Hyperspectral Reflectance and Fluorescence Imaging to Detect Scab Induced Stress in Apple Leaves
Abstract
:1. Introduction
2. Materials and Methods
2.1. Plant Material and Infections
2.2. Symptom Assessment
2.3. Chlorophyll Fluorescence Imaging
2.4. Hyperspectral Measurements
2.5. Chlorophyll Extraction
3. Data Analysis
3.1. Chlorophyll Fluorescence Imaging
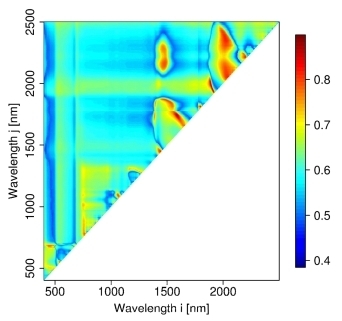
3.2. Hyperspectral Measurements
4. Results
4.1. Fluorescence Imaging
| 12 days after inoculation | 14 days after inoculation | 17 days after inoculation | |||||||
|---|---|---|---|---|---|---|---|---|---|
| S1 | S2 | S3 | S1 | S2 | S3 | S1 | S2 | S3 | |
| L1 | 2 | 0 | 1 | 2 | 0 | 1 | 4 | 3 | 1 |
| L2 | 3 | 1 | 1 | 4 | 4 | 1 | 4 | 5 | 1 |
| L3 | 4 | 3 | 1 | 4 | 5 | 2 | 5 | 6 | 2 |
| L4 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |

4.2. Hyperspectral Analysis

| DAI | c-values of considered indices | |||||
|---|---|---|---|---|---|---|
| R1480/R2135 | PSSRa | PRI | NPQI | R690/R600 | R740/R800 | |
| 3 | 0.78 | 0.52 | 0.53 | 0.59 | 0.52 | 0.68 |
| 4 | 0.82 | 0.58 | 0.58 | 0.80 | 0.73 | 0.84 |
| 6 | 0.85 | 0.62 | 0.59 | 0.58 | 0.52 | 0.74 |
| 7 | 0.85 | 0.60 | 0.56 | 0.64 | 0.65 | 0.59 |
| 10 | 0.89 | 0.72 | 0.57 | 0.67 | 0.66 | 0.53 |
| 12 | 0.66 | 0.78 | 0.59 | 0.62 | 0.79 | 0.53 |
| 14 | 0.64 | 0.85 | 0.79 | 0.73 | 0.75 | 0.62 |
| 17 | 0.53 | 0.86 | 0.67 | 0.71 | 0.57 | 0.65 |


5. Discussion and Conclusions
5.1. Fluorescence Imaging
5.2. Hyperspectral Analysis


Acknowledgements
References and Notes
- MacHardy, W.E. Physiological responses to infections. In Apple Scab: Biology, Epidemiology, and Management; APS Press: St. Paul, MN, USA, 1996; pp. 152–165. [Google Scholar]
- Szklarz, M. Evaluation of apple cultivars’resistance to apple scab (Venturia inaequalis Che.). J. Fruit Ornam. Plant Res. 2006, 14, 183–188. [Google Scholar]
- Brun, L.; Didelot, F.; Parisi, L. Effects of apple cultivar susceptibility to Venturia inaequalis on scab epidemics in apple orchards. Crop Prot. 2008, 27, 1009–1019. [Google Scholar] [CrossRef]
- Zarco-Tejada, P.J.; Miller, J.R.; Mohammed, G.H.; Noland, T.L.; Sampson, P.H. Chlorophyll fluorescence effects on vegetation apparent reflectance: II. Laboratory and airborne canopy-Level measurements with hyperspectral data. Remote Sens. Environ. 2000, 74, 596–608. [Google Scholar] [CrossRef]
- Chaerle, L.; Hagenbeek, D.; De Bruyne, E.; Valcke, R.; Van Der Straeten, D. Thermal and chlorophyll-fluorescence imaging distinguish plant–pathogen interactions at an early stage. Plant Cell Physiol. 2004, 45, 887–896. [Google Scholar] [CrossRef] [PubMed]
- Gielen, B.; Vandermeiren, K.; Horemans, N.; D’Haese, D.D.; Serneels, R.; Valcke, R. Clorophyll a fluorescence imaging of ozone-sressed Brassica napus L. plants differing in glucosinolate concentrations. Plant Biol. 2006, 8, 698–705. [Google Scholar] [CrossRef] [PubMed]
- Ciscato, M.; Valcke, R. Chlorophyll fluorescence imaging of heavy metal-treated plants. In Photosynthesis: Mechanisms and Effects; Garab, G., Ed.; Kluwer Academic: Dordrecht, The Netherlands, 1998; Volume 4, pp. 2661–2663. [Google Scholar]
- Delalieux, S.; van Aardt, J.A.N.; Keulemans, W.; Schrevens, E.; Coppin, P. Detection of biotic stress (Venturia inaequalis) in apple trees using hyperspectral data: Non-parametric statistical approaches and physiological implications. Eur. J. Agron. 2007, 27, 130–143. [Google Scholar] [CrossRef]
- Suárez, L.; Zarco-Tejada, P.J.; Sepulcre-Cantó, G.; Pérez-Priego, O.; Millar, J.R.; Jiménez-Muñoz, J.C.; Sobrino, J. Assessing canopy PRI for water stress detection with diurnal airborne imagery. Remote Sens. Environ. 2008, 112, 560–575. [Google Scholar] [CrossRef]
- Shi, R.H.; Zhuang, D.F.; Niu, Z. Physical investigation on biochemical prediction using continuum removal. Int. Geosci. Remote Sens. Symp. 2004, 2, 1463–1466. [Google Scholar]
- Gausman, H. Plant leaf optical parameters in visible and near-infrared light. Graduate Studies, Texas Tech University, Lubbock, TX, USA, 1985; p. 78. [Google Scholar]
- Merzlyak, M.; Gitelson, A.; Chivkunova, A.; Pogosyan, S. Application of reflectance spectroscopy for analysis of higher plant pigments. Russ. J. Plant Physl. 2003, 50, 704–710. [Google Scholar] [CrossRef]
- Zarco-Tejada, P.J.; Miller, J.; Morales, A.; Berjon, A.; Agüera, J. Hyperspectral indices and model simulation for chlorophyll estimation in open canopy tree crops. Remote Sens. Environ. 2004, 90, 463–476. [Google Scholar] [CrossRef]
- Lichtenthaler, H.K.; Miehe, J.A. Fluorescence imaging as a diagnostic tool for plant stress. Trends Plant Sci. 1997, 2, 316–320. [Google Scholar] [CrossRef]
- Lichtenthaler, H.K.; Wenzel, O.; Bushmann, C.; Gitelson, A. Plant stress detection by reflectance and fluorescence. Annals NY Acad. Sci. 1998, 851, 271–285. [Google Scholar] [CrossRef]
- Papageorgiou, G.C.; Govindjee, J. Chlorophyll a fluorescence, a signature of photosynthesis. In Advances in Photosynthesis and Respiration; Springer: Dordrecht, The Netherlands, 2004. [Google Scholar]
- Maxwell, K.; Johnson, G.N. Chlorophyll fluorescence—a practical guide. J. Exp. Bot. 2000, 51, 659–668. [Google Scholar] [CrossRef] [PubMed]
- Flexas, J.; Briantais, J.M.; Cerovic, Z.; Medrano, H.; Moya, I. Steady-state and maximum chlorophyll fluorescence responses to water stress in grapevine leaves: a new remote sensing system. Remote Sens. Environ. 2000, 73, 283–297. [Google Scholar] [CrossRef]
- Genty, B.; Briantais, J.M.; Baker, N.R. The relationship between quantum yield of photosynthetic electron transport and quenching of chlorophyll fluorescence. Acta Bioch. Biophys. 1989, 990, 87–92. [Google Scholar] [CrossRef]
- Fraser, N.J.; Hashimoto, H.; Richard, C. Carotenoids and bacterial photosynthesis: the story so far. Photosynth. Res. 2001, 70, 249–256. [Google Scholar] [CrossRef] [PubMed]
- Dodd, I.C.; Critchley, C.; Woodall, G.S.; Stewart, G.R. Photoinhibition in differently coloured juvenile leaves of Syzygium species. J. Exp. Bot. 1998, 49, 1437–1445. [Google Scholar] [CrossRef]
- Mendez, M.; Gwynn, J.D.; Manetas, Y. Enhanced UV-B radiation under field conditions increases anthocyanin and reduces the risk of photoinhibition but does not affect growth in the carnivorous plant Pinguicula vulgaris. New Phytol. 1999, 144, 275–282. [Google Scholar] [CrossRef]
- Sims, D.A.; Gamon, J.A. Relationships between leaf pigment content and spectral reflectance across a wide range of species, leaf structures and developmental stages. Remote Sens. Environ. 2002, 81, 337–354. [Google Scholar] [CrossRef]
- Chevalier, M.; Lespinasse, Y.; Renaudin, S. A microscopic study of the different classes of symptoms coded by the Vf gene in apple for resistance to scab (Venturia inaequalis). Plant Pathol. 1991, 40, 249–256. [Google Scholar] [CrossRef]
- Croxall, H.E.; Gwynne, D.C.; Jenkins, J.E.E. The rapid assessment of apple scab in leaves. Plant Pathol. 1952, 1, 39–41. [Google Scholar] [CrossRef]
- Parisi, L.; Lespinasse, Y.; Guillaumès, J.; Krüger, J. A new race of Venturia inaequalis virulent to apples with resistance due to Vf gene. Phytopathology 1993, 83, 533–537. [Google Scholar] [CrossRef]
- Kokaly, R.F.; Clark, R.N. Spectroscopic determination of leaf biochemistry using band-depth analysis of absorption features and stepwise multiple linear regression. Remote Sens. Environ. 1999, 67, 267–287. [Google Scholar] [CrossRef]
- Kokaly, R.F. Investigating a physical basis for spectroscopic estimates of leaf nitrogen concentration. Remote Sens. Environ. 2001, 75, 153–161. [Google Scholar] [CrossRef]
- Delalieux, S.; Somers, B.; Verstraeten, W.W.; van Aardt, J.A.N.; Keulemans, W.; Coppin, P. Hyperspectral indices to diagnose leaf biotic stress of apple plants considering leaf phenology. Int. J. Remote Sens. 2009, 30, 1887–1912. [Google Scholar] [CrossRef]
- Gamon, J.A.; Peñuelas, J.; Field, C.B. A narrow-waveband spectral index that tracks diurnal changes in photosynthetic efficiency. Remote Sens. Environ. 1992, 41, 35–44. [Google Scholar] [CrossRef]
- Barnes, J.D. A reappraisal of the use of DMSO for the extraction and determination of chlorophylls a and b in lichens and higher plants. Environ. Exp. Bot. 1992, 2, 85–100. [Google Scholar] [CrossRef]
- Dobrowsky, S.Z.; Pushnik, J.C.; Zarco-Tejada, P.J.; Ustin, S.L. Simple reflectance indices track heat and water stress-induced changes in steady-state chlorophyll fluorescence at the canopy scale. Remote Sens. Environ. 2005, 97, 403–414. [Google Scholar] [CrossRef]
- Blackburn, G.A. Spectral indices for estimating photosynthetic concentrations: a test using senescent tree leaves. Int. J. Remote Sens. 1998, 19, 657–675. [Google Scholar] [CrossRef]
- Baker, N.R.; Rosenqvist, E. Applications of chlorophyll fluorescence can improve crop production strategies: an examination of future possibilities. J. Exp. Bot. 2004, 55, 1607–1621. [Google Scholar] [CrossRef] [PubMed]
- Takahashi, T.; Yasuoka, Y.; Fujii, T. Hyperspectral remote sensing of riparian vegetation and leaf chemistry contents. In 23rd Asian Conference on Remote Sensing 2003; Kathmandu, Nepal.
- Kumar, L.; Schmidt, K.S.; Dury, S.; Skidmore, A.K. Imaging spectrometry and vegetation science. In Imaging Spectrometry; van de Meer, F., de Jong, S.M., Eds.; Kluwer Academic: Dordrecht, The Netherlands, 2001. [Google Scholar]
- Jacquemoud, S.; Ustin, S.L.; Verdebout, J.; Schmuck, G.; Andreoli, G.; Hosgood, B. Estimating leaf biochemistry using the PROSPECT leaf optical properties model. Remote Sens. Environ. 1996, 56, 194–202. [Google Scholar] [CrossRef]
- Win, J.; Greenwood, D.R.; Plummer, K.M. Characterisation of a protein from Venturia inaequalis that induces necrosis in Malus carrying the Vm resistance gene. Physiol. Mol. Plant P. 2003, 62, 193–202. [Google Scholar] [CrossRef]
- Gamon, J.A.; Serrano, L.; Surfus, J.S. The photochemical reflectance index: an optical indicator of photosynthetic radiation use efficiency across species, functional types, and nutrient levels. Oecologia 1997, 112, 492–501. [Google Scholar] [CrossRef]
- Peñuelas, J.; Filella, I.; Gamon, J.A. Assessment of photosynthetic radiation-use efficiency with spectral reflectance. New Phytol. 1995, 131, 291–296. [Google Scholar] [CrossRef]
- Peñuelas, J.; Llusia, J.; Pinol, J.; Filella, I. Photochemical reflectance index and leaf photosynthetic radiation-use-efficiency assessment in Mediterranean trees. Int. J. Remote Sens. 1997, 18, 2863–2868. [Google Scholar] [CrossRef]
- Sun, P.; Grignetti, A.; Liu, S.; Casaccia, R.; Salvatori, R.; Pietrini, F.; Loreto, F.; Centritto, M. Associated changes in physiological parameters and spectral reflectance indices in omiv (Olea europea L.) leaves in response to different levels of water stress. Int. J. Remote Sens. 2008, 29, 1725–1743. [Google Scholar] [CrossRef]
- Penuelas, J.; Filella, I.; Lloret, P.; Munoz, F.; Vilajeliu, M. Reflectance assessment of mite effects on apple-trees. Int. J. Remote Sens. 1995, 16, 2727–2733. [Google Scholar] [CrossRef]
- Lorenzen, B.; Jensen, A. Changes in leaf spectral properties induced in barley by cereal powdery mildew. Remote Sens. Environ. 1989, 27, 201–209. [Google Scholar]
- Ahern, F.J. The effects of bark beetle stress on the foliar spectral reflectance of lodgepole pine. Int. J. Remote Sens. 1988, 9, 1451–1468. [Google Scholar] [CrossRef]
© 2009 by the authors; licensee Molecular Diversity Preservation International, Basel, Switzerland. This article is an open-access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Delalieux, S.; Auwerkerken, A.; Verstraeten, W.W.; Somers, B.; Valcke, R.; Lhermitte, S.; Keulemans, J.; Coppin, P. Hyperspectral Reflectance and Fluorescence Imaging to Detect Scab Induced Stress in Apple Leaves. Remote Sens. 2009, 1, 858-874. https://doi.org/10.3390/rs1040858
Delalieux S, Auwerkerken A, Verstraeten WW, Somers B, Valcke R, Lhermitte S, Keulemans J, Coppin P. Hyperspectral Reflectance and Fluorescence Imaging to Detect Scab Induced Stress in Apple Leaves. Remote Sensing. 2009; 1(4):858-874. https://doi.org/10.3390/rs1040858
Chicago/Turabian StyleDelalieux, Stephanie, Annemarie Auwerkerken, Willem W. Verstraeten, Ben Somers, Roland Valcke, Stefaan Lhermitte, Johan Keulemans, and Pol Coppin. 2009. "Hyperspectral Reflectance and Fluorescence Imaging to Detect Scab Induced Stress in Apple Leaves" Remote Sensing 1, no. 4: 858-874. https://doi.org/10.3390/rs1040858