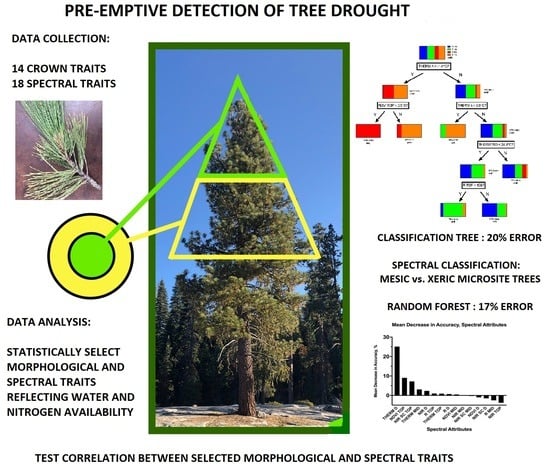
Pre-Emptive Detection of Mature Pine Drought Stress Using Multispectral Aerial Imagery
Abstract
:1. Introduction
2. Methods
2.1. Site Description
2.2. Study Overview
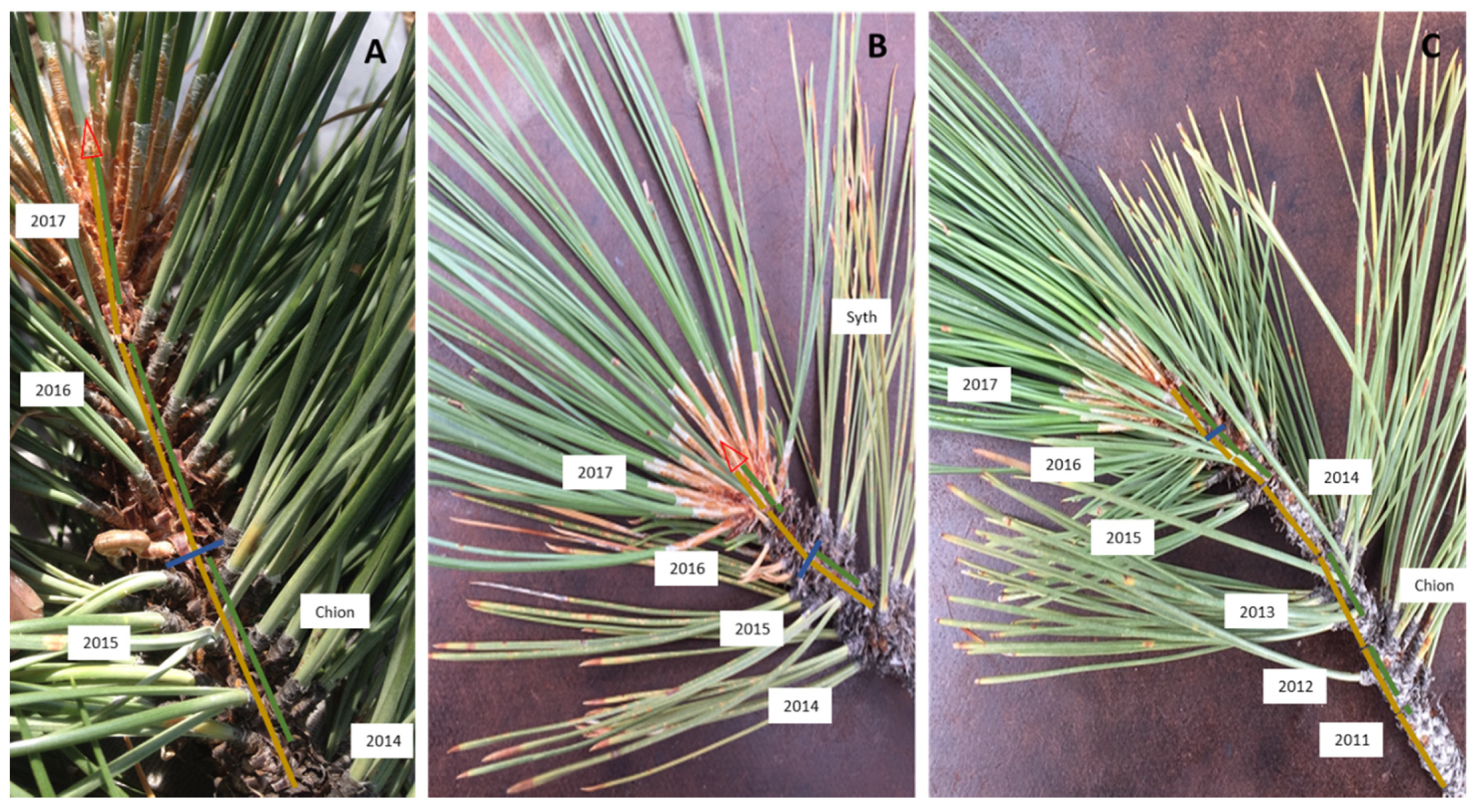
2.3. Crown Morphological Traits
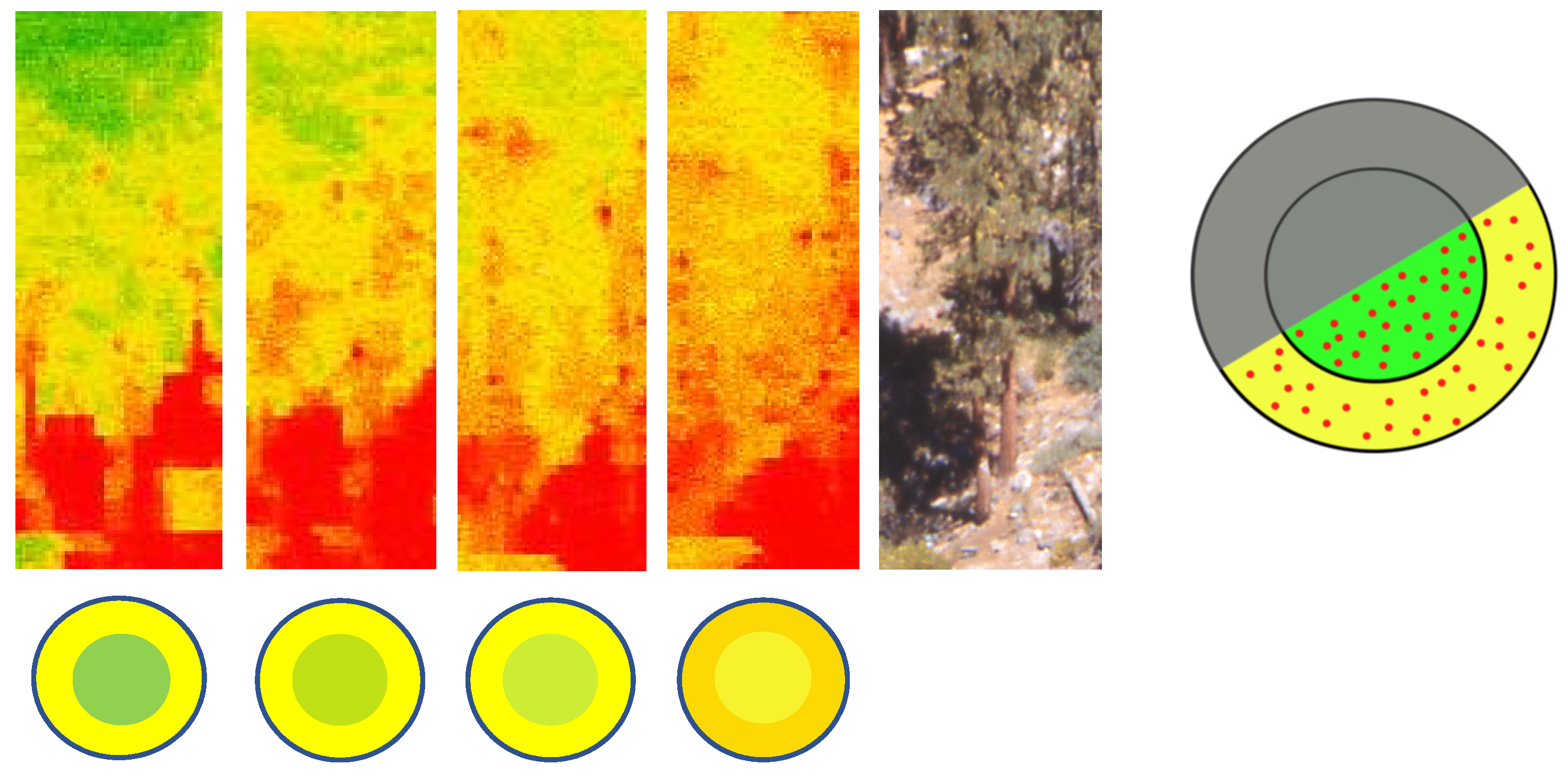
2.4. Remote Sensing and Imagery Processing
2.5. Analysis Approach
3. Results
3.1. Crown Morphology
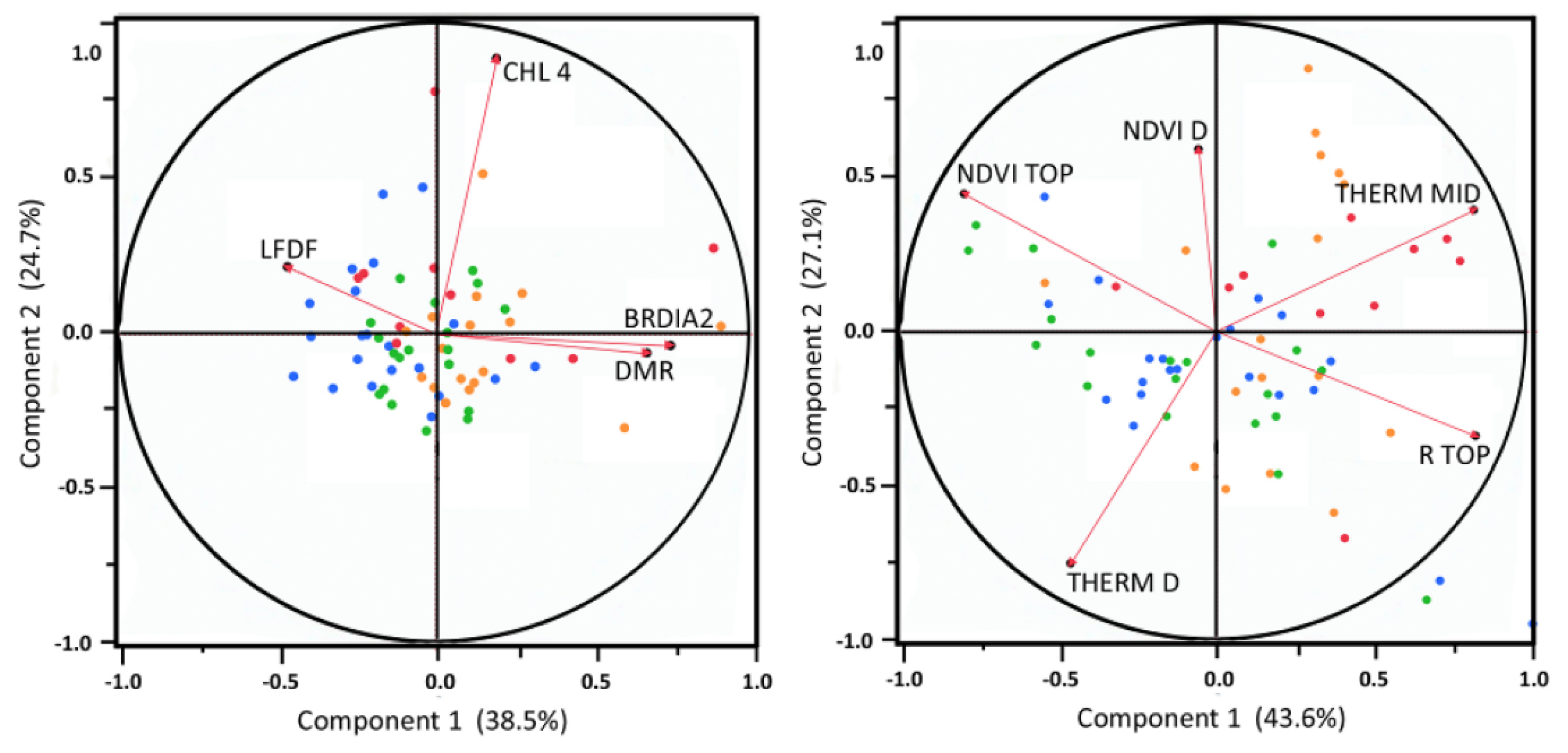
3.2. Multivariate Analysis of Morphological Traits
3.3. Crown Spectral Traits
3.4. Multivariate Analysis of Spectral Traits
3.5. Correlation of Morphological and Spectral Traits
3.6. Classification Tree Model Outcome
4. Discussion
4.1. Effects of Environmental Stresses on Crown Morphology
4.2. Spectral Delineation of Environmental Stresses
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Appendix A. Crown Morphological, and Insect and Disease Traits on Branchlets
Appendix B. List of All Whole Tree, Crown Morphological, and Spectral Traits
| TRAIT | M BN | M+N | X BN | X+N | DESCRIPTION |
|---|---|---|---|---|---|
| WHL | 5.2 | 5.4 | 5.1 | 5.4 | number of retained needle ages on branchlets |
| CHL1 | 0 | 1 | 0 | 0.4 | % chlorosis or chlorotic mottle on 1-year needles |
| CHL2 | 2 | 3 | 3 | 3 | % chlorosis or chlorotic mottle on 2-year needles |
| CHL4 | 12 | 14 | 16 | 15 | % chlorosis or chlorotic mottle on 4-year needles |
| CHL6 | 48 | 52 | 44 | 49 | % chlorosis or chlorotic mottle on 6-year needles |
| %MxNL1 | 81 | 80 | 77 | 79 | Relative current year needle length |
| %FOLLN | 59 | 57 | 61 | 59 | proportion of branchlet length with retained needles |
| BRDIA2 | 10.7 | 10.8 | 11.4 | 11.7 | prior year branchlet diameter at its base |
| ES | 1.2 | 0.9 | 1.2 | 0.9 | early senescence of needles, whole tree |
| Strobili ♂ | 0.02 | 0.02 | 0.02 | 0.00 | frequency of male strobili |
| LF DF | 1.72 | 0.01 | 1.34 | 0.00 | additive frequency of both needle defoliators |
| Elytroderma | 0.19 | 0.25 | 0.60 | 0.43 | needle cast fungi, presence/absence, whole tree |
| DMR | 0.07 | 0.00 | 0.50 | 0.31 | dwarf mistletoe rank |
| R TOP | 125.7 | 121.0 | 136.6 | 136.3 | intensity of R wavelength, tree-top |
| NDVI TOP | 0.57 | 0.57 | 0.53 | 0.54 | index of productivity, tree-top |
| NIR TOP | 475.0 | 459.2 | 444.4 | 452.9 | intensity of NIR wavelength, tree-top |
| NIR SC TOP | 0.79 | 0.79 | 0.76 | 0.77 | inverse of NDVI, indexed water content, tree-top |
| THERM TOP | 24.6 | 24.2 | 26.3 | 25.8 | needle temperature, tree-top |
| R MID | 129.1 | 124.6 | 138.2 | 135.1 | intensity of R wavelength, upper mid crown |
| NDVI MID | 0.58 | 0.58 | 0.54 | 0.55 | index of productivity, upper mid crown |
| NIR MID | 491.3 | 482 | 459.2 | 479.8 | intensity of NIR wavelength, upper mid crown |
| NIR SC MID | 0.79 | 0.79 | 0.77 | 0.78 | indexed water content, upper mid crown |
| THERM MID | 24.7 | 24.3 | 27.7 | 27.3 | needle temperature, upper mid crown |
| R Δ | −3.46 | −3.58 | −1.56 | −6.64 | [tree-top] − [upper mid crown] R |
| NDVI Δ | 0.00 | −0.01 | −0.01 | 0.00 | [tree-top] − [upper mid crown] NDVI |
| NIR Δ | −16.31 | −22.90 | −14.94 | −26.88 | [tree-top] − [upper mid crown] NIR |
| NIR SC Δ | 0.00 | 0.00 | 0.00 | 0.00 | [tree-top] − [upper mid crown] NIR SC |
| THERM Δ | −0.1 | −0.1 | −1.6 | −1.8 | [tree-top] − [upper mid crown] THERM |
References
- Allen, C.D.; Macalady, A.K.; Chenchouni, H.; Bachelet, D.; McDowell, N.; Vennetier, M.; Kitzberger, T.; Rigling, A.; Breshears, D.D.; Hogg, T.; et al. A global overview of drought and heat-induced tree mortality reveals emerging climate change risks for forests. For. Ecol. Manag. 2010, 259, 660–684. [Google Scholar] [CrossRef] [Green Version]
- Griffin, D.; Anchukaitis, K.J. How unusual is the 2012–2014 California drought? Geophys. Res. Lett. 2014, 41, 9017–9023. [Google Scholar] [CrossRef] [Green Version]
- Kolb, T.E.; Fettig, C.J.; Ayres, M.P.; Bentz, B.J.; Hicke, J.A.; Mathiasen, R.; Stewart, J.E.; Weed, A.S. Observed and anticipated impacts of drought on forest insects and diseases in the United States. For. Ecol. Manag. 2016, 380, 321–334. [Google Scholar] [CrossRef]
- Eitel, J.U.H.; Vierling, L.A.; Litvak, M.E.; Long, D.S.; Schulthess, U.; Ager, A.A.; Krofcheck, D.J.; Stoscheck, L. Broadband, red-edge information from satellites improves early stress detection in a New Mexico conifer woodland. Remote. Sens. Environ. 2011, 115, 3640–3646. [Google Scholar] [CrossRef]
- Pontius, J.; Hallett, R. Comprehensive Methods for Earlier Detection and Monitoring of Forest Decline. For. Sci. 2014, 60, 1156–1163. [Google Scholar] [CrossRef]
- Anderegg, W.R.; Anderegg, L.D.L.; Huang, C.-Y. Testing early warning metrics for drought-induced tree physiological stress and mortality. Glob. Chang. Boil. 2019, 25, 2459–2469. [Google Scholar] [CrossRef]
- De la Serrana, R.G.; Vilagrosa, A.; Alloza, J.A. Pine mortality in southeast Spain after an extreme dry and warm year: Interactions among drought stress, carbohydrates, and bark beetle attack. Trees 2015, 29, 1791–1804. [Google Scholar] [CrossRef]
- McDowell, N.G.; Pockman, W.; Allen, C.D.; Breshears, D.D.; Cobb, N.; Kolb, T.; Plaut, J.; Sperry, J.; West, A.; Williams, D.G.; et al. Mechanisms of plant survival and mortality during drought: Why do some plants survive while others succumb to drought? New Phytol. 2008, 178, 719–739. [Google Scholar] [CrossRef]
- Peñuelas, J.; Filella, I.; Biel, C.; Serrano, L.; Savé, R. The reflectance at the 950–970 nm region as an indicator of plant water status. Int. J. Remote. Sens. 1993, 14, 1887–1905. [Google Scholar] [CrossRef]
- Sims, D.A.; Gamon, J.A. Estimation of vegetation water content and photosynthetic tissue area from spectral reflectance: A comparison of indices based on liquid water and chlorophyll absorption features. Remote. Sens. Environ. 2003, 84, 526–537. [Google Scholar] [CrossRef]
- Marusig, D.; Petruzzellis, F.; Tomasella, M.; Napolitano, R.; Altobelli, A.; Nardini, A. Correlation of Field-Measured and Remotely Sensed Plant Water Status as a Tool to Monitor the Risk of Drought-Induced Forest Decline. Forests 2020, 11, 77. [Google Scholar] [CrossRef] [Green Version]
- Seidel, H.; Schunk, C.; Matiu, M.; Menzel, A. Diverging Drought Resistance of Scots Pine Provenances Revealed by Infrared Thermography. Front. Plant Sci. 2016, 7, 346. [Google Scholar] [CrossRef] [Green Version]
- Stimson, H.C.; Breshears, D.D.; Ustin, S.L.; Kefauver, S.C. Spectral sensing of foliar water conditions in two co-occurring conifer species: Pinus edulis and Juniperus monosperma. Remote. Sens. Environ. 2005, 96, 108–118. [Google Scholar] [CrossRef]
- Santesteban, L.G.; di Gennaro, S.F.; Herrero-Langreo, A.; Miranda, C.; Royo, J.; Matese, A. High-resolution UAV-based thermal imaging to estimate the instantaneous and seasonal variability of plant water status within a vineyard. Agric. Water Manag. 2017, 183, 49–59. [Google Scholar] [CrossRef]
- Bell, D.M.; Cohen, W.; Reilly, M.; Yang, Z. Visual interpretation and time series modeling of Landsat imagery highlight drought’s role in forest canopy declines. Ecosphere 2018, 9, e02195. [Google Scholar] [CrossRef]
- Peñuelas, J.; Savè, R.; Marfà, O.; Serrano, L. Remotely measured canopy temperature of greenhouse strawberries as an indicator of water status and yield under mild and very mild water stress conditions. Agric. For. Meteor. 1992, 58, 63–77. [Google Scholar] [CrossRef]
- Richardson, A.; Everitt, J.H. Monitoring water stress in buffelgrass using hand-held radiometers. Int. J. Remote. Sens. 1987, 8, 1797–1806. [Google Scholar] [CrossRef]
- Meddens, A.J.H.; Hicke, J.A.; Vierling, L.A. Evaluating the potential of multispectral imagery to map multiple stages of tree mortality. Remote. Sens. Environ. 2011, 115, 1632–1642. [Google Scholar] [CrossRef]
- Norman, S.P.; Hargrove, W.W.; Spruce, J.P.; Christie, W.M.; Schroeder, S.W. Highlights of Satellite-Based Forest Change Recognition and Tracking Using the ForWarn System; U.S. Department of Agriculture; Forest Service: Asheville, NC, USA, 2013; p. 30.
- Casas, A.; Riaño, D.; Ustin, S.; Dennison, P.E.; Salas, J. Estimation of water-related biochemical and biophysical vegetation properties using multitemporal airborne hyperspectral data and its comparison to MODIS spectral response. Remote. Sens. Environ. 2014, 148, 28–41. [Google Scholar] [CrossRef]
- Asner, G.P.; Brodrick, P.G.; Anderson, C.B.; Vaughn, N.; Knapp, D.E.; Martin, R.E. Progressive forest canopy water loss during the 2012–2015 California drought. Proc. Natl. Acad. Sci. USA 2015, 113, E249–E255. [Google Scholar] [CrossRef] [Green Version]
- Asner, G.P.; Martin, R.E. Spectral and chemical analysis of tropical forests: Scaling from leaf to canopy levels. Remote. Sens. Environ. 2008, 112, 3958–3970. [Google Scholar] [CrossRef]
- Housman, I.W.; Chastain, R.A.; Finco, M.V. An Evaluation of Forest Health Insect and Disease Survey Data and Satellite-Based Remote Sensing Forest Change Detection Methods: Case Studies in the United States. Remote. Sens. 2018, 10, 1184. [Google Scholar] [CrossRef] [Green Version]
- Slatyer, R.O. Plant-Water Relationships; Academic Press: London, UK, 1967; p. 366. [Google Scholar]
- Mirzaie, M.; Darvishzadeh, R.; Shakiba, A.; Matkan, A.; Atzberger, C.; Skidmore, A. Comparative analysis of different uni- and multi-variate methods for estimation of vegetation water content using hyper-spectral measurements. Int. J. Appl. Earth Obs. Geoinform. 2014, 26, 1–11. [Google Scholar] [CrossRef]
- Martin, R.E.; Asner, G.P.; Francis, E.; Ambrose, A.; Baxter, W.; Das, A.J.; Vaughn, N.R.; Paz-Kagan, T.; Dawson, T.; Nydick, K.; et al. Remote measurement of canopy water content in giant sequoias (Sequoiadendron giganteum) during drought. For. Ecol. Manag. 2018, 279–290. [Google Scholar] [CrossRef]
- Cook, B.D.; Corp, L.A.; Nelson, R.F.; Middleton, E.M.; Morton, D.C.; McCorkel, J.; Masek, J.G.; Ranson, K.J.; Ly, V.; Montesano, P.M. NASA Goddard’s LiDAR, Hyperspectral and Thermal (G-LiHT) Airborne Imager. Remote. Sens. 2013, 5, 4045–4066. [Google Scholar] [CrossRef] [Green Version]
- Levitt, J. Responses to Plants to Environmental Stresses; Academic Press: London, UK, 1980; p. 497. [Google Scholar]
- Grulke, N.E.; Johnson, R.; Jones, D.; Monschein, S.; Nikolova, P.; Tausz, M. Variation in morphological and biochemical O3 injury traits of Jeffrey pine within canopies and between microsites. Tree Phys. 2003, 23, 923–929. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Miller, P.R.; Stolte, K.W.; Duriscoe, D.; Pronos, J.; Tech, C. Monitoring Ozone Air Pollution Effects on Western Pine Forests; U.S. Department of Agriculture, Forest Service, Pacific Southwest Research Station: Albany, CA, USA, 1996; PSW-GTR-155; p. 79.
- Jones, M.E.; Paine, T.D. Detecting changes in insect herbivore communities along a pollution gradient. Environ. Pollut. 2006, 143, 377–387. [Google Scholar] [CrossRef] [PubMed]
- López, M.L.; Calderón, R.; Gonzalez-Dugo, V.; Zarco-Tejada, P.J.; Fereres, E. Early Detection and Quantification of Almond Red Leaf Blotch Using High-Resolution Hyperspectral and Thermal Imagery. Remote. Sens. 2016, 8, 276. [Google Scholar] [CrossRef] [Green Version]
- Mahlein, A.-K.; Rumpf, T.; Welke, P.; Dehne, H.-W.; Plümer, L.; Steiner, U.; Oerke, E.-C. Development of spectral indices for detecting and identifying plant diseases. Remote. Sens. Environ. 2013, 128, 21–30. [Google Scholar] [CrossRef]
- Maffei, H.M.; Filip, G.M.; Grulke, N.E.; Oblinger, B.W.; Margolis, E.Q.; Chadwick, K.L. Pruning high-value coastal Douglas-fir reduces dwarf mistletoe severity and increases longevity in Central Oregon. For. Ecol. Manag. 2016, 379, 11–19. [Google Scholar] [CrossRef]
- McLaughlin, B.C.; Blakey, R.; Weitz, A.P.; Feng, X.; Brown, B.J.; Ackerly, D.D.; Dawson, T.E.; Thompson, S. Weather underground: Subsurface hydrologic processes mediate tree vulnerability to extreme climatic drought. Glob. Chang. Boil. 2020, 26, 3091–3107. [Google Scholar] [CrossRef]
- Anderegg, W.R.L.; Hicke, J.A.; Fisher, R.A.; Allen, C.D.; Aukema, J.; Bentz, B.; Hood, S.M.; Lichstein, J.W.; Macalady, A.K.; McDowell, N.; et al. Tree mortality from drought, insects, and their interactions in a changing climate. New Phytol. 2015, 208, 674–683. [Google Scholar] [CrossRef]
- Cotrozzi, L.; Pellegrini, E.; Guidi, L.; Landi, M.; Lorenzini, G.; Massai, R.; Remorini, D.; Tonelli, M.; Trivellini, A.; Vernieri, P.; et al. Losing the Warning Signal: Drought Compromises the Cross-Talk of Signaling Molecules in Quercus ilex Exposed to Ozone. Front. Plant Sci. 2017, 8. [Google Scholar] [CrossRef] [Green Version]
- Sala, A.; Piper, F.I.; Hoch, G. Physiological mechanisms of drought-induced tree mortality are far from being resolved. New Phytol. 2010, 186, 274–281. [Google Scholar] [CrossRef]
- Grulke, N.E.; Heath, R.L. Ozone effects on plants in natural ecosystems. Plant Boil. 2019, 22, 12–37. [Google Scholar] [CrossRef]
- Adams, M.L.; Philpot, W.; Norvell, W.A. Yellowness index: An application of spectral second derivatives to estimate chlorosis of leaves in stressed vegetation. Int. J. Remote. Sens. 1999, 20, 3663–3675. [Google Scholar] [CrossRef]
- McMillin, J.D.; Wagner, M.R. Effects of water stress on biomass partitioning of ponderosa pine seedlings during primary root growth and shoot growth periods. Forest Sci. 1995, 41, 594–610. [Google Scholar]
- De Lucia, E.H.; Maherali, H.; Carey, E.V. Climate-driven changes in biomass allocation in pines. Glob. Chang. Boil. 2000, 6, 587–593. [Google Scholar] [CrossRef]
- Forest Vegetation Simulator; U.S. Department of Agriculture, Forest Service: Washington, DC, USA. Available online: https://www.fs.fed.us/fvs/ (accessed on 18 June 2020).
- Kim, J.B.; Monier, E.; Sohngen, B.; Pitts, G.S.; Drapek, R.; McFarland, J.; Ohrel, S.; Cole, J. Assessing climate change impacts, benefits of mitigation, and uncertainties on major global forest regions under multiple socioeconomic and emissions scenarios. Environ. Res. Lett. 2017, 12, 045001. [Google Scholar] [CrossRef]
- Preisler, H.K.; Grulke, N.E.; Heath, Z.; Smith, S.L. Analysis and out-year forecast of beetle, borer, and drought-induced tree mortality in California. For. Ecol. Manag. 2017, 399, 166–178. [Google Scholar] [CrossRef]
- Grulke, N.E.; Johnson, R.; Esperanza, A.; Jones, D.; Nguyen, T.; Posch, S.; Tausz, M. Canopy transpiration of Jeffrey pine in mesic and xeric microsites: O3 uptake and injury response. Trees 2003, 17, 292–298. [Google Scholar] [CrossRef]
- National Park Service. Air Resources Division, Sequoia National Park-Lodgepole. Available online: https://www.nps.gov/orgs/1971 (accessed on 18 June 2020).
- Grulke, N.E.; Minnich, R.A.; Paine, T.D.; Seybold, S.J.; Chavez, D.J.; Fenn, M.E.; Riggan, P.J.; Dunn, A. Chapter 17 Air Pollution Increases Forest Susceptibility to Wildfires: A Case Study in the San Bernardino Mountains in Southern California. Alta. Oil Sands 2008, 8, 365–403. [Google Scholar] [CrossRef]
- Belmecheri, S.; Babst, F.; Wahl, E.R.; Stahle, D.W.; Trouet, V. Multi-century evaluation of Sierra Nevada snowpack. Nat. Clim. Chang. 2015, 6, 2–3. [Google Scholar] [CrossRef]
- Fenn, M.E.; Baron, J.S.; Allen, E.; Rueth, H.M.; Nydick, K.R.; Geiser, L.; Bowman, W.D.; Sickman, J.O.; Meixner, T.; Johnson, D.W.; et al. Ecological Effects of Nitrogen Deposition in the Western United States. BioScience 2003, 53, 404. [Google Scholar] [CrossRef]
- Bytnerowicz, A.; Tausz, M.; Alonso, R.; Jones, D.; Johnson, R.; Grulke, N. Summer-time distribution of air pollutants in Sequoia National Park, California. Environ. Pollut. 2002, 118, 187–203. [Google Scholar] [CrossRef]
- Grulke, N.; Bienz, C.; Hrinkevich, K.; Maxfield, J.; Uyeda, K. Quantitative and qualitative approaches to assess tree vigor and stand health in dry pine forests. For. Ecol. Manag. 2020, 465, 118085. [Google Scholar] [CrossRef]
- Grulke, N.E.; Lee, E.H. Assessing ozone-induced foliar injury in ponderosa pine. Can. J. For. Res. 1997, 27, 1658–1668. [Google Scholar] [CrossRef]
- Staszak, J.; Grulke, N.E.; Marrett, M.; Prus-Glowacki, W. Isozyme markers associated with O3 tolerance indicate shift in genetic structure of ponderosa and Jeffrey pine in Sequoia National Park, California. Environ. Pollut. 2007, 149, 366–375. [Google Scholar] [CrossRef]
- Bridgwater, F.E.; Bramlett, D.L. Supplemental Mass Pollination to increase Seed Yields in Loblolly Pine Seed Orchards. South. J. Appl. For. 1982, 6, 100–104. [Google Scholar] [CrossRef]
- Whipple, A.V.; Cobb, N.S.; Gehring, C.A.; Mopper, S.; Flores-Rentería, L.; Whitham, T.G. Long-Term Studies Reveal Differential Responses to Climate Change for Trees Under Soil- or Herbivore-Related Stress. Front. Plant Sci. 2019, 10, 132. [Google Scholar] [CrossRef]
- Hawksworth, F.G.; Wiens, D. Dwarf Mistletoes: Biology, Pathology and Systematics; USDA Forest Service: Washington, DC, USA, 1996; Volume 709, p. 410. Available online: http://www.rms.nau.edu/publications/ah_709 (accessed on 18 June 2020).
- Riggan, P.; Hoffman, J.; Brass, J. Estimating fire properties by remote sensing. IEEE Aerosp. Electron. Syst. Mag. 2009, 24, 13–19. [Google Scholar] [CrossRef]
- Hoffman, J.W.; Coulter, L.L.; Riggan, P.J. Rapid turn-around mapping of wildfires and disasters with airborne infrared imagery from the new FireMapper 2.0 and Oilmapper systems. In Proceedings of the American Society Photogrammetry and Remote Sensing, Baltimore, MD, USA, 7–11 March 2005; p. 8. Available online: https://www.fs.fed.us/psw/publications/riggan/psw_2005_riggan001_hoffman.pdf (accessed on 18 June 2020).
- Immitzer, M.; Atzberger, C.; Koukal, T. Tree Species Classification with Random Forest Using Very High Spatial Resolution 8-Band WorldView-2 Satellite Data. Remote. Sens. 2012, 4, 2661–2693. [Google Scholar] [CrossRef] [Green Version]
- ESRI. ArcGIS 10.8. 2019. Available online: esri.com/en-us/arcgis/products/arcgis-desktop/ (accessed on 17 June 2020).
- Grulke, N.; Balduman, L. Deciduous Conifers: High N Deposition and O3 Exposure Effects on Growth and Biomass Allocation in Ponderosa Pine. Water Air Soil Pollut. 1999, 116, 235–248. [Google Scholar] [CrossRef]
- King, J.R.; Jackson, D.A. Variable selection in large environmental data sets using principal components analysis. Environmetrics 1999, 10, 67–77. [Google Scholar] [CrossRef]
- Cutler, D.R.; Edwards, T.C.; Beard, K.H.; Cutler, A.; Hess, K.T.; Gibson, J.; Lawler, J.J. Random Forests for classification in ecology. Ecology 2007, 88, 2783–2792. [Google Scholar] [CrossRef]
- Brieman, L.; Cutler, A. Random Forests, 201. Available online: https://www.stat.berkeley.edu/~breiman/RandomForests/cc_copyright.htm (accessed on 16 June 2020).
- Boehmke, B.; Greenwell, B.M. Hands-On Machine Learning With R; CRS Press: Boca Raton, FL, USA, 2019; p. 488. [Google Scholar]
- McCune, B.; Medford, M.J. Multivariate Analysis of Ecological Data; Version 6; MjM Software: Gleneden Beach, OR, USA, 2011. [Google Scholar]
- JMP 2020. Partition Platform. Available online: https://www.jmp.com/support/help/en/15.1/index.shtml#page/jmp/partition-models.shtml (accessed on 29 May 2020).
- Gordon, A.D.; Breiman, L.; Friedman, J.H.; Olshen, R.A.; Stone, C.J. Classification and Regression Trees; CRC Press: Boca Raton, FL, USA, 1984; Volume 40, p. 874. [Google Scholar] [CrossRef] [Green Version]
- Schrader-Patton, C.; Grulke, N.E.; Bienz, C. Assessments of ponderosa pine vigor using 4-Band aerial imagery in south central Oregon: Crown objects to landscapes. For. Ecol. Manag. 2004, 191–217. [Google Scholar]
- Andersen, C.P.; Wilson, R.; Plocher, M.; Hogsett, W.E. Carry-over effects of ozone on root growth and carbohydrate concentrations of ponderosa pine seedlings. Tree Physiol. 1997, 17, 805–811. [Google Scholar] [CrossRef] [Green Version]
- Grulke, N.E.; Andersen, C.P.; Fenn, M.P.; Miller, P.R. Ozone exposure and N deposition reduces root biomass in ponderosa pine across the San Bernardino Mountains, California. Environ. Poll. 1998, 103, 63–73. [Google Scholar] [CrossRef]
- Paoletti, E.; Grulke, N. Does living in elevated CO ameliorate tree response to ozone? A review on stomatal responses. Environ. Pollut. 2005, 137, 483–493. [Google Scholar] [CrossRef]
- Grulke, N.E.; Alonso, R.; Nguyen, T.; Cascio, C.; Dobrowolski, W. Stomata open at night in pole-sized and mature ponderosa pine: Implications for O3 exposure metrics. Tree Physiol. 2004, 24, 1001–1010. [Google Scholar] [CrossRef] [Green Version]
- Vanderheyden, D.; Skelly, J.; Innes, J.L.; Hug, C.; Zhang, J.; Landolt, W.; Bleuler, P. Ozone exposure thresholds and foliar injury on forest plants in Switzerland. Environ. Pollut. 2001, 111, 321–331. [Google Scholar] [CrossRef]
- Gerosa, G.; Marzuoli, R.; Desotgiu, R.; Bussotti, F.; Denti, A.B. Visible leaf injury in young trees of Fagus sylvatica L. and Quercus robur L. in relation to ozone uptake and ozone exposure. An Open-Top Chambers experiment in South Alpine environmental conditions. Environ. Pollut. 2008, 152, 274–284. [Google Scholar] [CrossRef]
- Mitchell, R.G.; Waring, R.H.; Pitman, G.B. Thinning lodgepole pine increases tree vigor and resistance to mountain pine beetle. Forest Sci. 1983, 29, 204–211. [Google Scholar]
- Keen, F.P. Ponderosa pine tree classes redefined. J. For. 1943, 41, 249–253. [Google Scholar]
- Karlsson, P.E.; Örlander, G.; Langvall, O.; Uddling, J.; Hjorth, U.; Wiklander, K.; Areskoug, B.; Grennfelt, P. Negative impact of ozone on the stem basal area increment of mature Norway spruce in south Sweden. For. Ecol. Manag. 2006, 232, 146–151. [Google Scholar] [CrossRef]
- Albaugh, T.J.; Allen, H.L.; Kress, L.W. Biomass-DBH relationship for young loblolly pine as affected by ozone. Biomass Bioenergy 1991, 1, 143–148. [Google Scholar] [CrossRef]
- Raffa, K.F.; Aukema, B.H.; Bentz, B.J.; Carroll, A.L.; Hicke, J.A.; Turner, M.G.; Romme, W.H. Cross-scale Drivers of Natural Disturbances Prone to Anthropogenic Amplification: The Dynamics of Bark Beetle Eruptions. BioScience 2008, 58, 501–517. [Google Scholar] [CrossRef] [Green Version]
- Mattson, W.J.; Haack, R.A. The Role of Drought in Outbreaks of Plant-Eating Insects. BioScience 1987, 37, 110–118. [Google Scholar] [CrossRef]
- Brewer, J.W.; O’Neill, K.M.; Deshon, R.E. Effects of artificially altered foliar nitrogen levels on development and survival of young instars of western spruce budworm, Choristoneura occidentalis Freeman. J. Appl. Èntomol. 1987, 104, 121–130. [Google Scholar] [CrossRef]
- Heliovaara, K. Insects and Pollution; Informa UK Limited: London, UK, 2018; p. 393. [Google Scholar]
- Manning, W.J.; Tiedemann, A.V. Climate change: Potential effects of increased atmospheric carbon dioxide (CO2), ozone (O3), and ultraviolet-B (UV-B) radiation on plant diseases. Environ. Poll. 1995, 88, 219–245. [Google Scholar] [CrossRef]
- Percy, K.E.; Awmack, C.S.; Lindroth, R.L.; Kubiske, M.E.; Kopper, B.J.; Isebrands, J.G.; Pregitzer, K.S.; Hendrey, G.R.; Dickson, R.E.; Zak, D.R.; et al. Altered performance of forest pests under atmospheres enriched by CO2 and O3. Nature 2002, 420, 403–407. [Google Scholar] [CrossRef] [PubMed]
- Jones, M.E.; Paine, T.D.; Fenn, M.E.; Poth, M.A. Influence of ozone and nitrogen deposition on bark beetle activity under drought conditions. For. Ecol. Manag. 2004, 200, 67–76. [Google Scholar] [CrossRef]
- Maffei, M.; Wing, B. How airborne lidar can help map root disease and individual tree decline. In Proceedings of the 64th Annulas Western International Forests Disease Work Conference, Sitka, AK, USA, 13 May 2016; p. 37. Available online: https://www.irp-cdn.multiscreensite.com/1463fd0a/files/uploaded/WIFDWC2016FFinal%20%281%29.pdf (accessed on 18 June 2020).
- Meddens, A.J.H.; Hicke, J.A.; Vierling, L.A.; Hudak, A.T. Evaluating methods to detect bark beetle-caused tree mortality using single-date and multi-date Landsat imagery. Remote. Sens. Environ. 2013, 132, 49–58. [Google Scholar] [CrossRef]
- Hanavan, R.; Pontius, J.; Hallett, R. A 10-Year Assessment of Hemlock Decline in the Catskill Mountain Region of New York State Using Hyperspectral Remote Sensing Techniques. J. Econ. Èntomol. 2015, 108, 339–349. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cotrozzi, L.; Couture, J.J.; Cavender-Bares, J.; Kingdon, C.C.; Fallon, B.; Pilz, G.; Pellegrini, E.; Nali, C.; A Townsend, P. Using foliar spectral properties to assess the effects of drought on plant water potential. Tree Physiol. 2017, 37, 1582–1591. [Google Scholar] [CrossRef]
- Schrader-Patton, C.; Grulke, N.E.; Dressen, M. Rapid Reconnaissance of a Forest Insect Outbreak in Colorado Using MODIS Phenology Data; U.S. Department of Agriculture, Pacific Northwest Research Station: Portland, OR, USA, 2016; PNW-GTR-940e; p. 35.
- Spruce, J.P.; Hicke, J.A.; Hargrove, W.W.; Grulke, N.E.; Meddens, A.J.H. Use of MODIS NDVI Products to Map Tree Mortality Levels in Forests Affected by Mountain Pine Beetle Outbreaks. Forests 2019, 10, 811. [Google Scholar] [CrossRef] [Green Version]
- Kefauver, S.C.; Peñuelas, J.; Ustin, S. Using topographic and remotely sensed variables to assess ozone injury to conifers in the Sierra Nevada (USA) and Catalonia (Spain). Remote. Sens. Environ. 2013, 139, 138–148. [Google Scholar] [CrossRef]
- Martinelli, F.; Scalenghe, R.; Davino, S.; Panno, S.; Scuderi, G.; Ruisi, P.; Villa, P.; Stroppiana, D.; Boschetti, M.; Goulart, L.R.; et al. Advanced methods of plant disease detection. A review. Agron. Sustain. Dev. 2014, 35, 1–25. [Google Scholar] [CrossRef] [Green Version]
- Leckie, D.G.; Cloney, E.; Joyce, S.P. Automated detection, and mapping of crown discolouration caused by jack pine budworm with 2.5 m resolution multispectral imagery. Int. J. Appl. Earth Obs. Geoinform. 2005, 7, 61–77. [Google Scholar] [CrossRef]
- Yamasaki, S.; Dillenburg, L.R. Measurements of leaf relative water content in Araucaria angustifolia. Rev. Brasill. Fisil. Feget. 1999, 11, 69–75. [Google Scholar]
- Barrs, H.; Weatherley, P. A Re-Examination of the Relative Turgidity Technique for Estimating Water Deficits in Leaves. Aust. J. Boil. Sci. 1962, 15, 413. [Google Scholar] [CrossRef] [Green Version]
- Costa, J.M.; Grant, O.M.; Chaves, M.M. Thermography to explore plant–environment interactions. J. Exp. Bot. 2013, 64, 3937–3949. [Google Scholar] [CrossRef] [PubMed]




| a. | Mesic/Xeric | BN/+N | H2O * N |
|---|---|---|---|
| CHL4 | 0.018 | 0.241 | 0.096 |
| BRDIA2 | 0.011 | 0.905 | 0.751 |
| LF DF | <0.0001 | <0.0001 | <0.0001 |
| DMR | 0.043 | 0.725 | 0.679 |
| b. | |||
| R TOP | 0.005 | 0.276 | 0.976 |
| NDVI TOP | 0.012 | 0.246 | 0.558 |
| NIR SC TOP | 0.005 | 0.274 | 0.590 |
| THERM Δ | <0.0001 | 0.348 | 0.327 |
| Test | Value | Approx. F | # DF | DenDF | Prob > F |
|---|---|---|---|---|---|
| Wilks’ λ | 0.4341 | 1.93 | 16 | 98.40 | 0.0150 |
| Pillai’s Trace | 0.6450 | 1.68 | 16 | 140.00 | 0.0269 |
| Hotelling-Lawley | 1.1275 | 2.19 | 16 | 58.25 | 0.0103 |
| Roy’s Max Root | 0.9550 | 8.36 | 4 | 35.00 | <0.0001 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Grulke, N.; Maxfield, J.; Riggan, P.; Schrader-Patton, C. Pre-Emptive Detection of Mature Pine Drought Stress Using Multispectral Aerial Imagery. Remote Sens. 2020, 12, 2338. https://doi.org/10.3390/rs12142338
Grulke N, Maxfield J, Riggan P, Schrader-Patton C. Pre-Emptive Detection of Mature Pine Drought Stress Using Multispectral Aerial Imagery. Remote Sensing. 2020; 12(14):2338. https://doi.org/10.3390/rs12142338
Chicago/Turabian StyleGrulke, Nancy, Jason Maxfield, Phillip Riggan, and Charlie Schrader-Patton. 2020. "Pre-Emptive Detection of Mature Pine Drought Stress Using Multispectral Aerial Imagery" Remote Sensing 12, no. 14: 2338. https://doi.org/10.3390/rs12142338