On the Potentiality of UAV Multispectral Imagery to Detect Flavescence dorée and Grapevine Trunk Diseases
Abstract
:1. Introduction
2. Materials and Methods
2.1. Data Acquisition
2.1.1. Experimental Sites
2.1.2. Field Data Acquisition
2.1.3. Multispectral Data Acquisition
2.2. Data Processing and Analysis
2.2.1. Computing Vegetation Indices and Biophysical Parameters
2.2.2. Extraction of Symptomatic and Asymptomatic Vine Vegetation Areas
2.2.3. Statistical Analysis
Step 1: Selection of the best discriminating variables
Step 2: Mapping of symptomatic vine vegetation at the whole vineyard scale
Step 3: Validation
3. Results
3.1. Selection of the Best Discriminating Variables
3.1.1. Best Vegetation Indices
3.1.2. Best Biophysical Parameters
3.2. Validation and Mapping at the Vineyard Scale
4. Discussion
4.1. Case 1: Discrimination of FD or GTD Symptomatic Vines from Asymptomatic Vines
4.2. Case 2: Discrimination of FD from GTD Vines
5. Conclusions and Perspectives
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Appendix A
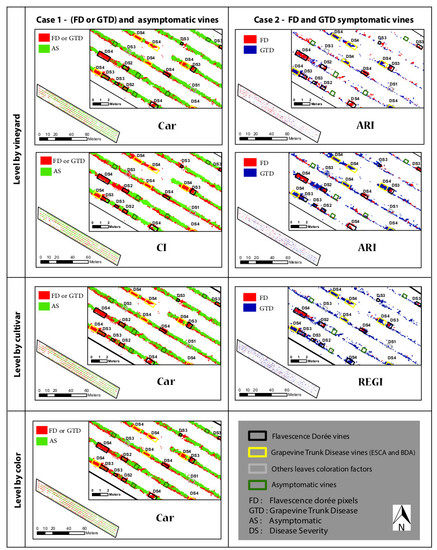
Appendix A.1. Map Disease Level of Vineyard Analysis

Appendix A.2. Map Disease Level of Cultivar Analysis

Appendix A.3. Map Disease Level of Berry Color Analysis

References
- Chuche, J.; Thiéry, D. Biology and ecology of the Flavescence dorée vector Scaphoideus titanus: A review. Agron. Sustain. Dev. 2014, 34, 381–403. [Google Scholar] [CrossRef]
- Bruez, E.; Lecomte, P.; Grosman, J.; Doublet, B.; Bertsch, C.; Fontaine, F.; Ugaglia, A.; Teissedre, P.L.; Da Costa, J.P.; Guerin-Dubrana, L.; et al. Overview of grapevine trunk diseases in France in the 2000s. Phytopathol. Mediterr. 2013, 52, 262–275. [Google Scholar]
- Fontaine, F.; Gramaje, D.; Armengol, J.; Smart, R.; Nagy, Z.A.; Borgo, M.; Rego, C.; Corio-Costet, M.F. Grapevine Trunk Diseases. A Review; Cahiers de recherche, OIV Publications: Paris, France, 2016. [Google Scholar]
- MAAF. Rapport annuel de la Surveillance biologique du territoire de l’année 2013; Technical Report; Ministère de l’Agriculture de l’Agroalimentaire et de la Forêt: Paris, France, 2013.
- MAAF. Rapport annuel de la Surveillance biologique du territoire de l’année 2015; Technical Report; Ministère de l’Agriculture de l’Agroalimentaire et de la Forêt: Paris, France, 2015.
- Schvester, D.; Carle, P.; Moutous, G. Transmission de la flavescence dorée de la vigne par Scaphoideus littoralis Ball. Annales des Epiphyties 1963, 14, 175–198. [Google Scholar]
- Mori, N.; Bressan, A.; Martin, M.; Guadagnini, M.; Girolami, V.; Bertaccini, A. Experimental transmission by Scaphoideus titanus Ball of two Flavescence doree-type phytoplasmas. VITIS J. Grapevine Res. 2002, 41, 99–102. [Google Scholar]
- Galet, P. Les maladies et les parasites de la vigne Tome 1; Tec & Doc Distribution: Montpellier, France, 1999. [Google Scholar]
- Bovey, R. Maladies à virus et affections similaires de la vigne; La Maison rustique: Paris, France, 1980. [Google Scholar]
- Chuche, J. Comportement de Scaphoideus Titanus, ConséQuences Spatiales et DéMographiques. Ph.D. Thesis, Université Victor Segalen Bordeaux 2, Bordeaux, France, 2010. [Google Scholar]
- Pueyo, C.; Carrara, J.; Parent, E. Flavescence dorée en Languedoc Roussillon: Bilan de 10 années de lutte (Synthese des données 1997–2007); Direction Régionale de l’Agriculture et de la Forêt Languedoc-Roussillon, Service Régional de la Protection des Végétaux: Montpellier, France, 2008. [Google Scholar]
- Vitali, M.; Chitarra, W.; Galetto, L.; Bosco, D.; Marzachì, C.; Gullino, M.; Spanna, F.; Lovisolo, C. Flavescence dorée phytoplasma deregulates stomatal control of photosynthesis in Vitis vinifera. Ann. Appl. Biol. 2013, 162, 335–346. [Google Scholar] [CrossRef]
- Mugnai, L.; Graniti, A.; Surico, G. Esca (black measles) and brown wood-streaking: Two old and elusive diseases of grapevines. Plant Dis. 1999, 83, 404–418. [Google Scholar] [CrossRef]
- Guerin-Dubrana, L.; Labenne, A.; Labrousse, J.C.; Bastien, S.; Rey, P.; Gégout-Petit, A. Statistical analysis of grapevine mortality associated with esca or Eutypa dieback foliar expression. Phytopathol. Mediterr. 2012, 52, 276–288. [Google Scholar] [CrossRef]
- Denizot, A.M.; Larignon, P. Description des symptômes des maladies du bois—Black Dead Arm; Institut Français de la Vigne et du Vin: Le Grau du Roi, France, 2008. [Google Scholar]
- Denizot, A.M.; Larignon, P. Description des symptômes des maladies du bois—ESCA; Institut Français de la Vigne et du Vin: Le Grau du Roi, France, 2008. [Google Scholar]
- Sankaran, S.; Mishra, A.; Ehsani, R.; Davis, C. A review of advanced techniques for detecting plant diseases. Comput. Electron. Agric. 2010, 72, 1–13. [Google Scholar] [CrossRef]
- Martinelli, F.; Scalenghe, R.; Davino, S.; Panno, S.; Scuderi, G.; Ruisi, P.; Villa, P.; Stroppiana, D.; Boschetti, M.; Goulart, L.R.; et al. Advanced methods of plant disease detection. A review. Agron. Sustain. Dev. 2015, 35, 1–25. [Google Scholar] [CrossRef]
- Yang, C.M.; Cheng, C.H.; Chen, R.K. Changes in spectral characteristics of rice canopy infested with brown planthopper and leaffolder. Crop Sci. 2007, 47, 329–335. [Google Scholar] [CrossRef]
- Naidu, R.A.; Perry, E.M.; Pierce, F.J.; Mekuria, T. The potential of spectral reflectance technique for the detection of Grapevine leafroll-associated virus-3 in two red-berried wine grape cultivars. Comput. Electron. Agric. 2009, 66, 38–45. [Google Scholar] [CrossRef]
- Meroni, M.; Rossini, M.; Colombo, R. Characterization of Leaf Physiology Using Reflectance and Fluorescence Hyperspectral Measurements; Optical Observation of Vegetation Properties and Characteristics; Research Signpost: Trivandrum, India, 2010; pp. 165–187. [Google Scholar]
- Mahlein, A.K.; Steiner, U.; Hillnhütter, C.; Dehne, H.W.; Oerke, E.C. Hyperspectral imaging for small-scale analysis of symptoms caused by different sugar beet diseases. Plant Methods 2012, 8, 3. [Google Scholar] [CrossRef] [PubMed]
- Mahlein, A.K. Plant Disease Detection by Imaging Sensors—Parallels and Specific Demands for Precision Agriculture and Plant Phenotyping. Plant Dis. 2015, 100, 241–251. [Google Scholar] [CrossRef]
- Steele, M.R.; Gitelson, A.A.; Rundquist, D.C.; Merzlyak, M.N. Nondestructive estimation of anthocyanin content in grapevine leaves. Am. J. Enol. Vitic. 2009, 60, 87–92. [Google Scholar]
- Gamon, J.A.; Surfus, J.S. Assessing leaf pigment content and activity with a reflectometer. New Phytol. 1999, 143, 105–117. [Google Scholar] [CrossRef] [Green Version]
- Zhang, M.; Qin, Z.; Liu, X.; Ustin, S.L. Detection of stress in tomatoes induced by late blight disease in California, USA, using hyperspectral remote sensing. Int. J. Appl. Earth Obs. Geoinf. 2003, 4, 295–310. [Google Scholar] [CrossRef]
- Mahlein, A.K.; Rumpf, T.; Welke, P.; Plümer, L.; Steiner, U.; Oerke, E.C. Development of spectral indices for detecting and identifying plant diseases. Remote Sens. Environ. 2013, 128, 21–30. [Google Scholar] [CrossRef]
- Hall, A.; Lamb, D.; Holzapfel, B.; Louis, J. Optical remote sensing applications in viticulture—A review. Aust. J. Grape Wine Res. 2002, 8, 36–47. [Google Scholar] [CrossRef]
- Lobitz, B.; Johnson, L.; Hlavka, C.; Armstrong, R.; Bell, C. Grapevine Remote Sensing Analysis of Phylloxera Early Stress (GRAPES): Remote Sensing Analysis Summary; Technical Report; National Aeronautics and Space Administration: Washington, DC, USA, 1997. [Google Scholar]
- MacDonald, S.L.; Staid, M.; Staid, M.; Cooper, M.L. Remote hyperspectral imaging of grapevine leafroll-associated virus 3 in cabernet sauvignon vineyards. Comput. Electron. Agric. 2016, 130, 109–117. [Google Scholar] [CrossRef]
- Gennaro, S.F.D.; Battiston, E.; Marco, S.D.; Facini, O.; Matese, A.; Nocentini, M.; Palliotti, A.; Mugnai, L. Unmanned Aerial Vehicle (UAV)-based remote sensing to monitor grapevine leaf stripe disease within a vineyard affected by esca complex. Phytopathol. Mediterr. 2016, 55, 262–275. [Google Scholar] [CrossRef]
- Al-Saddik, H.; Laybros, A.; Cointault, F. Using Image Texture and Spectral Reflectance Analysis to Detect Yellowness and Esca in Grapevines at Leaf-Level. Remote Sens. 2018, 10, 618. [Google Scholar] [CrossRef]
- Paindavoine, M.; Zunino, P.; Brossaud, F.; Cointault, F. Détection de foyers infectieux de Flavescence Dorée par imagerie de drone; Presented at 8e; Les Rencontres du Végétal: Angers, France, 2015. [Google Scholar]
- Al-Saddik, H.; Simon, J.; Cointault, F. Assessment of the optimal spectral bands for designing a sensor for vineyard disease detection: The case of Flavescence dorée. Precis. Agric. 2018, 1–25. [Google Scholar] [CrossRef]
- Albetis, J.; Duthoit, S.; Guttler, F.; Jacquin, A.; Goulard, M.; Poilvé, H.; Féret, J.B.; Dedieu, G. Detection of Flavescence dorée Grapevine Disease Using Unmanned Aerial Vehicle (UAV) Multispectral Imagery. Remote Sens. 2017, 9, 308. [Google Scholar] [CrossRef]
- Guttler, F.; Duthoit, S.; Fauvel, M.; Jacquin, A. Spectral analysis of Vitis vinifera leaves for the detection of the Flavescence dorée disease in red and white cultivars. Science 2018. Article in preparation. [Google Scholar]
- Poilvé, H. Towards an Operational GMES Land Monitoring Core Service—BioPar Product User Manual—MERIS FR Biophysical Products; Technical Report, European Research Project geoland2 (FP7, EC Proposal Reference No.: FP-7-218795); European Research: Brussels, Belgium, 2010. [Google Scholar]
- Féret, J.B.; Gitelson, A.A.; Noble, S.D.; Jacquemoud, S. PROSPECT-D: Towards modeling leaf optical properties through a complete lifecycle. Remote Sens. Environ. 2017, 193, 204–215. [Google Scholar] [CrossRef] [Green Version]
- Jacquemoud, S.; Verhoef, W.; Baret, F.; Bacour, C.; Zarco-Tejada, P.J.; Asner, G.P.; François, C.; Ustin, S.L. PROSPECT + SAIL models: A review of use for vegetation characterization. Remote Sens. Environ. 2009, 113, S56–S66. [Google Scholar] [CrossRef]
- Féret, J.B.; François, C.; Asner, G.P.; Gitelson, A.A.; Martin, R.E.; Bidel, L.P.; Ustin, S.L.; le Maire, G.; Jacquemoud, S. PROSPECT-4 and 5: Advances in the leaf optical properties model separating photosynthetic pigments. Remote Sens. Environ. 2008, 112, 3030–3043. [Google Scholar] [CrossRef]
- Margaria, P.; Ferrandino, A.; Caciagli, P.; Kedrina, O.; Schubert, A.; Palmano, S. Metabolic and transcript analysis of the flavonoid pathway in diseased and recovered Nebbiolo and Barbera grapevines (Vitis vinifera L.) following infection by Flavescence dorée phytoplasma. Plant Cell Environ. 2014, 37, 2183–2200. [Google Scholar] [CrossRef] [PubMed]
- Rouse, J., Jr.; Haas, R.H.; Schell, J.A.; Deering, D.W. Monitoring vegetation systems in the Great Plains with ERTS. NASA Spec. Publ. 1974, 351, 309. [Google Scholar]
- Gitelson, A.A.; Kaufman, Y.J.; Stark, R.; Rundquist, D. Novel algorithms for remote estimation of vegetation fraction. Remote Sens. Environ. 2002, 80, 76–87. [Google Scholar] [CrossRef] [Green Version]
- Gitelson, A.A.; Kaufman, Y.J.; Merzlyak, M.N. Use of a green channel in remote sensing of global vegetation from EOS-MODIS. Remote Sens. Environ. 1996, 58, 289–298. [Google Scholar] [CrossRef]
- Yuan, L.; Pu, R.; Zhang, J.; Wang, J.; Yang, H. Using high spatial resolution satellite imagery for mapping powdery mildew at a regional scale. Precis. Agric. 2016, 17, 332–348. [Google Scholar] [CrossRef]
- Richardson, A.J.; Wiegand, C.L. Distinguishing vegetation from soil background information. Photogramm. Eng. Remote Sens. 1977, 43, 1541–1552. [Google Scholar]
- Cui, D.; Zhang, Q.; Li, M.; Zhao, Y.; Hartman, G.L. Detection of soybean rust using a multispectral image sensor. Sens. Instrum. Food Qual. Saf. 2009, 3, 49–56. [Google Scholar] [CrossRef]
- Huete, A.R. A soil-adjusted vegetation index (SAVI). Remote Sens. Environ. 1988, 25, 295–309. [Google Scholar] [CrossRef]
- Gitelson, A.A.; Merzlyak, M.N.; Chivkunova, O.B. Optical properties and nondestructive estimation of anthocyanin content in plant leaves. Photochem. Photobiol. 2001, 74, 38–45. [Google Scholar] [CrossRef]
- Gitelson, A.A.; Keydan, G.P.; Merzlyak, M.N. Three-band model for noninvasive estimation of chlorophyll, carotenoids, and anthocyanin contents in higher plant leaves. Geophys. Res. Lett. 2006, 33, L11402. [Google Scholar] [CrossRef]
- Patrick, A.; Pelham, S.; Culbreath, A.; Holbrook, C.C.; Godoy, I.J.D.; Li, C. High throughput phenotyping of tomato spot wilt disease in peanuts using unmanned aerial systems and multispectral imaging. IEEE Instrum. Meas. Mag. 2017, 20, 4–12. [Google Scholar] [CrossRef]
- van den Berg, A.K.; Perkins, T.D. Nondestructive estimation of anthocyanin content in autumn sugar maple leaves. HortScience 2005, 40, 685–686. [Google Scholar]
- Steele, M.R.; Gitelson, A.A.; Rundquist, D.C. A comparison of two techniques for nondestructive measurement of chlorophyll content in grapevine leaves. Agron. J. 2008, 100, 779–782. [Google Scholar] [CrossRef]
- Zarco-Tejada, P.J.; Miller, J.R.; Noland, T.L.; Mohammed, G.H.; Sampson, P.H. Scaling-up and model inversion methods with narrowband optical indices for chlorophyll content estimation in closed forest canopies with hyperspectral data. IEEE Trans. Geosci. Remote Sens. 2001, 39, 1491–1507. [Google Scholar] [CrossRef] [Green Version]
- Peñuelas, J.; Gamon, J.A.; Fredeen, A.L.; Merino, J.; Field, C.B. Reflectance indices associated with physiological changes in nitrogen- and water-limited sunflower leaves. Remote Sens. Environ. 1994, 48, 135–146. [Google Scholar] [CrossRef]
- Inglada, J.; Christophe, E. The Orfeo Toolbox remote sensing image processing software. In Proceedings of the 2009 IEEE International Geoscience and Remote Sensing Symposium (IGARSS 2009), Cape Town, South Africa, 12–17 July 2009; IEEE: Piscataway, NJ, USA, 2009; Volume 4, pp. IV–733. [Google Scholar]
- Cortes, C.; Vapnik, V. Support-vector networks. Mach. Learn. 1995, 20, 273–297. [Google Scholar] [CrossRef] [Green Version]
- Hossin, M.; Sulaiman, M. A review on evaluation metrics for data classification evaluations. Int. J. Data Min. Knowl. Manag. Process 2015, 5, 1. [Google Scholar]
- Delacour, H.; Servonnet, A.; Perrot, A.; Vigezzi, J.F.; Ramirez, J.M. La courbe ROC (Receiver operating characteristic): Principes et principales applications en biologie clinique. Ann. De Biol. Clin. 2005, 63, 145–154. [Google Scholar]
- Fawcett, T. An introduction to ROC analysis. Pattern Recognit. Lett. 2006, 27, 861–874. [Google Scholar] [CrossRef]
- Saatchi, S.; Buermann, W.; Ter Steege, H.; Mori, S.; Smith, T.B. Modeling distribution of Amazonian tree species and diversity using remote sensing measurements. Remote Sens. Environ. 2008, 112, 2000–2017. [Google Scholar] [CrossRef]
- Hastie, T.; Tibshirani, R.; Friedman, J. The Elements of Statistical Learning New York; Springer: New York, NY, USA, 2001; pp. 115–163. [Google Scholar]
- Welch, B.L. The generalization of students’ problem when several different population variances are involved. Biometrika 1947, 34, 28–35. [Google Scholar]
- Weiss, M.; Baret, F. Using 3D Point Clouds Derived from UAV RGB Imagery to Describe Vineyard 3D Macro-Structure. Remote Sens. 2017, 9, 111. [Google Scholar] [CrossRef]
- Comba, L.; Gay, P.; Primicerio, J.; Ricauda Aimonino, D. Vineyard detection from unmanned aerial systems images. Comput. Electron. Agric. 2015, 114, 78–87. [Google Scholar] [CrossRef]
- Al-Saddik, H.; Simon, J.C.; Cointault, F. Development of Spectral Disease Indices for ’Flavescence Dorée’ Grapevine Disease Identification. Sensors 2017, 17, 2772. [Google Scholar] [CrossRef] [PubMed]










| FD | GTD | OF | AS | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ID Vineyard | Vineyard Size (ha) | Site n° | Orientation | Total Numb. of GPS Points | ds1 | ds2 | ds3 | ds4 | ds1 | ds2 | ds3 | ds4 | Total | Total |
| Fer_Servadou (A) | 0.1 | 1 | 132° | 142 | 19 | 41 | 33 | 13 | 1 | 0 | 3 | 6 | 3 | 23 |
| Fer_Servadou (B) | 0.1 | 2 | 88° | 140 | 30 | 29 | 14 | 6 | 3 | 4 | 7 | 7 | 15 | 25 |
| Gamay (A) | 0.2 | 2 | 132° | 183 | 17 | 38 | 37 | 23 | 2 | 3 | 4 | 13 | 9 | 37 |
| Gamay (B) | 0.5 | 3 | 165° | 121 | 3 | 6 | 7 | 14 | 3 | 14 | 19 | 22 | 10 | 23 |
| Duras | 0.3 | 1 | 115° | 168 | 17 | 30 | 15 | 12 | 3 | 8 | 10 | 10 | 24 | 39 |
| Mourvèdre | 0.5 | 4 | 139° | 102 | 17 | 17 | 6 | 13 | 1 | 6 | 4 | 2 | 1 | 35 |
| Grenache | 0.4 | 5 | 102° | 130 | 19 | 12 | 6 | 8 | 14 | 19 | 8 | 3 | 5 | 36 |
| Characteristic Name | Description |
|---|---|
| Platform | Long range DT-18 |
| Sensor | DT-5Bands |
| Number of bands | 5 |
| Spectral wavelengths | Blue (455–495 nm) |
| Green (540–580 nm) | |
| Red (658–678 nm) | |
| Red-edge (707–727 nm) | |
| NIR (800–880 nm) | |
| Focal length | 5.5 mm |
| Field of view | 47.2° |
| Flight altitude Above Ground Level (AGL) | 150 m |
| Ground resolution | 0.08 m/pixel |
| Index Name | Formula | Biophysical Indicator | References |
|---|---|---|---|
| - Normalized Difference Vegetation Index (NDVI) | (NIR − Red)/(NIR + Red) | Biomass | [31,42] |
| - Green-Red Vegetation Index (GRVI) | (Green + Red)/(Green + Red) | [36,43] | |
| - Green Normalized Difference Vegetation Index (GNDVI) | (NIR − Green)/(NIR + Green) | [44,45] | |
| - Difference Vegetation Index (DVI) | NIR − Red | [46,47] | |
| - Soil Adjusted Vegetation Index (SAVI) | (NIR − Red) × (1 + L)/(NIR + Red + L) L = 0.5 | [19,48] | |
| - Anthocyanin Reflectance Index (ARI) | Green − RedEdge | Anthocyanins Content | [24,49] |
| - Modified Anthocyanin Reflectance Index (MARI) | (Green − RedEdge) × NIR | [24,50] | |
| - Red Green Index (RGI) | Red/Green | [25] | |
| - Modified Anthocyanin Content Index (MACI) | NIR/Green | [24,51] | |
| - Anthocyanin Content Index (ACI) | Green/NIR | [52] | |
| - Chlorophyll Index (CI) | (NIR/RedEdge) − 1 | Chlorophyll content | [43,53] |
| - Normalized Pigment Chlorophyll Index (NPCI) | (RedEdge − Blue)/(RedEdge + Blue) | [51,54,55] | |
| - Normalized Difference Red-edge Index (NDRE) | (NIR − RedEdge)/(NIR + RedEdge) | [36,51] | |
| - Red-edge Green Index (REGI) | (RedEdge − Green)/(RedEdge + Green) | Not defined | - |
| - Red-edge Rouge Index (RERI) | (RedEdge − Red)/(RedEdge + Red) | - |
| Parameter Name | Acronym | Description | Unit and Typical Range |
|---|---|---|---|
| fCover | fCover | Fractional cover of green vegetation (interception in vertical view) | 0.0 to 1.0 |
| Leaf Anthocyanin content | Ant | Anthocyanin content in the leaves (per leaf unit area) | 0 to 12 μg/cm2 |
| Leaf Carotenoid content | Car | Carotenoid content of the leaves (per leaf unit area) | 0 to 15 μg/cm2 |
| Leaf Chlorophyll content | Chl | Chlorophyll content in the leaves (per leaf unit area) | 20 to 80 μg/cm2 |
| Vineyard | Accuracy Metric (%) |
|---|---|
| Fer_Servadou (A) | 90.13 |
| Fer_Servadou (B) | 95.87 |
| Gamay (A) | 98.43 |
| Gamay (B) | 99.02 |
| Duras | 92.53 |
| Mourvèdre | 99.64 |
| Grenache | 99.51 |
| Study Case | Case 1 | Case 2 | ||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Statical Analysis | Training | Validation | Training | Validation | ||||||||||||||
| Disease Severity | AS | FD or GTD | AS | FD or GTD | FD | GTD | FD | GTD | ||||||||||
| - | DS4 | - | DS1 | DS2 | DS3 | DS4 | DS4 | DS4 | DS1 | DS2 | DS3 | DS4 | DS1 | DS2 | DS3 | DS4 | ||
| Vineyard | ||||||||||||||||||
| Fer_Servadou (A) | VV | 14 | 14 | 9 | 20 | 41 | 36 | 5 | 4 | 4 | 19 | 41 | 33 | 9 | 1 | - | 3 | 2 |
| (VP) | (285) | (634) | (184) | (819) | (1917) | (1813) | (254) | (196) | (228) | (762) | (1917) | (1715) | (323) | (57) | (98) | (141) | ||
| Fer_Servadou (B) | VV | 9 | 9 | 16 | 33 | 33 | 21 | 4 | 4 | 4 | 30 | 29 | 14 | 2 | 3 | 4 | 7 | 3 |
| (VP) | (295) | (340) | (503) | (934) | (1187) | (723) | (164) | (81) | (181) | (828) | (1011) | (410) | (97) | (106) | (176) | (313) | (145) | |
| Gamay (A) | VV | 28 | 28 | 9 | 19 | 41 | 41 | 8 | 10 | 10 | 17 | 38 | 37 | 13 | 2 | 3 | 4 | 3 |
| (VP) | (608) | (1003) | (201) | (583) | (1481) | (1468) | (336) | (409) | (372) | (484) | (1393) | (1262) | (449) | (99) | (88) | (206) | (109) | |
| Gamay (B) | VV | 18 | 18 | 5 | 6 | 20 | 26 | 18 | 11 | 11 | 3 | 6 | 7 | 3 | 3 | 14 | 19 | 11 |
| (VP) | (291) | (710) | (78) | (149) | (777) | (867) | (504) | (443) | (405) | (78) | (146) | (181) | (81) | (71) | (631) | (686) | (285) | |
| Duras | VV | 17 | 17 | 22 | 20 | 38 | 25 | 5 | 8 | 8 | 17 | 30 | 15 | 4 | 3 | 8 | 10 | 2 |
| (VP) | (355) | (539) | (421) | (542) | (1224) | (787) | (126) | (228) | (291) | (466) | (1026) | (410) | (103) | (76) | (198) | (377) | (43) | |
| Cultivar | ||||||||||||||||||
| Fer_Servadou | VV | 25 | 25 | 23 | 53 | 74 | 57 | 7 | 10 | 10 | 49 | 70 | 47 | 9 | 4 | 4 | 10 | 3 |
| (VP) | (635) | (1117) | (632) | (1753) | (3104) | (2536) | (275) | (387) | (513) | (1590) | (2928) | (2125) | (310) | (163) | (176) | (411) | (182) | |
| Gamay | VV | 57 | 57 | 3 | 25 | 61 | 67 | 15 | 28 | 28 | 20 | 44 | 44 | 9 | 5 | 17 | 23 | 7 |
| (VP) | (1124) | (1955) | (54) | (732) | (2258) | (2335) | (598) | (996) | (944) | (562) | (1539) | (1443) | (386) | (170) | (719) | (892) | (227) | |
| Berry color | ||||||||||||||||||
| Red | VV | 121 | 121 | 97 | 149 | 227 | 173 | 31 | 50 | 50 | 122 | 173 | 118 | 39 | 27 | 54 | 55 | 13 |
| (VP) | (2460) | (4488) | (1930) | (4448) | (8203) | (6438) | (1130) | (1757) | (1844) | (3603) | (6402) | (4437) | (1535) | (845) | (1801) | (2001) | (482) | |
| Reference Data | |||
|---|---|---|---|
| Symptomatic (FD or GTD) | Asymptomatic | ||
| Classification results | Symptomatic (FD or GTD) | True positive (TP) (Symptomatic pixel classified as Symptomatic) | False positive (FP) (Asymptomatic pixel classified as Symptomatic) |
| asymptomatic | False negative (FP) (Symptomatic pixel classified as Asymptomatic) | True negative (TN) (Asymptomatic pixel classified as Asymptomatic) | |
| Study Case | Case 1—FD or GTD and Asymptomatic Vines | ||||
|---|---|---|---|---|---|
| scale of Analysis | Best VI or BS | Best BP | |||
| Position | Variable | (AUC, Sens./Spe.) | Variable | (AUC, Sens./Spe.) | |
| Vineyard | |||||
| Fer Servadou (A) | 1 | REGI | (0.92, 0.83/0.87) | Car | (0.93, 0.84/0.91) |
| 2 | GRVI | (0.90, 0.82/0.82) | AUC < 0.70 | - | |
| Fer Servadou (B) | 1 | RGI | (0.94, 0.85/0.85 ) | fCover | (0.89, 0.7/0.92) |
| 2 | GRVI | (0.94, 0.84/0.87) | AUC < 0.70 | - | |
| Gamay (A) | 1 | CI, NDRE | (0.89, 0.85/0.75) | Car | (0.90, 084/0.82) |
| 2 | NDVI | (0.80, 0.89/0.63) | fCover | (0.82, 0.87/0.70) | |
| Gamay (B) | 1 | RGI, GRVI | (0.86, 0.81/0.74) | Car | (0.90, 0.82/0.79) |
| 2 | CI, NDRE | (0.81, 0.64/0.76) | fCover | (0.86, 0.85/0.75) | |
| Duras | 1 | GRVI | (0.95, 0.93/0.85 ) | Car | (0.92, 0.81/0.89) |
| 2 | RGI | (0.95, 0.92/0.85) | fCover | (0.91, 0.84/0.81) | |
| Cultivar | |||||
| Fer Servadou | 1 | RGI, GRVI | (0.89, 0.81/0.81) | Car | (0.87, 0.75/0.94 ) |
| 2 | REGI | (0.88, 0.73/0.89) | fCover | (0.86, 0.75/0.84) | |
| Gamay | 1 | CI, NDRE | (0.88, 0.77/0.8) | Car | (0.84, 0.71, 0.84) |
| 2 | NDVI | (0.80, 0.84/0.64) | fCover | (0.78, 0.72/0.74) | |
| Berry color | |||||
| Red | 1 | GRVI, RGI | (0.77, 0.77/0.66) | Car | (0.84, 0.75/0.80) |
| 2 | CI, NDRE | (0.71, 0.80/0.55) | fCover | (0.74, 0.78/0.62) | |
| Study Case | Case 2—FD and GTD Vines | ||||
|---|---|---|---|---|---|
| Scale of Analysis | Best VI or SB | Best BP | |||
| Position | Variable | (AUC, Sens./Spe.) | Variable | (AUC, Sens./Spe.) | |
| Vineyard | |||||
| Fer Servadou (A) | 1 | RERI | (0.84, 0.76/0.74) | fCover | (0.79, 0.76/0.62) |
| 2 | GRVI | (0.78, 0.69/0.69) | Car | (0.78, 0.67/0.72) | |
| Fer Servadou (B) | 1 | AUC < 0.70 | - | Ant | (0.77, 0.64/0.82) |
| 2 | AUC < 0.70 | - | |||
| Gamay (A) | 1 | ARI | (0.85, 0.79/0.76) | AUC < 0.70 | - |
| 2 | RED | (0.80, 0.71/0.79) | |||
| Gamay (B) | 1 | REGI | (0.90, 0.77/0.9) | Car | (0.77, 0.67/0.79) |
| 2 | ARI | (0.81, 0.69/0.81) | AUC < 0.70 | - | |
| Duras | 1 | REGI | (0.84, 0.76/0.80) | AUC < 0.70 | - |
| 2 | MARI | (0.81, 0.72/0.75) | |||
| Cultivar | |||||
| Fer Servadou | 1 | RERI | (0.79, 0.76/0.67) | Car | (0.74, 0.72/0.70) |
| 2 | RED | (0.75, 0.79/0.55) | AUC < 0.70 | - | |
| Gamay | 1 | REGI | (0.78, 0.56/0.94) | AUC < 0.70 | - |
| 2 | MARI | (0.72, 0.5/0.80) | AUC < 0.70 | - | |
| Berry color | |||||
| Red | 1 | AUC < 0.70 | - | AUC < 0.70 | - |
| 2 | |||||
| ID Vineyard | Random Pixels (%) | Edges of Vines Rows (%) | Drop Shadow (%) | Absence (%) | Total AS Vines |
|---|---|---|---|---|---|
| Fer_Servadou (A) | 36.36 | 54.55 | 0.00 | 9.09 | 11 |
| Fer_servadou (B) | 0.00 | 63.64 | 9.09 | 27.27 | 11 |
| Gamay (A) | 25.00 | 56.25 | 0.00 | 18.75 | 16 |
| Gamay (B) | 0.00 | 12.50 | 0.00 | 87.50 | 8 |
| Duras | 6.25 | 43.75 | 6.25 | 43.75 | 16 |
| Mourvedre | 0.00 | 69.23 | 0.00 | 30.77 | 13 |
| Grenache | 80.00 | 0.00 | 0.00 | 20.00 | 20 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Albetis, J.; Jacquin, A.; Goulard, M.; Poilvé, H.; Rousseau, J.; Clenet, H.; Dedieu, G.; Duthoit, S. On the Potentiality of UAV Multispectral Imagery to Detect Flavescence dorée and Grapevine Trunk Diseases. Remote Sens. 2019, 11, 23. https://doi.org/10.3390/rs11010023
Albetis J, Jacquin A, Goulard M, Poilvé H, Rousseau J, Clenet H, Dedieu G, Duthoit S. On the Potentiality of UAV Multispectral Imagery to Detect Flavescence dorée and Grapevine Trunk Diseases. Remote Sensing. 2019; 11(1):23. https://doi.org/10.3390/rs11010023
Chicago/Turabian StyleAlbetis, Johanna, Anne Jacquin, Michel Goulard, Hervé Poilvé, Jacques Rousseau, Harold Clenet, Gerard Dedieu, and Sylvie Duthoit. 2019. "On the Potentiality of UAV Multispectral Imagery to Detect Flavescence dorée and Grapevine Trunk Diseases" Remote Sensing 11, no. 1: 23. https://doi.org/10.3390/rs11010023