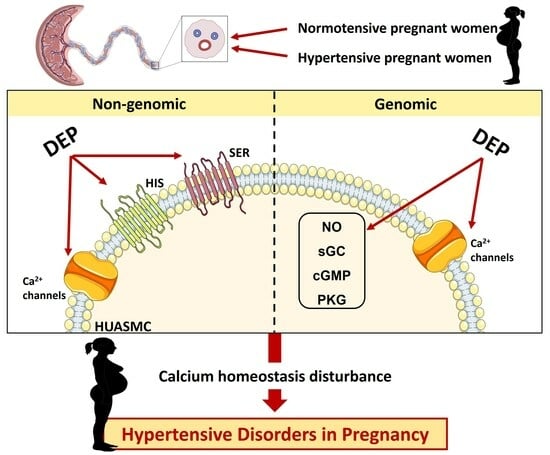
Exposure to DEP Modifies the Human Umbilical Artery Vascular Resistance Contributing to Hypertension in Pregnancy
Abstract
:1. Introduction
2. Materials and Methods
2.1. Tissue Collection
2.2. Umbilical Arteries Isolation
2.3. Artery Tension Recordings
2.4. Vascular Reactivity Protocols
2.5. Isolation of the HUA for Smooth Muscle Cell Culture
2.6. Cell Viability Determination
2.7. Drugs and Solutions
- -
- PSS (used for umbilical cord storage and isolation): CaCl2 (0.15 mM); KCl (5 mM); KH2PO4 (0.5 mM); NaCl (110 mM); MgCl2 (2 mM); NaHCO3 (10 mM); NaH2PO4 (0.5 mM); glucose (10 mM); EDTA (0.49 mM); HEPES (10 mM).
- -
- Krebs’ modified solution (used for the vascular reactivity experiments): NaCl (119 mM), KCl (5 mM), CaCl2⋅2H2O (0.5 mM), MgSO4⋅7H2O (1.2 mM), KH2PO4 (1.2 mM), NaHCO3 (25 mM), EDTA-Na2 (0.03 mM), l-(+)-ascorbic acid (0.6 mM), and glucose (11 mM)—pH 7.4.
- -
- Cell culture medium: DMEM-F12 (Dulbecco’s Modified Eagle’s Medium/Nutrient Mixture F-12 Hams) supplemented with NaHCO3 (1.2 g/L), L-ascorbic acid (20 mg/L), BSA (0.25%), FBS; (5%), antibiotics solution (1%), epidermal growth factor (EGF, 5 μg/mL), fibroblast growth factor (FGF, 0.5 ng/mL), heparin (2 μg/mL), insulin (5 μg/mL)—pH 7.4.
2.8. Statistical Data Analysis
3. Results
3.1. Nongenomic Effects of DEP in HUA
3.2. DEP Effects on HUASMC Viability
3.3. Genomic Effects of DEP in HUA
3.3.1. Contractile Response
3.3.2. Cyclic Guanosine Monophosphate Signaling
3.3.3. L-Type Calcium Channels Activity
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Corrigan, L.; O’Farrell, A.; Moran, P.; Daly, D. Hypertension in pregnancy: Prevalence, risk factors and outcomes for women birthing in Ireland. Pregnancy Hypertens. 2021, 24, 1–6. [Google Scholar] [CrossRef]
- Cifkova, R. Hypertension in Pregnancy: A Diagnostic and Therapeutic Overview. High. Blood Press. Cardiovasc. Prev. 2023, 30, 289–303. [Google Scholar] [CrossRef] [PubMed]
- Metoki, H.; Iwama, N.; Hamada, H.; Satoh, M.; Murakami, T.; Ishikuro, M.; Obara, T. Hypertensive disorders of pregnancy: Definition, management, and out-of-office blood pressure measurement. Hypertens. Res. 2022, 45, 1298–1309. [Google Scholar] [CrossRef] [PubMed]
- Soomro, M.H.; England-Mason, G.; Liu, J.; Reardon, A.J.F.; MacDonald, A.M.; Kinniburgh, D.W.; Martin, J.W.; Dewey, D.; APron Study Team. Associations between the chemical exposome and pregnancy induced hypertension. Environ. Res. 2023, 237, 116838. [Google Scholar] [CrossRef] [PubMed]
- U.S.E.P.A. Priority Pollutant List. 2014. Available online: https://www.epa.gov/sites/default/files/2015-09/documents/priority-pollutant-list-epa.pdf (accessed on 1 February 2023).
- Tsatsakis, A.M.; Katsikantami, I.; Kalantzi, O.; Sevim, Ç.; Tsarouhas, K.; Sarigiannis, D.; Tzatzarakis, M.N.; Rizos, A.K. Phthalates: Exposure and Health Effects. In Encyclopedia of Environmental Health, 2nd ed.; Elsevier: Amsterdam, The Netherlands, 2019; pp. 163–173. [Google Scholar]
- Fruh, V.; Preston, E.V.; Quinn, M.R.; Hacker, M.R.; Wylie, B.J.; O’Brien, K.; Hauser, R.; James-Todd, T.; Mahalingaiah, S. Urinary phthalate metabolite concentrations and personal care product use during pregnancy—Results of a pilot study. Sci. Total Environ. 2022, 835, 155439. [Google Scholar] [CrossRef] [PubMed]
- Werner, E.F.; Braun, J.M.; Yolton, K.; Khoury, J.C.; Lanphear, B.P. The association between maternal urinary phthalate concentrations and blood pressure in pregnancy: The HOME Study. Environ. Health 2015, 14, 75. [Google Scholar] [CrossRef]
- Soomro, M.H.; Maesano, C.N.; Heude, B.; Bornehag, C.G.; Annesi-Maesano, I. The association between maternal urinary phthalate metabolites concentrations and pregnancy induced hypertension: Results from the EDEN Mother-Child Cohort. J. Gynecol. Obs. Obstet. Hum. Reprod. 2021, 50, 102216. [Google Scholar] [CrossRef]
- Bedell, S.M.; Lyden, G.R.; Sathyanarayana, S.; Barrett, E.S.; Ferguson, K.K.; Santilli, A.; Bush, N.R.; Swan, S.H.; McElrath, T.F.; Nguyen, R.H.N. First- and Third-Trimester Urinary Phthalate Metabolites in the Development of Hypertensive Diseases of Pregnancy. Int. J. Environ. Res. Public. Health 2021, 18, 10627. [Google Scholar] [CrossRef]
- Hirke, A.; Varghese, B.; Varade, S.; Adela, R. Exposure to endocrine-disrupting chemicals and risk of gestational hypertension and preeclampsia: A systematic review and meta-analysis. Environ. Pollut. 2023, 317, 120828. [Google Scholar] [CrossRef]
- Trowbridge, J.; Abrahamsson, D.; Bland, G.D.; Jiang, T.; Wang, M.; Park, J.S.; Morello-Frosch, R.; Sirota, M.; Lee, H.; Goin, D.E.; et al. Extending Nontargeted Discovery of Environmental Chemical Exposures during Pregnancy and Their Association with Pregnancy Complications-A Cross-Sectional Study. Environ. Health Perspect. 2023, 131, 77003. [Google Scholar] [CrossRef]
- Lee, K.I.; Chiang, C.W.; Lin, H.C.; Zhao, J.F.; Li, C.T.; Shyue, S.K.; Lee, T.S. Maternal exposure to di-(2-ethylhexyl) phthalate exposure deregulates blood pressure, adiposity, cholesterol metabolism and social interaction in mouse offspring. Arch. Toxicol. 2016, 90, 1211–1224. [Google Scholar] [CrossRef] [PubMed]
- Tain, Y.L.; Hou, C.Y.; Chang-Chien, G.P.; Lin, S.; Hsu, C.N. Resveratrol Butyrate Ester Supplementation Blunts the Development of Offspring Hypertension in a Maternal Di-2-ethylhexyl Phthalate Exposure Rat Model. Nutrients 2023, 15, 697. [Google Scholar] [CrossRef] [PubMed]
- Evaristo Rodrigues da Silva, R.; de Alencar Silva, A.; Pereira-de-Morais, L.; de Sousa Almeida, N.; Iriti, M.; Kerntopf, M.R.; Menezes, I.R.A.; Coutinho, H.D.M.; Barbosa, R. Relaxant Effect of Monoterpene (−)-Carveol on Isolated Human Umbilical Cord Arteries and the Involvement of Ion Channels. Molecules 2020, 25, 2681. [Google Scholar] [CrossRef] [PubMed]
- Gajic Bojic, M.; Dukanovic, D.; Marinkovic, S.; Jovicic, S.; Stojiljkovic, M.P.; Djuric, D.M.; Skrbic, R. Methodological challenges in using human umbilical artery as a model for in vitro studies. Exp. Physiol. 2023, 108, 1578. [Google Scholar] [CrossRef] [PubMed]
- Cairrao, E.; Alvarez, E.; Santos-Silva, A.J.; Verde, I. Potassium channels are involved in testosterone-induced vasorelaxation of human umbilical artery. Naunyn Schmiedebergs Arch. Pharmacol. 2008, 376, 375–383. [Google Scholar] [CrossRef] [PubMed]
- Gloria, S.; Marques, J.; Feiteiro, J.; Marcelino, H.; Verde, I.; Cairrao, E. Tributyltin role on the serotonin and histamine receptors in human umbilical artery. Toxicol. In Vitro 2018, 50, 210–216. [Google Scholar] [CrossRef] [PubMed]
- Lorigo, M.; Quintaneiro, C.; Maia, C.J.; Breitenfeld, L.; Cairrao, E. UV-B filter octylmethoxycinnamate impaired the main vasorelaxant mechanism of human umbilical artery. Chemosphere 2021, 277, 130302. [Google Scholar] [CrossRef]
- Wittassek, M.; Koch, H.M.; Angerer, J.; Bruning, T. Assessing exposure to phthalates—The human biomonitoring approach. Mol. Nutr. Food Res. 2011, 55, 7–31. [Google Scholar] [CrossRef] [PubMed]
- Lu, L.; Rong, H.; Wu, C.; Cui, B.; Huang, Y.; Tan, Y.; Zhang, L.; Peng, Y.; Garcia, J.M.; Chen, J.A. Levels of phthalate acid esters and sex hormones and their possible sources in traffic-patrol policemen in Chongqing. Environ. Sci. Pollut. Res. Int. 2019, 26, 9005–9013. [Google Scholar] [CrossRef]
- Arbuckle, T.E.; Davis, K.; Marro, L.; Fisher, M.; Legrand, M.; LeBlanc, A.; Gaudreau, E.; Foster, W.G.; Choeurng, V.; Fraser, W.D.; et al. Phthalate and bisphenol A exposure among pregnant women in Canada--results from the MIREC study. Environ. Int. 2014, 68, 55–65. [Google Scholar] [CrossRef]
- Assens, M.; Frederiksen, H.; Petersen, J.H.; Larsen, T.; Skakkebaek, N.E.; Juul, A.; Andersson, A.M.; Main, K.M. Variations in repeated serum concentrations of UV filters, phthalates, phenols and parabens during pregnancy. Environ. Int. 2019, 123, 318–324. [Google Scholar] [CrossRef]
- Bu, H.; Tang, S.; Liu, G.; Miao, C.; Zhou, X.; Yang, H.; Liu, B. In silico, in vitro and in vivo studies: Dibutyl phthalate promotes prostate cancer cell proliferation by activating Forkhead Box M1 and remission after Natura-alpha pretreatment. Toxicology 2023, 488, 153465. [Google Scholar] [CrossRef]
- Xu, Q.; Zhao, T.; Ri, H.; Ye, J.; Zhao, W.; Zhang, Y.; Ye, L. Di(2-ethylhexyl) phthalate induced thyroid toxicity via endoplasmic reticulum stress: In vivo and in vitro study. Environ. Toxicol. 2022, 37, 2924–2936. [Google Scholar] [CrossRef] [PubMed]
- Schaffert, A.; Karkossa, I.; Ueberham, E.; Schlichting, R.; Walter, K.; Arnold, J.; Bluher, M.; Heiker, J.T.; Lehmann, J.; Wabitsch, M.; et al. Di-(2-ethylhexyl) phthalate substitutes accelerate human adipogenesis through PPARgamma activation and cause oxidative stress and impaired metabolic homeostasis in mature adipocytes. Environ. Int. 2022, 164, 107279. [Google Scholar] [CrossRef]
- Liu, C.; Qin, Q.; Xu, J.; Li, X.; Cong, H. Phthalate promotes atherosclerosis through interacting with long-non coding RNA and induces macrophage foam cell formation and vascular smooth muscle damage. Chemosphere 2022, 308, 136383. [Google Scholar] [CrossRef] [PubMed]
- Mariana, M.; Lorigo, M.; Feiteiro, J.; Castelo-Branco, M.; Soares, A.M.; Cairrao, E. Adverse cardiovascular effects of long-term exposure to diethyl phthalate in the rat aorta. Chemosphere 2023, 340, 139904. [Google Scholar] [CrossRef] [PubMed]
- Hengstler, J.G.; Sjogren, A.K.; Zink, D.; Hornberg, J.J. In vitro prediction of organ toxicity: The challenges of scaling and secondary mechanisms of toxicity. Arch. Toxicol. 2020, 94, 353–356. [Google Scholar] [CrossRef]
- Rosen, E.M.; Stevens, D.R.; Ramos, A.M.; McNell, E.E.; Wood, M.E.; Engel, S.M.; Keil, A.P.; Calafat, A.M.; Botelho, J.C.; Sinkovskaya, E.; et al. Personal care product use patterns in association with phthalate and replacement biomarkers across pregnancy. J. Expo. Sci. Environ. Epidemiol. 2024. [Google Scholar] [CrossRef]
- Koniecki, D.; Wang, R.; Moody, R.P.; Zhu, J. Phthalates in cosmetic and personal care products: Concentrations and possible dermal exposure. Environ. Res. 2011, 111, 329–336. [Google Scholar] [CrossRef]
- Rameshrad, M.; Babaei, H.; Azarmi, Y.; Fouladi, D.F. Rat aorta as a pharmacological tool for in vitro and in vivo studies. Life Sci. 2016, 145, 190–204. [Google Scholar] [CrossRef]
- Đukanović, D.; Gajić, M.; Škrbić, R. Time-Dependent and Force-Dependent Vasoreactivity of Isolated Human Umbilical Arteries. Scr. Med. 2020, 51, 134–140. [Google Scholar] [CrossRef]
- Chang, W.H.; Herianto, S.; Lee, C.C.; Hung, H.; Chen, H.L. The effects of phthalate ester exposure on human health: A review. Sci. Total Environ. 2021, 786, 147371. [Google Scholar] [CrossRef] [PubMed]
- Gore, A.C.; Chappell, V.A.; Fenton, S.E.; Flaws, J.A.; Nadal, A.; Prins, G.S.; Toppari, J.; Zoeller, R.T. Executive Summary to EDC-2: The Endocrine Society’s Second Scientific Statement on Endocrine-Disrupting Chemicals. Endocr. Rev. 2015, 36, 593–602. [Google Scholar] [CrossRef] [PubMed]
- Pereira-de-Morais, L.; Silva, A.A.; Bastos, C.M.S.; Calixto, G.L.; Araujo, I.M.; Araujo, M.C.; Barbosa, R.; Leal-Cardoso, J.H. The preeclampsia condition alters external potassium-evoked contraction of human umbilical vessels. Placenta 2023, 138, 68–74. [Google Scholar] [CrossRef] [PubMed]
- Sena Bastos, C.M.; Pereira de Morais, L.; Silva, A.A.; Menezes Dantas, D.; Batista, P.R.; Lima Gomes, M.F.; Araujo Delmondes, G.; Alencar de Menezes, I.R.; Rodrigues da Silva, R.E.; Barbosa, R. Perillyl Alcohol Promotes Relaxation in Human Umbilical Artery. Curr. Med. Chem. 2024, in press. [Google Scholar] [CrossRef]
- Sato, N.; Tanaka, K.A.; Szlam, F.; Tsuda, A.; Arias, M.E.; Levy, J.H. The vasodilatory effects of hydralazine, nicardipine, nitroglycerin, and fenoldopam in the human umbilical artery. Anesth. Analg. 2003, 96, 539–544. [Google Scholar] [CrossRef] [PubMed]
- Santos-Silva, A.J.; Cairrao, E.; Marques, B.; Verde, I. Regulation of human umbilical artery contractility by different serotonin and histamine receptors. Reprod. Sci. 2009, 16, 1175–1185. [Google Scholar] [CrossRef] [PubMed]
- Brew, O.; Sullivan, M.H. The links between maternal histamine levels and complications of human pregnancy. J. Reprod. Immunol. 2006, 72, 94–107. [Google Scholar] [CrossRef]
- Gupta, S.; Hanff, L.M.; Visser, W.; Steegers, E.A.; Saxena, P.R.; Vulto, A.G.; MaassenVanDenBrink, A. Functional reactivity of 5-HT receptors in human umbilical cord and maternal subcutaneous fat arteries after normotensive or pre-eclamptic pregnancy. J. Hypertens. 2006, 24, 1345–1353. [Google Scholar] [CrossRef]
- Houten, S.M.; Chen, J.; Belpoggi, F.; Manservisi, F.; Sanchez-Guijo, A.; Wudy, S.A.; Teitelbaum, S.L. Changes in the Metabolome in Response to Low-Dose Exposure to Environmental Chemicals Used in Personal Care Products during Different Windows of Susceptibility. PLoS ONE 2016, 11, e0159919. [Google Scholar] [CrossRef]
- Schneider, A.; Riess, P.; Elbers, A.; Neugebauer, E.; Schaefer, U. Polyclonal anti-histamine H2 receptor antibodies detect differential expression of H2 receptor protein in primary vascular cell types. Inflamm. Res. 2004, 53, 223–229. [Google Scholar] [CrossRef] [PubMed]
- Azevedo, R.; Oliveira, N.; Maia, C.; Verde, I. Effects of di(2-etilhexil) phthalate on human umbilical artery. Chemosphere 2019, 228, 278–286. [Google Scholar] [CrossRef]
- Lite, C.; Raja, G.L.; Juliet, M.; Sridhar, V.V.; Subhashree, K.D.; Kumar, P.; Chakraborty, P.; Arockiaraj, J. In utero exposure to endocrine-disrupting chemicals, maternal factors and alterations in the epigenetic landscape underlying later-life health effects. Environ. Toxicol. Pharmacol. 2022, 89, 103779. [Google Scholar] [CrossRef] [PubMed]
- Oh, B.S.; Jung, Y.J.; Oh, Y.J.; Yoo, Y.S.; Kang, J.W. Application of ozone, UV and ozone/UV processes to reduce diethyl phthalate and its estrogenic activity. Sci. Total Environ. 2006, 367, 681–693. [Google Scholar] [CrossRef]
- Kumar, N.; Sharan, S.; Srivastava, S.; Roy, P. Assessment of estrogenic potential of diethyl phthalate in female reproductive system involving both genomic and non-genomic actions. Reprod. Toxicol. 2014, 49, 12–26. [Google Scholar] [CrossRef]
- Fiocchetti, M.; Bastari, G.; Cipolletti, M.; Leone, S.; Acconcia, F.; Marino, M. The Peculiar Estrogenicity of Diethyl Phthalate: Modulation of Estrogen Receptor alpha Activities in the Proliferation of Breast Cancer Cells. Toxics 2021, 9, 237. [Google Scholar] [CrossRef] [PubMed]
- Mugge, A.; Riedel, M.; Barton, M.; Kuhn, M.; Lichtlen, P.R. Endothelium independent relaxation of human coronary arteries by 17 beta-oestradiol in vitro. Cardiovasc. Res. 1993, 27, 1939–1942. [Google Scholar] [CrossRef]
- White, R.E. Estrogen and vascular function. Vasc. Pharmacol. 2002, 38, 73–80. [Google Scholar] [CrossRef]
- Li, Y.; Han, B.; Salmeron, A.G.; Bai, J.; Chen, D.B. Estrogen-Induced Uterine Vasodilation in Pregnancy and Preeclampsia. Matern. Fetal Med. 2022, 4, 52–60. [Google Scholar] [CrossRef]
- Simard, M.; Drolet, R.; Blomquist, C.H.; Tremblay, Y. Human type 2 17beta-hydroxysteroid dehydrogenase in umbilical vein and artery endothelial cells: Differential inactivation of sex steroids according to the vessel type. Endocrine 2011, 40, 203–211. [Google Scholar] [CrossRef]
- Sathishkumar, K.; Elkins, R.; Yallampalli, U.; Yallampalli, C. Protein restriction during pregnancy induces hypertension in adult female rat offspring--influence of oestradiol. Br. J. Nutr. 2012, 107, 665–673. [Google Scholar] [CrossRef] [PubMed]
- Yin, G.; Zhu, X.; Guo, C.; Yang, Y.; Han, T.; Chen, L.; Yin, W.; Gao, P.; Zhang, H.; Geng, J.; et al. Differential expression of estradiol and estrogen receptor alpha in severe preeclamptic pregnancies compared with normal pregnancies. Mol. Med. Rep. 2013, 7, 981–985. [Google Scholar] [CrossRef] [PubMed]
- Feng, X.; Zhou, L.; Mao, X.; Tong, C.; Chen, X.; Zhao, D.; Baker, P.N.; Xia, Y.; Zhang, H. Association of a reduction of G-protein coupled receptor 30 expression and the pathogenesis of preeclampsia. Mol. Med. Rep. 2017, 16, 5997–6003. [Google Scholar] [CrossRef] [PubMed]
- Park, M.N.; Park, K.H.; Lee, J.E.; Shin, Y.Y.; An, S.M.; Kang, S.S.; Cho, W.S.; An, B.S.; Kim, S.C. The expression and activation of sex steroid receptors in the preeclamptic placenta. Int. J. Mol. Med. 2018, 41, 2943–2951. [Google Scholar] [CrossRef]
- Kuo, I.Y.; Wolfle, S.E.; Hill, C.E. T-type calcium channels and vascular function: The new kid on the block? J. Physiol. 2011, 589, 783–795. [Google Scholar] [CrossRef]







Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mariana, M.; Soares, A.M.V.M.; Castelo-Branco, M.; Cairrao, E. Exposure to DEP Modifies the Human Umbilical Artery Vascular Resistance Contributing to Hypertension in Pregnancy. J. Xenobiot. 2024, 14, 497-515. https://doi.org/10.3390/jox14020030
Mariana M, Soares AMVM, Castelo-Branco M, Cairrao E. Exposure to DEP Modifies the Human Umbilical Artery Vascular Resistance Contributing to Hypertension in Pregnancy. Journal of Xenobiotics. 2024; 14(2):497-515. https://doi.org/10.3390/jox14020030
Chicago/Turabian StyleMariana, Melissa, Amadeu M. V. M. Soares, Miguel Castelo-Branco, and Elisa Cairrao. 2024. "Exposure to DEP Modifies the Human Umbilical Artery Vascular Resistance Contributing to Hypertension in Pregnancy" Journal of Xenobiotics 14, no. 2: 497-515. https://doi.org/10.3390/jox14020030