Assessing the Impact of Satellite Revisit Rate on Estimation of Corn Phenological Transition Timing through Shape Model Fitting
Abstract
:1. Introduction
2. Materials and Methods
2.1. Field Research Site and Data Collection
2.2. VI Selection
2.2.1. VI–Ground Truth Comparison
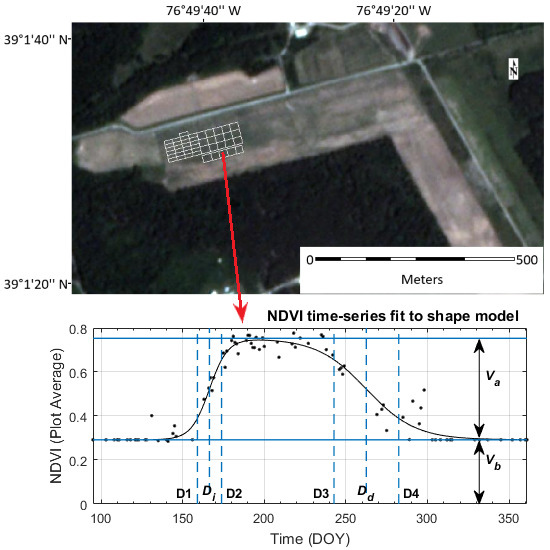
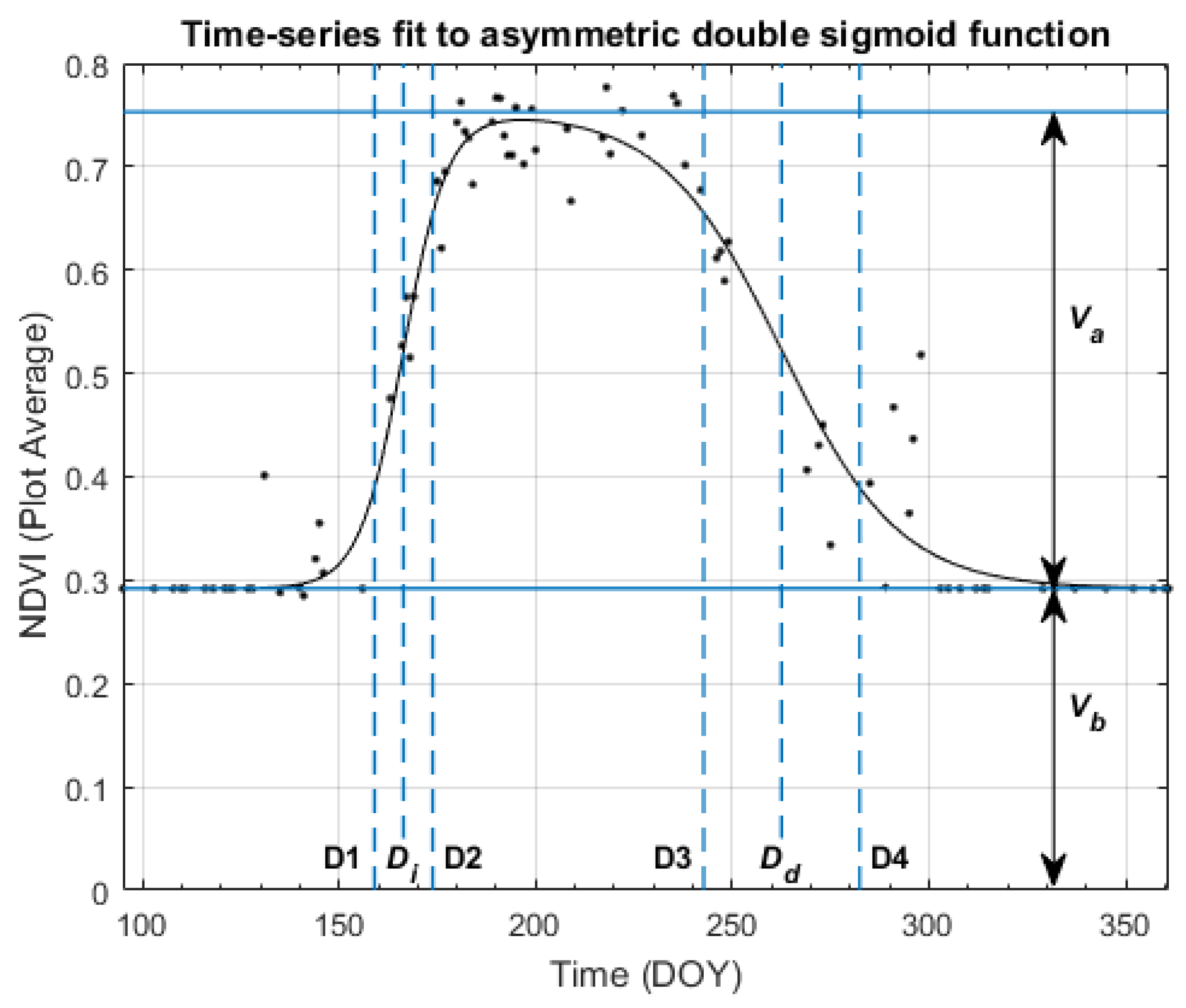
2.2.2. Shape Model Fitting
2.3. Temporal Sampling and Analysis
3. Results
3.1. VI–Ground Truth Comparison
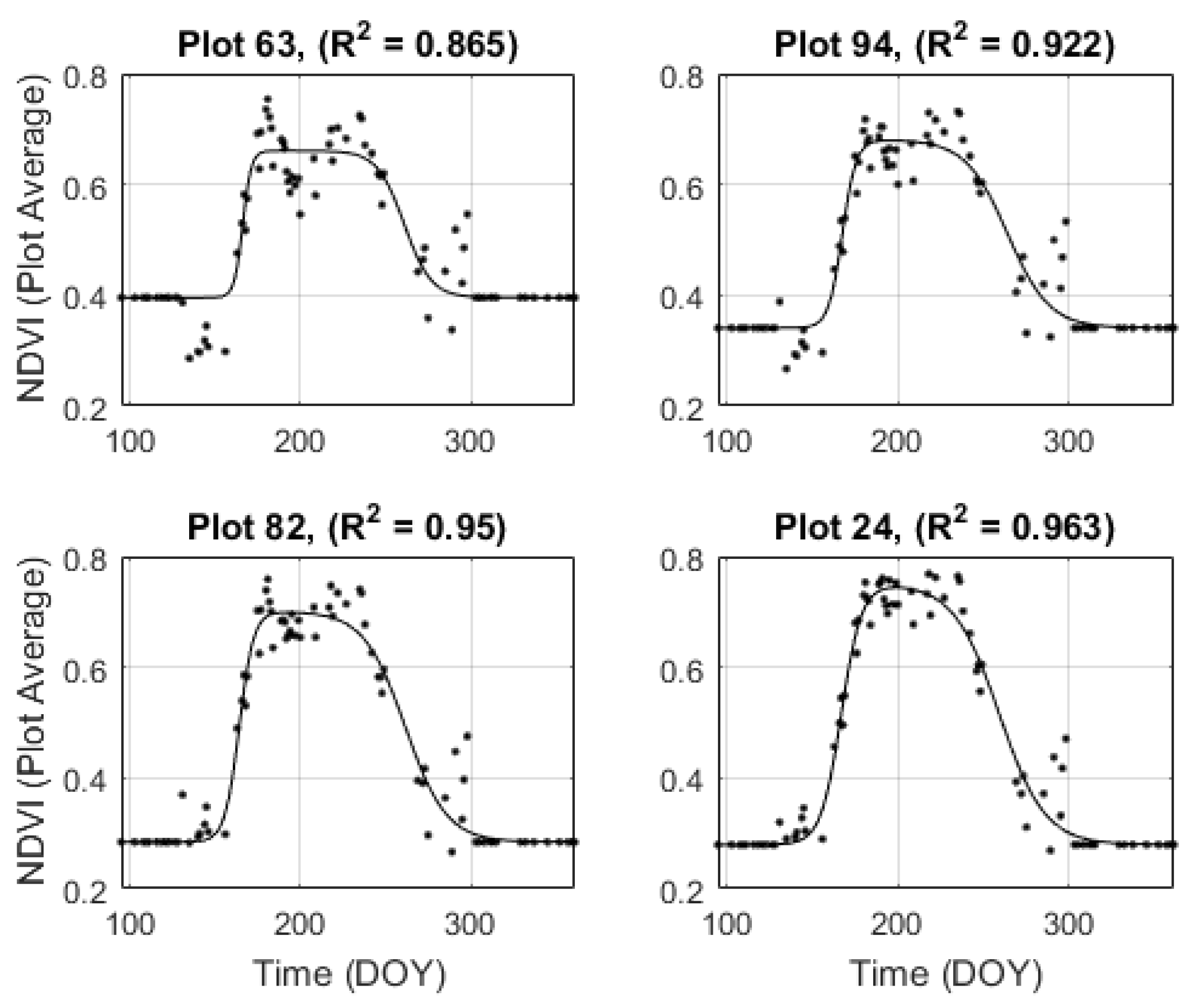
3.2. Shape Model Fitting
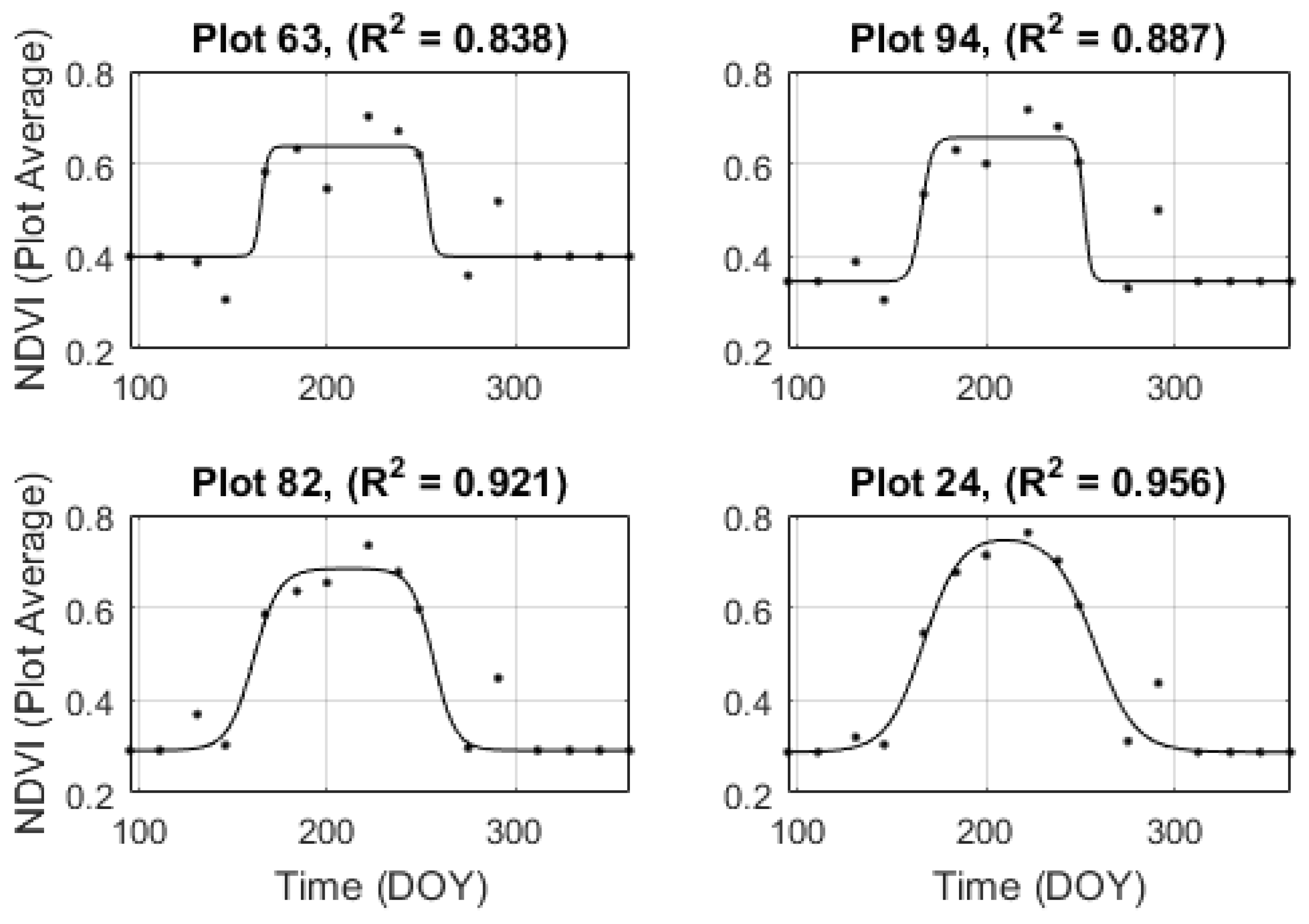
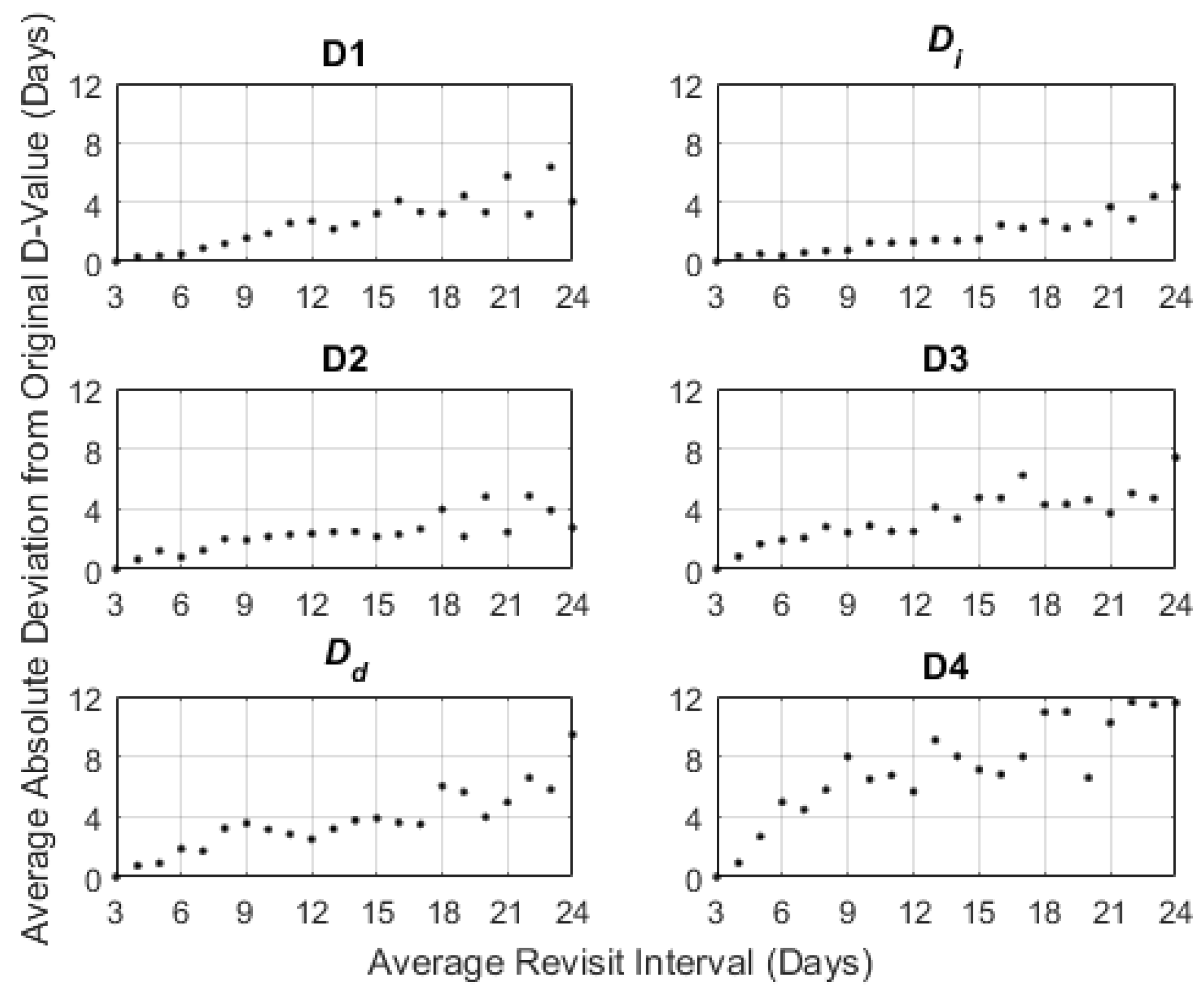
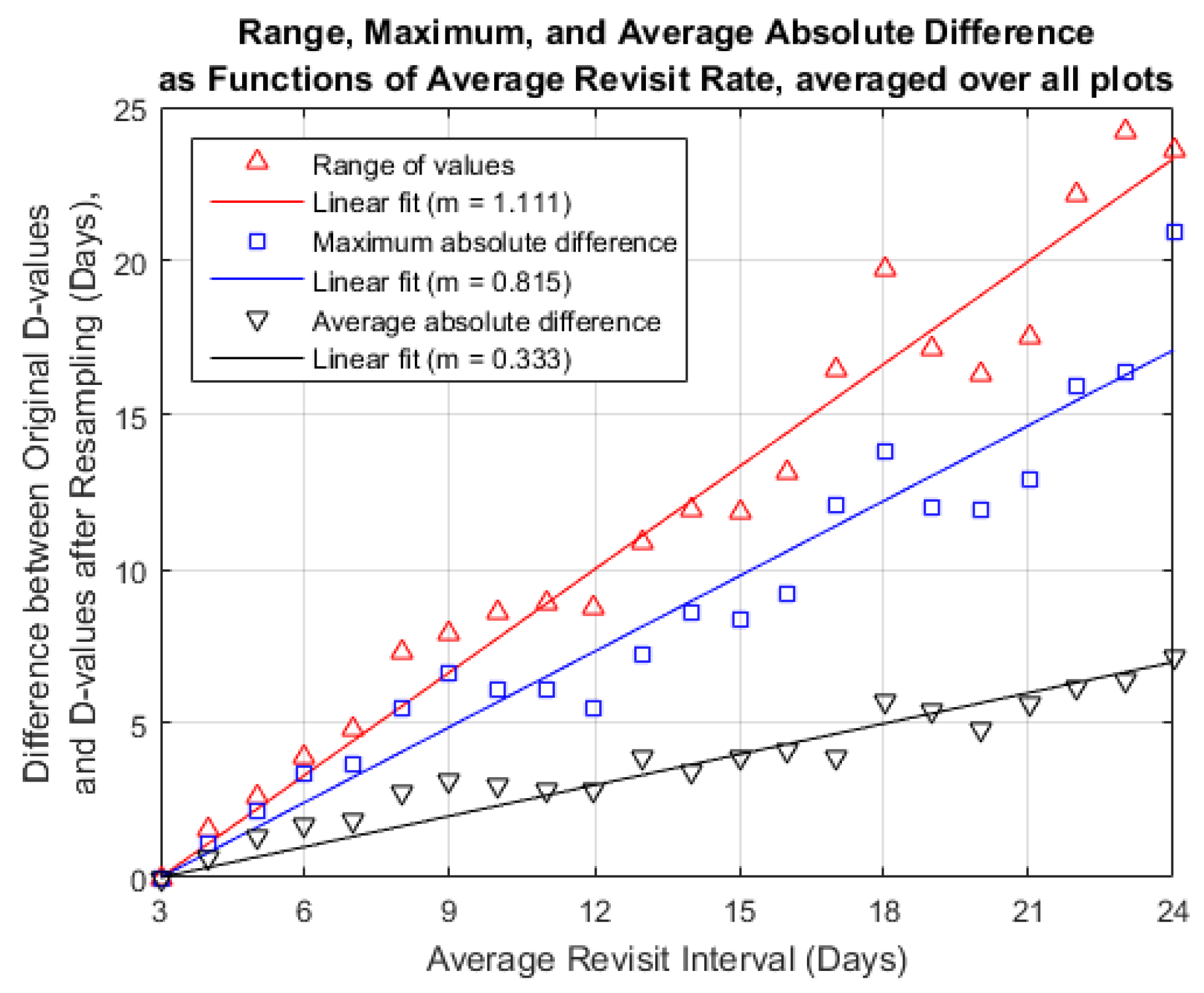
3.3. Temporal Resampling
4. Discussion
4.1. VI–Ground Truth Correlation and Initial Shape Model Fitting
4.2. Temporal Resampling
4.3. Limitations and Sources of Error
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Zhang, X.; Zhang, Q. Monitoring interannual variation in global crop yield using long-term AVHRR and MODIS observations. ISPRS J. Photogramm. Remote. Sens. 2016, 114, 191–205. [Google Scholar] [CrossRef]
- Sakamoto, T. Refined shape model fitting methods for detecting various types of phenological information on major U.S. crops. ISPRS J. Photogramm. Remote. Sens. 2018, 138, 176–192. [Google Scholar] [CrossRef]
- Zhang, X.; Wang, J.; Gao, F.; Liu, Y.; Schaaf, C.; Friedl, M.; Yu, Y.; Jayavelu, S.; Gray, J.; Liu, L.; et al. Exploration of scaling effects on coarse resolution land surface phenology. Remote Sens. Environ. 2017, 190, 318–330. [Google Scholar] [CrossRef] [Green Version]
- González-Sanpedro, M.; Le Toan, T.; Moreno, J.; Kergoat, L.; Rubio, E. Seasonal variations of leaf area index of agricultural fields retrieved from Landsat data. Remote Sens. Environ. 2008, 112, 810–824. [Google Scholar] [CrossRef] [Green Version]
- Jönsson, P.; Cai, Z.; Melaas, E.; Friedl, M.; Eklundh, L. A Method for Robust Estimation of Vegetation Seasonality from Landsat and Sentinel-2 Time Series Data. Remote Sens. 2018, 10, 635. [Google Scholar] [CrossRef]
- Zhang, X.; Friedl, M.A.; Schaaf, C.B. Sensitivity of vegetation phenology detection to the temporal resolution of satellite data. Int. J. Remote. Sens. 2009, 30, 2061–2074. [Google Scholar] [CrossRef]
- Goward, S.N.; Loboda, T.V.; Williams, D.L.; Huang, C. Landsat Orbital Repeat Frequency and Cloud Contamination: A Case Study for Eastern United States. Photogramm. Eng. Remote Sens. 2019, 85, 109–118. [Google Scholar] [CrossRef]
- Whitcraft, A.; Becker-Reshef, I.; Justice, C. A Framework for Defining Spatially Explicit Earth Observation Requirements for a Global Agricultural Monitoring Initiative (GEOGLAM). Remote Sens. 2015, 7, 1461–1481. [Google Scholar] [CrossRef] [Green Version]
- Löw, F.; Duveiller, G. Defining the Spatial Resolution Requirements for Crop Identification Using Optical Remote Sensing. Remote Sens. 2014, 6, 9034–9063. [Google Scholar] [CrossRef] [Green Version]
- Gao, F.; Masek, J.; Schwaller, M.; Hall, F. On the blending of the Landsat and MODIS surface reflectance: predicting daily Landsat surface reflectance. IEEE Trans. Geosci. Remote Sens. 2006, 44, 2207–2218. [Google Scholar] [CrossRef]
- Meng, J.; Du, X.; Wu, B. Generation of high spatial and temporal resolution NDVI and its application in crop biomass estimation. Int. J. Digit. Earth 2013, 6, 203–218. [Google Scholar] [CrossRef]
- Walker, J.; de Beurs, K.; Wynne, R.; Gao, F. Evaluation of Landsat and MODIS data fusion products for analysis of dryland forest phenology. Remote Sens. Environ. 2012, 117, 381–393. [Google Scholar] [CrossRef]
- Li, J.; Roy, D. A Global Analysis of Sentinel-2A, Sentinel-2B and Landsat-8 Data Revisit Intervals and Implications for Terrestrial Monitoring. Remote Sens. 2017, 9, 902. [Google Scholar] [CrossRef]
- Claverie, M.; Ju, J.; Masek, J.G.; Dungan, J.L.; Vermote, E.F.; Roger, J.C.; Skakun, S.V.; Justice, C. The Harmonized Landsat and Sentinel-2 surface reflectance data set. Remote Sens. Environ. 2018, 219, 145–161. [Google Scholar] [CrossRef]
- Liu, Y.; Hill, M.J.; Zhang, X.; Wang, Z.; Richardson, A.D.; Hufkens, K.; Filippa, G.; Baldocchi, D.D.; Ma, S.; Verfaillie, J.; et al. Using data from Landsat, MODIS, VIIRS and PhenoCams to monitor the phenology of California oak/grass savanna and open grassland across spatial scales. Agric. For. Meteorol. 2017, 237-238, 311–325. [Google Scholar] [CrossRef]
- Wu, Z.; Snyder, G.; Vadnais, C.; Arora, R.; Babcock, M.; Stensaas, G.; Doucette, P.; Newman, T. User needs for future Landsat missions. Remote Sens. Environ. 2019, 231, 111214. [Google Scholar] [CrossRef]
- Planet Team. Planet Application Program Interface: In Space for Life on Earth; Planet Team: San Francisco, CA, USA, 2017; Available online: https://api.planet.com (accessed on 1 May 2019).
- Sadeh, Y.; Zhu, X.; Chenu, K.; Dunkerley, D. Sowing date detection at the field scale using CubeSats remote sensing. Comput. Electron. Agric. 2019, 157, 568–580. [Google Scholar] [CrossRef]
- Helman, D.; Bahat, I.; Netzer, Y.; Ben-Gal, A.; Alchanatis, V.; Peeters, A.; Cohen, Y. Using Time Series of High-Resolution Planet Satellite Images to Monitor Grapevine Stem Water Potential in Commercial Vineyards. Remote Sens. 2018, 10, 1615. [Google Scholar] [CrossRef]
- Burke, M.; Lobell, D.B. Satellite-based assessment of yield variation and its determinants in smallholder African systems. Proc. Natl. Acad. Sci. USA 2017, 114, 2189–2194. [Google Scholar] [CrossRef] [Green Version]
- Kross, A.; McNairn, H.; Lapen, D.; Sunohara, M.; Champagne, C. Assessment of RapidEye vegetation indices for estimation of leaf area index and biomass in corn and soybean crops. Int. J. Appl. Earth Obs. Geoinf. 2015, 34, 235–248. [Google Scholar] [CrossRef] [Green Version]
- Houborg, R.; McCabe, M.F. A Cubesat enabled Spatio-Temporal Enhancement Method (CESTEM) utilizing Planet, Landsat and MODIS data. Remote Sens. Environ. 2018, 209, 211–226. [Google Scholar] [CrossRef]
- Houborg, R.; McCabe, M. Daily Retrieval of NDVI and LAI at 3 m Resolution via the Fusion of CubeSat, Landsat, and MODIS Data. Remote Sens. 2018, 10, 890. [Google Scholar] [CrossRef]
- Gao, F.; Anderson, M.C.; Zhang, X.; Yang, Z.; Alfieri, J.G.; Kustas, W.P.; Mueller, R.; Johnson, D.M.; Prueger, J.H. Toward mapping crop progress at field scales through fusion of Landsat and MODIS imagery. Remote Sens. Environ. 2017, 188, 9–25. [Google Scholar] [CrossRef] [Green Version]
- Zhong, L.; Hu, L.; Yu, L.; Gong, P.; Biging, G.S. Automated mapping of soybean and corn using phenology. ISPRS J. Photogramm. Remote Sens. 2016, 119, 151–164. [Google Scholar] [CrossRef] [Green Version]
- Zhang, X.; Friedl, M.A.; Schaaf, C.B.; Strahler, A.H.; Hodges, J.C.; Gao, F.; Reed, B.C.; Huete, A. Monitoring vegetation phenology using MODIS. Remote Sens. Environ. 2003, 84, 471–475. [Google Scholar] [CrossRef]
- Hong, S.Y.; Sudduth, K.A.; Kitchen, N.R.; Fraisse, C.W.; Palm, H.L.; Wiebold, W.J. Comparison of Remote Sensing and Crop Growth Models for Estimating Within-Field LAI Variability. Korean J. Remote Sens. 2004, 20, 14. [Google Scholar]
- White, K.; Pontius, J.; Schaberg, P. Remote sensing of spring phenology in northeastern forests: A comparison of methods, field metrics and sources of uncertainty. Remote Sens. Environ. 2014, 148, 97–107. [Google Scholar] [CrossRef]
- Uddling, J.; Gelang-Alfredsson, J.; Piikki, K.; Pleijel, H. Evaluating the relationship between leaf chlorophyll concentration and SPAD-502 chlorophyll meter readings. Photosynth. Res. 2007, 91, 37–46. [Google Scholar] [CrossRef]
- Xiong, D.; Chen, J.; Yu, T.; Gao, W.; Ling, X.; Li, Y.; Peng, S.; Huang, J. SPAD-based leaf nitrogen estimation is impacted by environmental factors and crop leaf characteristics. Sci. Rep. 2015, 5. [Google Scholar] [CrossRef]
- Bullock, D.G.; Anderson, D.S. Evaluation of the Minolta SPAD-502 chlorophyll meter for nitrogen management in corn. J. Plant Nutr. 1998, 21, 741–755. [Google Scholar] [CrossRef]
- Çakir, R. Effect of water stress at different development stages on vegetative and reproductive growth of corn. Field Crop. Res. 2004, 89, 1–16. [Google Scholar] [CrossRef]
- Ritchie, S.; Hanway, J.; Benson, G. How a Corn Plant Develops; Special Report No. 48; Iowa State University, Cooperative Extension Service: Ames, IA, USA, 2005. [Google Scholar]
- Daughtry, C.S.T.; Cochran, J.C.; Hollinger, S.E. Estimating Silking and Maturity Dates of Corn for Large Areas1. Agron. J. 1984, 76, 415. [Google Scholar] [CrossRef]
- Marta, S. Planet Imagery Product Specifications; Planet Labs: San Francisco, CA, USA, 2018; p. 91. [Google Scholar]
- Qi, J.; Chehbouni, A.; Huete, A.; Kerr, Y.; Sorooshian, S. A modified soil adjusted vegetation index. Remote Sens. Environ. 1994, 48, 119–126. [Google Scholar] [CrossRef]
- Huete, A.; Didan, K.; Miura, T.; Rodriguez, E.; Gao, X.; Ferreira, L. Overview of the radiometric and biophysical performance of the MODIS vegetation indices. Remote Sens. Environ. 2002, 83, 195–213. [Google Scholar] [CrossRef]
- Jiang, Z.; Huete, A.; Didan, K.; Miura, T. Development of a two-band enhanced vegetation index without a blue band. Remote Sens. Environ. 2008, 112, 3833–3845. [Google Scholar] [CrossRef]
- Gitelson, A.A.; Gritz, Y.; Merzlyak, M.N. Relationships between leaf chlorophyll content and spectral reflectance and algorithms for non-destructive chlorophyll assessment in higher plant leaves. J. Plant Physiol. 2003, 160, 271–282. [Google Scholar] [CrossRef]
- Rouse, J.; Haas, R.; Schell, J.; Deering, D. Monitoring Vegetation Systems in the Great Plains with ERTS; NASA: Washington, DC, USA, 1973.
- Huete, A. A soil-adjusted vegetation index (SAVI). Remote Sens. Environ. 1988, 25, 295–309. [Google Scholar] [CrossRef]
- Gitelson, A.A. Wide Dynamic Range Vegetation Index for Remote Quantification of Biophysical Characteristics of Vegetation. J. Plant Physiol. 2004, 161, 165–173. [Google Scholar] [CrossRef] [Green Version]
- Sakamoto, T.; Gitelson, A.A.; Arkebauer, T.J. MODIS-based corn grain yield estimation model incorporating crop phenology information. Remote. Sens. Environ. 2013, 131, 215–231. [Google Scholar] [CrossRef]
- Henebry, G.M.; Viña, A.; Gitelson, A.A. The Wide Dynamic Range Vegetation Index and its Potential Utility for Gap Analysis. Gap Anal. Bull. 2004, 12, 50–56. [Google Scholar]
- Zhang, X. Reconstruction of a complete global time series of daily vegetation index trajectory from long-term AVHRR data. Remote Sens. Environ. 2015, 156, 457–472. [Google Scholar] [CrossRef]
- Collison, A.; Wilson, N. Planet Surface Reflectance Product; Planet Labs: San Francisco, CA, USA, 2018. [Google Scholar]





| Index | Equation | Originating Research | Citing Research |
|---|---|---|---|
| EVI | [37] | [25,26] | |
| EVI2 | [38] | [1] | |
| GCI | [39] | [20] | |
| GNDVI | [39] | [19] | |
| MSAVI2 | [36] | ||
| NDVI | [40] | [24] | |
| SAVI | [41] | [27] | |
| WDRVI, = 0.1 | [42] | [43] | |
| WDRVI, = 0.2 | [42,44] | [43] |
| Vegetation Index | Bands Used | SPAD Value | LAI Value |
|---|---|---|---|
| EVI | Blue, Red, NIR | 0.37 | 0.78 |
| EVI2 | Red, NIR | 0.54 | 0.76 |
| GCI | Green, NIR | 0.45 | 0.40 |
| GNDVI | Green, NIR | 0.46 | 0.46 |
| MSAVI2 | Red, NIR | 0.34 | 0.50 |
| NDVI | Red, NIR | 0.54 | 0.77 |
| SAVI | Red, NIR | 0.54 | 0.77 |
| WDRVI, = 0.1 | Red, NIR | 0.54 | 0.74 |
| WDRVI, = 0.2 | Red, NIR | 0.53 | 0.75 |
| Vegetation Index | Value | RMSE |
|---|---|---|
| EVI | 0.765 | 0.313 |
| EVI2 | 0.917 | 0.108 |
| GCI | 0.836 | 0.513 |
| GNDVI | 0.869 | 0.046 |
| MSAVI2 | 0.502 | 869.378 |
| NDVI | 0.922 | 0.045 |
| SAVI | 0.922 | 0.067 |
| WDRVI, = 0.1 | 0.898 | 0.052 |
| WDRVI, = 0.2 | 0.908 | 0.062 |
| Function Parameter | DOY | Associated Development Stage | Description |
|---|---|---|---|
| 161 ± 1 | V6–7 | Beginning of rapid vegetative growth. | |
| 166 ± 1 | V9–10 | Rapid growth, with a new fully expanded leaf every 2–4 days. | |
| 171 ± 2 | Rapid stalk elongation | Corn plants are growing taller. | |
| Maximum | 193 ± 4 | VT-R1 | Tassel fully emerged and all leaves fully expanded. End of vegetative growth—reproductive stages start when any silks become visible. |
| 248 ± 5 | R5—Mid-late Dent | Starch in kernels is drying and hardening. | |
| 264 ± 3 | R6—Physiological Maturity | Maximum grain dry matter accumulation, with a grain moisture content of 30–35%. | |
| 280 ± 4 | Harvest Maturity | Harvest occurs after grain is dried to <20% moisture; grain is safely stored at <15% moisture. |
| Metric (Days) | Slope of Linear Fit | |
|---|---|---|
| Median difference | −0.049 | −0.12 |
| Mean difference | −0.029 | −0.06 |
| Average absolute difference | 0.333 | 0.91 |
| Maximum absolute difference | 0.864 | 0.93 |
| Difference range (Maximum − Minimum) | 1.111 | 0.96 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Myers, E.; Kerekes, J.; Daughtry, C.; Russ, A. Assessing the Impact of Satellite Revisit Rate on Estimation of Corn Phenological Transition Timing through Shape Model Fitting. Remote Sens. 2019, 11, 2558. https://doi.org/10.3390/rs11212558
Myers E, Kerekes J, Daughtry C, Russ A. Assessing the Impact of Satellite Revisit Rate on Estimation of Corn Phenological Transition Timing through Shape Model Fitting. Remote Sensing. 2019; 11(21):2558. https://doi.org/10.3390/rs11212558
Chicago/Turabian StyleMyers, Emily, John Kerekes, Craig Daughtry, and Andrew Russ. 2019. "Assessing the Impact of Satellite Revisit Rate on Estimation of Corn Phenological Transition Timing through Shape Model Fitting" Remote Sensing 11, no. 21: 2558. https://doi.org/10.3390/rs11212558