene-bisphenol A, F and triclosan interaction analyses on urinary oxidative stress markers. Sci. Total Environ. 2022, 807, 150753. [Google Scholar] [CrossRef]
- Adhikari, A.; Das, B.K.; Ganguly, S.; Nag, S.K.; Sadhukhan, D.; Raut, S.S. Emerging contaminant triclosan incites endocrine disruption, reproductive impairments and oxidative stress in the commercially important carp, Catla (Labeo catla): An insight through molecular, histopathological and bioinformatic approach. Comp. Biochem. Physiol. C Toxicol. Pharmacol. 2023, 268, 109605. [Google Scholar] [CrossRef]
- Cui, Z.; He, F.; Li, X.; Li, Y.; Huo, C.; Wang, H.; Qi, Y.; Tian, G.; Zong, W.; Liu, R. Response pathways of superoxide dismutase and catalase under the regulation of triclocarban-triggered oxidative stress in Eisenia foetida: Comprehensive mechanism analysis based on cytotoxicity and binding model. Sci. Total Environ. 2023, 854, 158821. [Google Scholar] [CrossRef] [PubMed]
- Alfhili, M.A.; Lee, M.H. Triclosan: An Update on Biochemical and Molecular Mechanisms. Oxid. Med. Cell Longev. 2019, 2019, 1607304. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.S.; Oh, Y.; Park, H.E.; Lee, J.S.; Kim, H.S. Synergistic toxic mechanisms of microplastics and triclosan via multixenobiotic resistance (MXR) inhibition-mediated autophagy in the freshwater water flea Daphnia magna. Sci. Total Environ. 2023, 896, 165214. [Google Scholar] [CrossRef] [PubMed]
- Pashaei, R.; Dzingelevičienė, R.; Putna-Nimane, I.; Overlinge, D.; Błaszczyk, A.; Walker, T.R. Acute toxicity of triclosan, caffeine, nanoplastics, microplastics, and their mixtures on Daphnia magna. Mar. Pollut. Bull. 2023, 192, 115113. [Google Scholar] [CrossRef] [PubMed]
- Qu, H.; Barrett, H.; Wang, B.; Han, J.; Wang, F.; Gong, W.; Wu, J.; Wang, W.; Yu, G. Co-occurrence of antiseptic triclocarban and chiral anti-inflammatory ibuprofen in environment: Association between biological effect in sediment and risk to human health. J. Hazard. Mater. 2021, 407, 124871. [Google Scholar] [CrossRef]
- Zhang, H.; Sanidad, K.Z.; Zhang, J.; Wang, G.; Zhang, R.; Hu, C.; Lin, Y.; Haggerty, T.D.; Parsonnet, J.; Zheng, Y.; et al. Microbiota-mediated reactivation of triclosan oxidative metabolites in colon tissues. J. Hazard. Mater. 2023, 445, 130509. [Google Scholar] [CrossRef]
- Oliver, M.; Kudłak, B.; Wieczerzak, M.; Reis, S.; Lima, S.A.C.; Segundo, M.A.; Miró, M. Ecotoxicological equilibria of triclosan in Microtox, XenoScreen YES/YAS, Caco2, HEPG2 and liposomal systems are affected by the occurrence of other pharmaceutical and personal care emerging contaminants. Sci. Total Environ. 2020, 719, 137358. [Google Scholar] [CrossRef]
- Kenda, M.; Kuželički, N.K.; Iida, M.; Kojima, H.; Dolenc, M.S. Triclocarban, Triclosan, Bromochlorophene, Chlorophene, and Climbazole effects on nuclear receptors: An in silico and in vitro study. Environ. Health Perspect. 2020, 128, 107005. [Google Scholar] [CrossRef]
- Pujol, E.; Blanco-Cabra, N.; Julián, E.; Leiva, R.; Torrents, E.; Vázquez, S. Pentafluorosulfanyl-containing triclocarban analogs with potent antimicrobial activity. Molecules 2018, 23, 2853. [Google Scholar] [CrossRef]
- Sreevidya, V.S.; Lenz, K.A.; Svoboda, K.R.; Ma, H. Benzalkonium chloride, benzethonium chloride, and chloroxylenol—Three replacement antimicrobials are more toxic than triclosan and triclocarban in two model organisms. Environ. Pollut. 2018, 235, 814–824. [Google Scholar] [CrossRef]
| Triclosan | ||||||||
|---|---|---|---|---|---|---|---|---|
| S. typhimurium strain | TA 98 | TA 100 | TA 1535 | TA 1537 | ||||
| S9 −/+ | S9− | S9+ | S9− | S9+ | S9− | S9+ | S9− | S9+ |
| 0.5 µg/mL | 0.00 | 4.33 | 0.00 | 3.33 | 0.00 | 0.33 | 0.00 | 2.00 |
| FI | 0.00 | 0.84 | 0.00 | 0.48 | 0.00 | 0.33 | 0.00 | 2.00 * |
| 1.0 µg/mL | 0.00 | 6.00 | 0.00 | 2.00 | 0.00 | 0.00 | 0.00 | 1.33 |
| FI | 0.00 | 1.16 | 0.00 | 0.29 | 0.00 | 0.00 | 0.00 | 1.33 |
| 2.0 µg/mL | 0.00 | 6.67 | 0.00 | 0.00 | 0.00 | 0.67 | 0.00 | 0.33 |
| FI | 0.00 | 1.29 | 0.00 | 0.00 | 0.00 | 0.67 | 0.00 | 0.33 |
| NC | 2.67 | 2.67 | 4.00 | 4.00 | 0.33 | 0.33 | 1.33 | 0.33 |
| Baseline | 5.18 | 5.18 | 7.00 | 7.00 | 1.00 | 1.00 | 2.86 | 1.00 |
| PC | 39.67 ** | 48.00 | 48.00 | 48.00 | 48.00 | 48.00 | 48.00 | 44.00 |
| FI | 7.65 | 9.26 | 6.86 | 6.86 | 48.00 | 48.00 | 16.78 | 44.00 |
| Triclocarban | ||||||||
| S. typhimurium strain | TA 98 | TA 100 | TA 1535 | TA 1537 | ||||
| S9 −/+ | S9− | S9+ | S9− | S9+ | S9− | S9+ | S9− | S9+ |
| 2.5 µg/mL | 0.00 | 4.00 | 1.67 | 5.33 | 0.00 | 0.67 | 2.67 | 2.33 |
| FI | 0.00 | 0.64 | 0.19 | 0.91 | 0.00 | 0.14 | 0.89 | 1.17 |
| 5.0 µg/mL | 2.33 | 4.33 | 3.33 | 4.67 | 0.67 | 1.00 | 2.33 | 1.33 |
| FI | 0.39 | 0.69 | 0.37 | 0.80 | 0.33 | 0.21 | 0.78 | 0.67 |
| 10.0 µg/mL | 3.33 | 4.33 | 2 | 5.67 | 0.00 | 0.67 | 1.00 | 1.67 |
| FI | 0.56 | 0.69 | 0.22 | 0.97 | 0.00 | 0.14 | 0.33 | 0.83 |
| NC | 3.67 | 5.67 | 9.00 | 3.33 | 1.00 | 3.00 | 3.00 | 2.00 |
| Baseline | 5.98 | 6.24 | 9.00 | 5.85 | 2.00 | 4.73 | 3.00 | 2.00 |
| PC | 41.00 | 40.67 | 47.33 | 42.67 | 44.33 | 39.67 | 45.67 | 37.67 |
| FI | 6.86 | 6.51 | 5.26 | 7.29 | 22.17 | 8.38 | 15.22 | 18.83 |
| Samples/ Controls | Concentration µg/mL | % Abberant Cells | Evaluation | ||
|---|---|---|---|---|---|
| 4 h + S9 | 4 h − S9 | 26 h − S9 | |||
| Triclosan | 2.5 | 5 | 6 | 4 | negative |
| 5 | 4 | 5 | NE | negative | |
| 10 | 21 | 10 | NE | positive | |
| Triclocarban | 2.5 | 4 | 3 | 3 | negative |
| 5 | 7 | 7 | NE | borderline | |
| 10 | 12 | 8 | NE | positive | |
| Negative control 1 (culture medium) | 4 | 3 | 4 | negative | |
| Positive control 1 (Thio-TEPA, 10−6 M) | 9 | 10 | positive | ||
| Negative control 2 (+S9) | 3 | negative | |||
| Positive control 2 (cyclophosphamide 10−4 M) | 10 | positive | |||
| Samples/ Controls | Concentration µg/mL | % DNA in Tail Mean ± SD | p-Value |
|---|---|---|---|
| Triclosan | 2.5 | 3.75 ± 0.34 | 0.060 |
| 5 | 13.87 ± 1.51 * | <0.0001 | |
| 10 | 15.99 ± 1.64 * | <0.0001 | |
| Triclocarban | 2.5 | 6.08 ± 0.20 * | 0.006 |
| 5 | 7.86 ± 0.98 * | 0.001 | |
| 10 | 11.92 ± 2.28 * | 0.0005 | |
| Negative control (culture medium) | 0.63 ± 0.07 | - | |
| Positive control (1% H2O2) | 91.48 ± 0.75 | - |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chrz, J.; Dvořáková, M.; Kejlová, K.; Očadlíková, D.; Svobodová, L.; Malina, L.; Hošíková, B.; Jírová, D.; Bendová, H.; Kolářová, H. The Potential for Genotoxicity, Mutagenicity and Endocrine Disruption in Triclosan and Triclocarban Assessed through a Combination of In Vitro Methods. J. Xenobiot. 2024, 14, 15-30. https://doi.org/10.3390/jox14010002
Chrz J, Dvořáková M, Kejlová K, Očadlíková D, Svobodová L, Malina L, Hošíková B, Jírová D, Bendová H, Kolářová H. The Potential for Genotoxicity, Mutagenicity and Endocrine Disruption in Triclosan and Triclocarban Assessed through a Combination of In Vitro Methods. Journal of Xenobiotics. 2024; 14(1):15-30. https://doi.org/10.3390/jox14010002
Chicago/Turabian StyleChrz, Jan, Markéta Dvořáková, Kristina Kejlová, Danuše Očadlíková, Lada Svobodová, Lukáš Malina, Barbora Hošíková, Dagmar Jírová, Hana Bendová, and Hana Kolářová. 2024. "The Potential for Genotoxicity, Mutagenicity and Endocrine Disruption in Triclosan and Triclocarban Assessed through a Combination of In Vitro Methods" Journal of Xenobiotics 14, no. 1: 15-30. https://doi.org/10.3390/jox14010002
APA StyleChrz, J., Dvořáková, M., Kejlová, K., Očadlíková, D., Svobodová, L., Malina, L., Hošíková, B., Jírová, D., Bendová, H., & Kolářová, H. (2024). The Potential for Genotoxicity, Mutagenicity and Endocrine Disruption in Triclosan and Triclocarban Assessed through a Combination of In Vitro Methods. Journal of Xenobiotics, 14(1), 15-30. https://doi.org/10.3390/jox14010002






