First Results of Phytoplankton Spatial Dynamics in Two NW-Mediterranean Bays from Chlorophyll-a Estimates Using Sentinel 2: Potential Implications for Aquaculture
Abstract
:1. Introduction
- Generate 20 m2 resolution chl-a maps from Sentinel-2 MSI imagery covering the whole system;
- Understand the spatiotemporal phytoplankton biomass dynamics by using the derived chl-a maps and to relate them to environmental variables and the rice farming year;
- Assess the applicability of the results to shellfish aquaculture management in the area.
2. Materials and Methods
2.1. Study Sites
2.2. In situ Data: chl-a
2.3. Sentinel 2 Data
2.4. Atmospheric Correction: ACOLITE
- (a)
- Rhot: top of atmosphere reflectance (TOA) derived from the original input file.
- (b)
- Rhorc: Rayleigh corrected reflectance. This is the Rhot with removed and corrected reflectance for two-way Rayleigh transmittance. An additional pre-processing step was made to avoid high reflectance pixels by fixing a maximum threshold (Rhorc reflectance at 492 nm or 560 nm ≥ 0.11) above which pixels were assigned as invalids.
- (c)
- Rrs: remote sensing reflectance (sr−1) for water pixels (Rrs = Rhow/π).
- (d)
- Rhow: surface reflectance for water pixels.
2.5. Chlorophyll-a Estimation Algorithms
- BG: The Ratio between Blue and Green spectra uses the reflectance at 490 nm (blue) and 560 nm (green). At 490 nm carotenoids absorb light strongly, while at 560 nm the absorption of all photosynthetic pigments is minimal (i.e., green reflection). This algorithm was initially proposed by [33]. R stands for Rhot, Rhorc, Rrs, or Rhow reflectance.
- BR: The Blue–Red ratio is based on the two chl-a maximal absorption peaks.
- NR: The ratio between Red and NIR assumes that the absorption by non-algal particles, yellow substances and the backscattering are insignificant when compared to chl-a absorption at red wavelengths (665 nm) [35]. Between 700 and 720 nm, the absorption due to water constituents is minimal.
- NDCI: The Normal Difference Chlorophyll Index developed by Mishra et al. [36] for turbid productive waters uses the information of the reflectance peak centered at 700 nm, which is maximally sensitive to variations in chl-a concentration in water. Furthermore, a wide spectral absorption peak between 665 nm and 675 nm is generally associated to the absorption by chl-a pigments. The normalization through the NDCI eliminates uncertainties in the estimation of the remote sensing reflectance, seasonal solar azimuth differences, and atmospheric contributions.
- DO5: Dall’Olmo and Gitelson [37] presented a three-band model using Red and NIR bands. It assumes that (i) the absorption by coloured dissolved organic matter (CDOM) and total suspended matter (TSM) beyond 700 nm is approximately equal to that at 665–675 nm and the difference between them can be neglected; (ii) total chlorophyll, CDOM, and TSM absorption beyond 730 nm is almost 0; and (iii) backscattering coefficient of chl-a is spectrally invariant [36,37].
- MCI: The Maximum Chlorophyll Index allows the detection of red tides and aquatic vegetation [24]. For Sentinel 2, it uses the band 5 (705 nm), perfectly located to detect high biomass water bodies against the baselines of the bands 4 and 6 (665 and 740 nm). In Equation (7), k is the thin cloud correction factor fixed at 1.005 for thin clouds.
2.6. Model Calibration and Validation
2.7. Time-Series Estimation
2.8. Workflow
3. Results
3.1. In situ Data: chl-a
3.2. Atmospheric Correction and Outlier Removal
3.3. Model Calibration and Validation
4. Discussion
4.1. In situ chl-a Data
4.2. Atmospheric Correction and chl-a Estimation Algorithms
4.3. Model Calibration and Validation
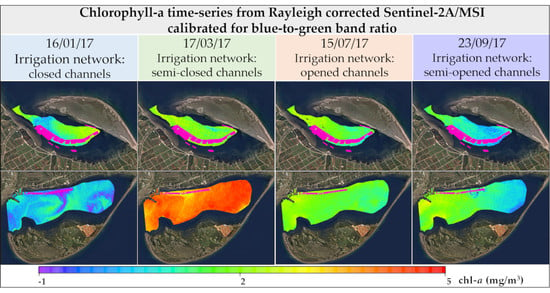
4.4. Spatiotemporal chl-a Dynamics
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Duarte, P.; Fernández-Reiriz, M.J.; Labarta, U. Modelling Mussel Growth in Ecosystems with Low Suspended Matter Loads Using a Dynamic Energy Budget Approach. J. Sea Res. 2012, 67, 44–57. [Google Scholar] [CrossRef]
- Ramón, M.; Cano, J.; Peña, J.B.; Campos, M.J. Current Status and Perspectives of Mollusc (Bivalves and Gastropods) Culture in the Spanish Mediterranean. Boletín Inst. Español Oceanogr. 2005, 21, 361–373. [Google Scholar]
- Prat, N.; Muñoz, I.; Camp, J.; Comin, F.A.; Lucena, J.R.; Romero, J.; Vidal, M. Seasonal Changes in Particulate Organic Carbon and Nitrogen in the River and Drainage Channels of the Ebro Delta (N.E. Spain). SIL Proc. 1988, 23, 1344–1349. [Google Scholar] [CrossRef]
- Llebot, C.; Rueda, F.J.; Solé, J.; Artigas, M.L.; Estrada, M. Hydrodynamic States in a Wind-Driven Microtidal Estuary (Alfacs Bay). J. Sea Res. 2014, 85, 263–276. [Google Scholar] [CrossRef]
- Artigas, M.L.; Llebot, C.; Ross, O.N.; Neszi, N.Z.; Rodellas, V.; Garcia-Orellana, J.; Masqué, P.; Piera, J.; Estrada, M.; Berdalet, E. Understanding the Spatio-Temporal Variability of Phytoplankton Biomass Distribution in a Microtidal Mediterranean Estuary. Deep Res. Part II Top. Stud. Oceanogr. 2014, 101, 180–192. [Google Scholar] [CrossRef]
- Camp, J.; Delgado, M. Hidrografía de Las Bahías Del Delta Del Ebro. In Investigación Pesquera; Instituto de Ciencias del Mar: Barcelona, Spain, 1987; pp. 351–369. [Google Scholar]
- Forget, M.-H.; Stuart, V.; Platt, T. Reports and Monographs of the International Ocean-Colour Coordinating Group Remote Sensing in Fisheries and Aquaculture. Aquaculture 2009, 1998, 1–128. [Google Scholar]
- Garcia, L.E.; Rodriguez, D.J.; Wijen, M.; Pakulski, I. (Eds.) Earth Observation for Water Resources Management: Current Use and Future Opportunities for the Water Sector; World Bank Group: Washington, DC, USA, 2016. [Google Scholar]
- Gregor, J.; Maršálek, B. Freshwater Phytoplankton Quantification by Chlorophyll a: A Comparative Study of in Vitro, in Vivo and in Situ Methods. Water Res. 2004, 38, 517–522. [Google Scholar] [CrossRef] [PubMed]
- Del López-Rodríguez, M.C.; Leira, M.; Valle, R.; Moyà-Niell, G. El Fitoplancton Como Indicador de Calidad de Masas de Agua Muy Modificadas En La DMA. El Lago Artificial de As Pontes (A Coruña. España). Nov. Acta Cient. Compostel. 2016, 23, 85–97. [Google Scholar]
- Kutser, T.; Paavel, B.; Verpoorter, C.; Ligi, M.; Soomets, T.; Toming, K.; Casal, G. Remote Sensing of Black Lakes and Using 810 Nm Reflectance Peak for Retrieving Water Quality Parameters of Optically Complex Waters. Remote Sens. 2016, 8, 497. [Google Scholar] [CrossRef]
- Gurlin, D.; Gitelson, A.A.; Moses, W.J. Remote Estimation of Chl-a Concentration in Turbid Productive Waters-Return to a Simple Two-Band NIR-Red Model? Remote Sens. Environ. 2011, 115, 3479–3490. [Google Scholar] [CrossRef]
- Blondeau-Patissier, D.; Gower, J.F.R.; Dekker, A.G.; Phinn, S.R.; Brando, V.E. A Review of Ocean Color Remote Sensing Methods and Statistical Techniques for the Detection, Mapping and Analysis of Phytoplankton Blooms in Coastal and Open Oceans. Prog. Oceanogr. 2014, 123, 23–144. [Google Scholar] [CrossRef]
- Matthews, M.W. A Current Review of Empirical Procedures of Remote Sensing in Inland and Near-Coastal Transitional Waters. Int. J. Remote Sens. 2011, 32, 6855–6899. [Google Scholar] [CrossRef]
- Gholizadeh, M.; Melesse, A.; Reddi, L. A Comprehensive Review on Water Quality Parameters Estimation Using Remote Sensing Techniques. Sensors 2016, 16, 1298. [Google Scholar] [CrossRef] [PubMed]
- Volpe, G.; Santoleri, R.; Vellucci, V.; Ribera d’Alcalà, M.; Marullo, S.; D’Ortenzio, F. The Colour of the Mediterranean Sea: Global versus Regional Bio-Optical Algorithms Evaluation and Implication for Satellite Chlorophyll Estimates. Remote Sens. Environ. 2007, 107, 625–638. [Google Scholar] [CrossRef]
- Campbell, J.W.; O’Reilly, J.E. Metrics for Quantifying the Uncertainty in a Chlorophyll Algorithm: Explicit Equations and Examples Using the OC4.v4 Algorithm and NOMAD Data. Ocean Color Bio-Opt. Algorithm Mini-Workshop 2006, 4, 1–15. [Google Scholar]
- Gitelson, A.A.; Yacobi, Y.Z.; Karnieli, A.; Nurit, K. Reflectance Spectra of Polluted Marine Waters in Haifa Bay, Southeastern Mediterranean:Features and Application for Remote Estimation of Chlorophyll Concentration. J. Earth Sci. 1996, 45, 127–136. [Google Scholar]
- Odermatt, D.; Gitelson, A.; Brando, V.E.; Schaepman, M. Review of Constituent Retrieval in Optically Deep and Complex Waters from Satellite Imagery. Remote Sens. Environ. 2012, 118, 116–126. [Google Scholar] [CrossRef]
- Le, C.; Hu, C.; Cannizzaro, J.; English, D.; Muller-Karger, F.; Lee, Z. Evaluation of Chlorophyll-a Remote Sensing Algorithms for an Optically Complex Estuary. Remote Sens. Environ. 2013, 129, 75–89. [Google Scholar] [CrossRef]
- Gitelson, A.A.; Dall’Olmo, G.; Moses, W.; Rundquist, D.C.; Barrow, T.; Fisher, T.R.; Gurlin, D.; Holz, J. A Simple Semi-Analytical Model for Remote Estimation of Chlorophyll-a in Turbid Waters: Validation. Remote Sens. Environ. 2008, 112, 3582–3593. [Google Scholar] [CrossRef]
- Gitelson, A.A.; Kondratyev, K.Y. Optical Models of Mesotrophic and Eutrophic Water Bodies. Int. J. Remote Sens. 1991, 12, 373–385. [Google Scholar] [CrossRef]
- Oliveira, E.N.; Fernandes, A.M.; Kampel, M.; Cordeiro, R.C.; Brandini, N.; Vinzon, S.B.; Grassi, R.M.; Pinto, F.N.; Fillipo, A.M.; Paranhos, R. Assessment of Remotely Sensed Chlorophyll- a Concentration in Guanabara Bay, Brazil. J. Appl. Remote Sens. 2016, 10, 026003. [Google Scholar] [CrossRef]
- Gower, J.F.R.; Doerffer, R.; Borstad, G.A. Interpretation of the 685nm Peak in Water-Leaving Radiance Spectra in Terms of Fluorescence, Absorption and Scattering, and Its Observation by MERIS. Int. J. Remote Sens. 1999, 20, 1771–1786. [Google Scholar] [CrossRef]
- Matthews, M.W.; Bernard, S.; Robertson, L. An Algorithm for Detecting Trophic Status (Chlorophyll-a), Cyanobacterial-Dominance, Surface Scums and Floating Vegetation in Inland and Coastal Waters. Remote Sens. Environ. 2012, 124, 637–652. [Google Scholar] [CrossRef]
- Joshi, I.D.; D’Sa, E.J.; Osburn, C.L.; Bianchi, T.S. Turbidity in Apalachicola Bay, Florida from Landsat 5 TM and Field Data: Seasonal Patterns and Response to Extreme Events. Remote Sens. 2017, 9, 367. [Google Scholar] [CrossRef]
- Sutherland, T.F.; Leonard, C.; Taylor, F.J.R. A Segmented Pipe Sampler for Integrated Profiling of the Upper Water Column. J. Plankton Res. 1992, 14, 915–923. [Google Scholar] [CrossRef]
- Lorenzen, C.J. A Method for the Continuous Measurement of in Vivo Chlorophyll Concentration. Deep Res. 1996, 13, 223–227. [Google Scholar] [CrossRef]
- Yentsch, C.S.; Menzel, D.W. A Method for the Determination of Phytoplankton Chlorophyll and Phaeophytin by Fluorescence. Deep Res. Oceanogr. Abstr. 1963, 10, 221–231. [Google Scholar] [CrossRef]
- Jeffrey, S.W.; Humphrey, G.F. New Spectrophotometric Equations for Determining Chlorophylls a, b, C1 and C2 in Higher Plants, Algae and Natural Phytoplankton. Biochem. Physiol. Pflanz. 1975, 167, 191–194. [Google Scholar] [CrossRef]
- Vanhellemont, Q.; Ruddick, K. Atmospheric Correction of Metre-Scale Optical Satellite Data for Inland and Coastal Water Applications. Remote Sens. Environ. 2018, 216, 586–597. [Google Scholar] [CrossRef]
- Harmel, T.; Chami, M.; Tormos, T.; Reynaud, N.; Danis, P.A. Sunglint Correction of the Multi-Spectral Instrument (MSI)-SENTINEL-2 Imagery over Inland and Sea Waters from SWIR Bands. Remote Sens. Environ. 2018, 204, 308–321. [Google Scholar] [CrossRef]
- Morel, A.; Prieur, L. Analysis of Variations in Ocean Color. Limnol. Oceanogr. 1977, 22, 709–722. [Google Scholar] [CrossRef]
- Gitelson, A.; Nikanorov, A.; Szabo, G.; Szilagyi, F. Etude de La Qualite Des Eaux de Surface Télédétéction. In Monitoring to Detect Chamges in Water Quality Series; IAHS Publication: Budapest, Hungary, 1986; Volume 157, pp. 111–121. [Google Scholar]
- Lins, R.; Martinez, J.M.; Motta Marques, D.; Cirilo, J.; Fragoso, C. Assessment of Chlorophyll-a Remote Sensing Algorithms in a Productive Tropical Estuarine-Lagoon System. Remote Sens. 2017, 9, 516. [Google Scholar] [CrossRef]
- Mishra, S.; Mishra, D.R. Normalized Difference Chlorophyll Index: A Novel Model for Remote Estimation of Chlorophyll-a Concentration in Turbid Productive Waters. Remote Sens. Environ. 2012, 117, 394–406. [Google Scholar] [CrossRef]
- Dall’Olmo, G.; Gitelson, A.A. Effect of Bio-Optical Parameter Variability on the Remote Estimation of Chlorophyll-a Concentration in Turbid Productive Waters: Experimental Results. Appl. Opt. 2005, 44, 412. [Google Scholar] [CrossRef]
- Seegers, B.N.; Stumpf, R.P.; Schaeffer, B.A.; Loftin, K.A.; Jeremy Werdell, P. Performance Metrics for the Assessment of Satellite Data Products: An Ocean Color Case Study. Opt. Express 2018, 26, 7404–7422. [Google Scholar] [CrossRef]
- Cannizzaro, J.P.; Carder, K.L. Estimating Chlorophyll a Concentrations from Remote-Sensing Reflectance in Optically Shallow Waters. Remote Sens. Environ. 2006, 101, 13–24. [Google Scholar] [CrossRef]
- Gons, H.J.; Auer, M.T.; Effler, S.W. MERIS Satellite Chlorophyll Mapping of Oligotrophic and Eutrophic Waters in the Laurentian Great Lakes. Remote Sens. Environ. 2008, 112, 4098–4106. [Google Scholar] [CrossRef]
- Ramón, M.; Fernández, M.; Galimany, E. Development of Mussel (Mytilus Galloprovincialis) Seed from Two Different Origins in a Semi-Enclosed Mediterranean Bay (N.E. Spain). Aquaculture 2007, 264, 148–159. [Google Scholar] [CrossRef]
- Dogliotti, A.; Ruddick, K. Improving Water Reflectance Retrieval from MODIS Imagery in the Highly Turbid Waters of La Plata River. In Proceedings of the VI International Conference in Current Problems in Optics of Natural Waters, St. Petersburg, Russia, 10 September 2011. [Google Scholar]
- Caballero, I.; Steinmetz, F.; Navarro, G. Evaluation of the First Year of Operational Sentinel-2A Data for Retrieval of Suspended Solids in Medium- to High-Turbiditywaters. Remote Sens. 2018, 10, 982. [Google Scholar] [CrossRef]
- Novoa, S.; Doxaran, D.; Ody, A.; Vanhellemont, Q.; Lafon, V.; Lubac, B.; Gernez, P. Atmospheric Corrections and Multi-Conditional Algorithm for Multi-Sensor Remote Sensing of Suspended Particulate Matter in Low-to-High Turbidity Levels Coastal Waters. Remote Sens. 2017, 9, 61. [Google Scholar] [CrossRef]
- Sei, A. Efficient Correction of Adjacency Effects for High-Resolution Imagery: Integral Equations, Analytic Continuation, and Padé Approximants. Appl. Opt. 2015, 54, 3748. [Google Scholar] [CrossRef]
- Gons, H.J.; Rijkeboer, M.; Ruddick, K.G. A Chlorophyll-Retrieval Algorithm for Satellite Imagery (Medium Resolution Imaging Spectrometer) of Inland and Coastal Waters. J. Plankton Res. 2002, 24, 947–951. [Google Scholar] [CrossRef]
- Gernez, P.; Doxaran, D.; Barillé, L. Shellfish Aquaculture from Space: Potential of Sentinel2 to Monitor Tide-Driven Changes in Turbidity, Chlorophyll Concentration and Oyster Physiological Response at the Scale of an Oyster Farm. Front. Mar. Sci. 2017, 4, 1–15. [Google Scholar] [CrossRef]
- Busch, J.A. Phytoplankton Dynamics and Bio-Optical Variables Associated with Harmful Algal Blooms in Aquaculture Zones. Ph.D. Thesis, Universität Bremen, Bremen, Germany, 2013. [Google Scholar]
- Llebot, C.; Solé, J.; Delgado, M.; Fernández-Tejedor, M.; Camp, J.; Estrada, M. Hydrographical Forcing and Phytoplankton Variability in Two Semi-Enclosed Estuarine Bays. J. Mar. Syst. 2011, 86, 69–86. [Google Scholar] [CrossRef]










| Date | Sampling Grid | Bay | Number of Samples per Method | ||
|---|---|---|---|---|---|
| In Vivo | Fluorimeter | Spectrophotometer | |||
| 11 April 2016 | 1 | Fangar | 5 | 1 | 0 |
| 20 June 2016 | 1 | Alfacs | 7 | 1 | 0 |
| Fangar | 5 | 1 | 0 | ||
| 16 January 2017 | 1 | Alfacs | 7 | 1 | 1 |
| 17 March 2017 | 1 | Alfacs | 7 | 7 | 7 |
| Fangar | 5 | 5 | 5 | ||
| 6 April 2017 | 1 | Alfacs | 7 | 7 | 7 |
| Fangar | 5 | 5 | 5 | ||
| 26 May 2017 | 2 | Alfacs | 6 | 6 | 6 |
| Fangar | 6 | 6 | 6 | ||
| 15 June 2017 | 2 | Alfacs | 6 | 6 | 6 |
| 25 July 2017 | 3 | Fangar | 40a | 6 | 0 |
| 4 August 2017 | 3 | Fangar | 40 a | 6 | 0 |
| Bay | Method | N | Minimum | Maximum | Median | Mean | SD | CV |
|---|---|---|---|---|---|---|---|---|
| Fangar | In vivo | 66 | 0.512 | 6.553 | 2.719 | 2.716 | 1.497 | 0.551 |
| FL | 30 | 0.170 | 4.992 | 1.365 | 1.836 | 1.278 | 0.696 | |
| SP | 16 | 0.222 | 2.597 | 1.604 | 1.501 | 0.732 | 0.487 | |
| Alfacs | In vivo | 40 | 0.774 | 8.880 | 2.807 | 3.197 | 1.867 | 0.584 |
| FL | 28 | 1.010 | 4.750 | 1.705 | 2.206 | 1.131 | 0.513 | |
| SP | 27 | 1.373 | 5.596 | 2.613 | 2.988 | 1.291 | 0.432 |
| Date | Sampling Grid | Bay | Number of Samples per Method | ||
|---|---|---|---|---|---|
| In Vivo | FL | SP | |||
| 11 April 2016 | 1 | Fangar | 5 | 1 | 0 |
| 20 June 2016 | 1 | Alfacs | 5 | 1 | 0 |
| Fangar | 4 | 1 | 0 | ||
| 16 January 2017 | 1 | Alfacs | 7 | 1 | 1 |
| 17 March 2017 | 1 | Alfacs | 7 | 7 | 7 |
| Fangar | 4 | 4 | 4 | ||
| 6 April 2017 | 1 | Alfacs | 7 | 7 | 7 |
| Fangar | 3 | 3 | 3 | ||
| 26 May 2017 | 2 | Alfacs | 6 | 6 | 6 |
| Fangar | 6 | 6 | 6 | ||
| 15 June 2017 | 2 | Alfacs | 6 | 6 | 6 |
| 25 July 2017 | 3 | Fangar | 12 | 5 | 0 |
| 4 August 2017 | 3 | Fangar | 16 | 5 | 0 |
| Bay | Algorithm | Min chl-a | Max chl-a | Intercept | Slope | N | MAE | APD | R2 | AIC |
|---|---|---|---|---|---|---|---|---|---|---|
| Fangar | NDCI | 0.46 | 2.39 | 0.56 | –12.80 | 9 | 0.41 | 0.27 | 0.43 | 17.12 |
| Alfacs | BG | 1.83 | 5.12 | 13.88 | –12.24 | 18 | 0.71 | 3.30 | 0.61 | 49.54 |
| Both | BG | 1.37 | 5.60 | 13.93 | –12.50 | 27 | 0.63 | 5.58 | 0.58 | 72.17 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Soriano-González, J.; Angelats, E.; Fernández-Tejedor, M.; Diogene, J.; Alcaraz, C. First Results of Phytoplankton Spatial Dynamics in Two NW-Mediterranean Bays from Chlorophyll-a Estimates Using Sentinel 2: Potential Implications for Aquaculture. Remote Sens. 2019, 11, 1756. https://doi.org/10.3390/rs11151756
Soriano-González J, Angelats E, Fernández-Tejedor M, Diogene J, Alcaraz C. First Results of Phytoplankton Spatial Dynamics in Two NW-Mediterranean Bays from Chlorophyll-a Estimates Using Sentinel 2: Potential Implications for Aquaculture. Remote Sensing. 2019; 11(15):1756. https://doi.org/10.3390/rs11151756
Chicago/Turabian StyleSoriano-González, Jesús, Eduard Angelats, Margarita Fernández-Tejedor, Jorge Diogene, and Carles Alcaraz. 2019. "First Results of Phytoplankton Spatial Dynamics in Two NW-Mediterranean Bays from Chlorophyll-a Estimates Using Sentinel 2: Potential Implications for Aquaculture" Remote Sensing 11, no. 15: 1756. https://doi.org/10.3390/rs11151756