Rapid Determination of Soil Class Based on Visible-Near Infrared, Mid-Infrared Spectroscopy and Data Fusion
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Area and Soil Sampling Sites
2.2. Chemical Analysis
2.3. Spectroscopic Measurement and Pre-Processing
2.4. Outer Product Analysis (OPA)
2.5. Model Construction
3. Results
3.1. Extracting Auxiliary Soil Information
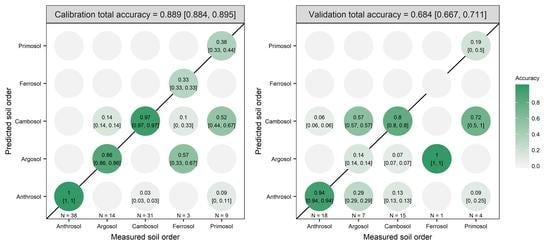
3.2. Model Prediction Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- McBratney, A.B.; Odeh, I.O.A.; Bishop, T.F.A.; Dunbar, M.S.; Shatar, T.M. An overview of pedometric techniques for use in soil survey. Geoderma 2000, 97, 293–327. [Google Scholar] [CrossRef]
- Vasques, G.M.; Dematte, J.A.M.; Viscarra Rossel, R.A.; Ramirez-Lopez, L.; Terra, F.S. Soil classification using visible/near-infrared diffuse reflectance spectra from multiple depths. Geoderma 2014, 223, 73–78. [Google Scholar] [CrossRef]
- Craswell, E.T.; Lefroy, R.D.B. The role and function of organic matter in tropical soils. Nutr. Cycl. Agroecosyst. 2001, 61, 7–18. [Google Scholar] [CrossRef]
- McCarty, G.W.; Reeves, J.B.; Reeves, V.B.; Follett, R.F.; Kimble, J.M. Mid-infrared and near-infrared diffuse reflectance spectroscopy for soil carbon measurement. Soil Sci. Soc. Am. J. 2002, 66, 640–646. [Google Scholar]
- Nocita, M.; Stevens, A.; van Wesemael, B.; Aitkenhead, M.; Bachmann, M.; Barthes, B.; Ben Dor, E.; Brown, D.J.; Clairotte, M.; Csorba, A.; et al. Soil Spectroscopy: An alternative to wet chemistry for soil monitoring. Adv. Agron. 2015, 132, 139–159. [Google Scholar]
- Viscarra Rossel, R.A.; Walvoort, D.J.J.; McBratney, A.B.; Janik, L.J.; Skjemstad, J.O. Visible, near infrared, mid infrared or combined diffuse reflectance spectroscopy for simultaneous assessment of various soil properties. Geoderma 2006, 131, 59–75. [Google Scholar] [CrossRef]
- Stenberg, B.; Viscarra Rossel, R.A.; Mouazen, A.M.; Wetterlind, J. Visible and near infrared spectroscopy in soil science. Adv. Agron. 2010, 107, 163–215. [Google Scholar]
- Soriano-Disla, J.M.; Janik, L.J.; Viscarra Rossel, R.A.; Macdonald, L.M.; McLaughlin, M.J. The performance of visible, near-, and mid-infrared reflectance spectroscopy for prediction of soil physical, chemical, and biological properties. Appl. Spectrosc. Rev. 2014, 49, 139–186. [Google Scholar] [CrossRef]
- Ji, W.; Li, S.; Chen, S.; Shi, Z.; Viscarra Rossel, R.A.; Mouazen, A.M. Prediction of soil attributes using the Chinese soil spectral library and standardized spectra recorded at field conditions. Soil Tillage Res. 2016, 155, 492–500. [Google Scholar] [CrossRef]
- Bilgili, A.V.; van Es, H.M.; Akbas, F.; Durak, A.; Hively, W.D. Visible-near infrared reflectance spectroscopy for assessment of soil properties in a semi-arid area of Turkey. J. Arid Environ. 2010, 74, 229–238. [Google Scholar] [CrossRef]
- Shi, Z.; Wang, Q.; Peng, J.; Ji, W.; Liu, H.; Li, X.; Viscarra Rossel, R.A. Development of national VNIR soil-spectral library for soil classification and the predictions of organic matter. Sci. China Earth Sci. 2014, 57, 1671–1680. [Google Scholar] [CrossRef]
- Ng, W.; Minasny, B.; Montazerolghaem, M.; Padarian, J.; Ferguson, R.; Bailey, S.; McBratney, A.B. Convolutional neural network for simultaneous prediction of several soil properties using visible/near-infrared, mid-infrared, and their combined spectra. Geoderma 2019, 352, 251–267. [Google Scholar] [CrossRef]
- Shepherd, K.D.; Walsh, M.G. Development of reflectance spectral libraries for characterization of soil properties. Soil Sci. Soc. Am. J. 2002, 66, 988–998. [Google Scholar] [CrossRef]
- Islam, K.; Singh, B.; McBratney, A. Simultaneous estimation of several soil properties by ultra-violet, visible, and near-infrared reflectance spectroscopy. Aust. J. Soil Res. 2003, 41, 1101–1114. [Google Scholar] [CrossRef]
- McDowell, M.L.; Bruland, G.L.; Deenik, J.L.; Grunwald, S.; Knox, N.M. Soil total carbon analysis in Hawaiian soils with visible, near-infrared and mid-infrared diffuse reflectance spectroscopy. Geoderma 2012, 189–190, 312–320. [Google Scholar] [CrossRef]
- Dematte, J.A.M.; Terra, F.D. Spectral pedology: A new perspective on evaluation of soils along pedogenetic alterations. Geoderma 2014, 217, 190–200. [Google Scholar] [CrossRef]
- Yang, M.H.; Xu, D.Y.; Chen, S.C.; Li, H.Y.; Shi, Z. Evaluation of Machine Learning Approaches to Predict Soil Organic Matter and pH Using vis-NIR Spectra. Sensors 2019, 19, 263. [Google Scholar] [CrossRef] [Green Version]
- Chang, C.W.; Laird, D.A. Near-infrared reflectance spectroscopic analysis of soil C and N. Soil Sci. 2002, 167, 110–116. [Google Scholar] [CrossRef]
- Cozzolino, D.; Moron, A. The potential of near-infrared reflectance spectroscopy to analyse soil chemical and physical characteristics. J. Agric. Sci. 2003, 140, 65–71. [Google Scholar] [CrossRef]
- Terra, F.S.; Dematte, J.A.M.; Viscarra Rossel, R.A. Proximal spectral sensing in pedological assessments: Vis-NIR spectra for soil classification based on weathering and pedogenesis. Geoderma 2018, 318, 123–136. [Google Scholar] [CrossRef]
- Janik, L.J.; Merry, R.H.; Skjemstad, J.O. Can mid infra-red diffuse reflectance analysis replace soil extractions? Aust. J. Exp. Agric. 1998, 38, 681–696. [Google Scholar] [CrossRef]
- Reeves, J.B., III. Near- versus mid-infrared diffuse reflectance spectroscopy for soil analysis emphasizing carbon and laboratory versus on-site analysis: Where are we and what needs to be done? Geoderma 2010, 158, 3–14. [Google Scholar] [CrossRef]
- Terra, F.S.; Dematte, J.A.M.; Viscarra Rossel, R.A. Spectral libraries for quantitative analyses of tropical Brazilian soils: Comparing vis-NIR and mid-IR reflectance data. Geoderma 2015, 255, 81–93. [Google Scholar] [CrossRef]
- Wang, S.; Li, W.; Li, J.; Liu, X. Prediction of soil texture using FT-NIR spectroscopy and PXRF spectrometry with data fusion. Soil Sci. 2013, 178, 626–638. [Google Scholar] [CrossRef]
- Aldabaa, A.A.A.; Weindorf, D.C.; Chakraborty, S.; Sharma, A.; Li, B. Combination of proximal and remote sensing methods for rapid soil salinity quantification. Geoderma 2015, 239, 34–46. [Google Scholar] [CrossRef] [Green Version]
- O’Rourke, S.M.; Stockmann, U.; Holden, N.M.; McBratney, A.B.; Minasny, B. An assessment of model averaging to improve predictive power of portable vis-NIR and XRF for the determination of agronomic soil properties. Geoderma 2016, 279, 31–44. [Google Scholar] [CrossRef]
- Xu, D.; Chen, S.; Viscarra Rossel, R.A.; Biswas, A.; Li, S.; Zhou, Y.; Shi, Z. X-ray fluorescence and visible near infrared sensor fusion for predicting soil chromium content. Geoderma 2019, 352, 61–69. [Google Scholar] [CrossRef]
- Xu, D.; Zhao, R.; Li, S.; Chen, S.; Jiang, Q.; Zhou, L.; Shi, Z. Multi-sensor fusion for the determination of several soil properties in the Yangtze River Delta, China. Eur. J. Soil Sci. 2019, 70, 162–173. [Google Scholar] [CrossRef] [Green Version]
- Wang, D.; Chakraborty, S.; Weindorf, D.C.; Li, B.; Sharma, A.; Paul, S.; Ali, M.N. Synthesized use of VisNIR DRS and PXRF for soil characterization: Total carbon and total nitrogen. Geoderma 2015, 243, 157–167. [Google Scholar] [CrossRef]
- Terra, F.S.; Viscarra Rossel, R.A.; Dematte, J.A.M. Spectral fusion by Outer Product Analysis (OPA) to improve predictions of soil organic C. Geoderma 2019, 335, 35–46. [Google Scholar] [CrossRef]
- Chakraborty, S.; Weindorf, D.C.; Li, B.; Ali Aldabaa, A.A.; Ghosh, R.K.; Paul, S.; Nasim Ali, M. Development of a hybrid proximal sensing method for rapid identification of petroleum contaminated soils. Sci. Total Environ. 2015, 514, 399–408. [Google Scholar] [CrossRef] [PubMed]
- O’Rourke, S.M.; Minasny, B.; Holden, N.M.; McBratney, A.B. Synergistic use of vis-NIR, MIR, and XRF spectroscopy for the determination of soil geochemistry. Soil Sci. Soc. Am. J. 2016, 80, 888–899. [Google Scholar] [CrossRef] [Green Version]
- Ji, W.; Adamchuk, V.I.; Chen, S.; Su, A.S.M.; Ismail, A.; Gan, Q.; Shi, Z.; Biswas, A. Simultaneous measurement of multiple soil properties through proximal sensor data fusion: A case study. Geoderma 2019, 341, 111–128. [Google Scholar] [CrossRef]
- Condit, H.R. The spectral reflectance of American soils. Photogramm. Eng. 1970, 36, 955–966. [Google Scholar]
- Cipra, J.E.; Stoner, E.R.; Baumgardner, M.F.; Macdonald, R.B. Measuring radiance characteristics of soil with a spectroradiometer. Soil Sci. Soc. Am. Proc. 1971, 35, 1014–1017. [Google Scholar] [CrossRef]
- Stoner, E.R.; Baumgardner, M.F. Characteristic variations in reflectance of surface soils. Soil Sci. Soc. Am. J. 1981, 45, 1161–1165. [Google Scholar] [CrossRef] [Green Version]
- Wang, R.C.; Su, H.P.; Wang, S.F. Studies on spectral reflectance of typical soils and its fuzzy category in soil classification in Zhejiang province. J. Zhejiang Univ. Sci. B 1986, 12, 464–471. [Google Scholar]
- Xu, B.B. Research of China’s soil spectral line. J. Remote Sens. 1991, 1, 61–71. [Google Scholar]
- Ji, W.J.; Shi, Z.; Zhou, Q.; Zhou, L.Q. VIS-NIR reflectance spectroscopy of the organic matter in several types of soils. J. Infrared Millim. Terahertz Waves 2012, 31, 277–282. [Google Scholar] [CrossRef]
- Linker, R. Soil classification via mid-infrared spectroscopy. In Computer and Computing Technologies in Agriculture; Springer: New York, NY, USA, 2008; Volume 259, pp. 1137–1146. [Google Scholar]
- Mouazen, A.M.; Karoui, R.; De Baerdemaeker, J.; Ramon, H. Classification of soil texture classes by using soil visual near infrared spectroscopy and factorial discriminant analysis techniques. J. Near Infrared Spectrosc. 2005, 13, 231–240. [Google Scholar] [CrossRef]
- Oliveira, J.F.; Brossard, M.; Siqueira Vendrame, P.R.; Mayi, S., III; Corazza, E.J.; Marchao, R.L.; Guimaraes, M.d.F. Soil discrimination using diffuse reflectance Vis-NIR spectroscopy in a local toposequence. Comptes Rendus Geosci. 2013, 345, 446–453. [Google Scholar] [CrossRef]
- Teng, H.; Viscarra Rossel, R.A.; Shi, Z.; Behrens, T. Updating a national soil classification with spectroscopic predictions and digital soil mapping. Catena 2018, 164, 125–134. [Google Scholar] [CrossRef]
- Viscarra Rossel, R.A.; Webster, R. Discrimination of Australian soil horizons and classes from their visible-near infrared spectra. Eur. J. Soil Sci. 2011, 62, 637–647. [Google Scholar] [CrossRef]
- Chen, S.; Li, S.; Ma, W.; Ji, W.; Xu, D.; Shi, Z.; Zhang, G. Rapid determination of soil classes in soil profiles using vis-NIR spectroscopy and multiple objectives mixed support vector classification. Eur. J. Soil Sci. 2019, 70, 42–53. [Google Scholar] [CrossRef] [Green Version]
- Dotto, A.; Demattê, J.; Viscarra Rossel, R.A.; Rizzo, R. Soil classification based on spectral and environmental variables. SOIL Dis. 2019, 1–20. [Google Scholar] [CrossRef]
- Shi, X.Z.; Yu, D.S.; Yang, G.X.; Wang, H.J.; Sun, W.X.; Du, G.H.; Gong, Z.T. Cross-reference benchmarks for translating the Genetic Soil Classification of China into the Chinese Soil Taxonomy. Pedosphere 2006, 16, 147–153. [Google Scholar] [CrossRef]
- IUSS Working Group WRB. World Reference Base for Soil Resources 2014, Update 2015 International Soil Classification System for Naming Soils and Creating Legends for Soil Maps. World Soil Resour. Rep. 2015, 106, 192. [Google Scholar]
- Xu, D.; Ma, W.; Chen, S.; Jiang, Q.; He, K.; Shi, Z. Assessment of important soil properties related to Chinese Soil Taxonomy based on vis-NIR reflectance spectroscopy. Comput. Electron. Agric. 2018, 144, 1–8. [Google Scholar] [CrossRef]
- Institute of Soil Science, Chinese Academy of Sciences. Chinese Soil Taxonomy, 1st ed.; China Agricultural Science and Technology Press: Beijing, China, 1995; pp. 19–37. [Google Scholar]
- Beaudette, D.E.; Roudier, P.; O’Geen, A.T. Algorithms for quantitative pedology: A toolkit for soil scientists. Comput. Geosci. 2013, 52, 258–268. [Google Scholar] [CrossRef]
- The R Development Core Team. In R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2015.
- Barros, A.S.; Safar, M.; Devaux, M.F.; Robert, P.; Bertrand, D.; Rutledge, D.N. Relations between mid-infrared and near-infrared spectra detected by analysis of variance of an intervariable data matrix. Appl. Spectrosc. 1997, 51, 1384–1393. [Google Scholar] [CrossRef]
- Jaillais, B.; Ottenhof, M.A.; Farhat, I.A.; Rutledge, D.N. Outer-product analysis (OPA) using PLS regression to study the retrogradation of starch. Vib. Spectrosc. 2006, 40, 10–19. [Google Scholar] [CrossRef]
- Joachims, T. Text categorization with support vector machines: Learning with many relevant features. In Proceedings of the European Conference on Machine Learning, Berlin/Heidelberg, Germany, 21–23 April 1998; Springer: Berlin/Heidelberg, Germany. [Google Scholar]
- Hsu, C.W.; Lin, C.J. A comparison of methods for multiclass support vector machines. IEEE Trans. Neural Netw. 2002, 13, 415–425. [Google Scholar] [PubMed] [Green Version]
- Li, S.; Ji, W.; Chen, S.; Peng, J.; Zhou, Y.; Shi, Z. Potential of VIS-NIR-SWIR spectroscopy from the Chinese soil spectral library for assessment of nitrogen fertilization rates in the paddy-rice region, China. Remote Sens. 2015, 7, 7029–7043. [Google Scholar] [CrossRef] [Green Version]
- Chen, S.C.; Peng, J.; Ji, W.J.; Zhou, Y.; He, J.X.; Shi, Z. Study on the characterization of VNIR-MIR spectra and prediction of soil organic matter in paddy soil. Spectrosc. Spect. Anal. 2016, 36, 1712–1716. [Google Scholar]
- Borras, E.; Ferre, J.; Boque, R.; Mestres, M.; Acena, L.; Busto, O. Data fusion methodologies for food and beverage authentication and quality assessment—A review. Anal. Chim. Acta 2015, 891, 1–14. [Google Scholar] [CrossRef]
- Knox, N.M.; Grunwald, S.; McDowell, M.L.; Bruland, G.L.; Myers, D.B.; Harris, W.G. Modelling soil carbon fractions with visible near-infrared (VNIR) and mid-infrared (MIR) spectroscopy. Geoderma 2015, 239, 229–239. [Google Scholar] [CrossRef]
- Chawla, N.; Bowyer, K.; Hall, L.; Kegelmeyer, W. SMOTE: Synthetic minority over-sampling technique. J. Artif. Intell. Res. 2002, 16, 321–357. [Google Scholar] [CrossRef]
- Viscarra Rossel, R.A.; Behrens, T.; Ben-Dor, E.; Brown, D.J.; Dematte, J.A.M.; Shepherd, K.D.; Shi, Z.; Stenberg, B.; Stevens, A.; Adamchuk, V.; et al. A global spectral library to characterize the world’s soil. Earth-Sci. Rev. 2016, 155, 198–230. [Google Scholar] [CrossRef] [Green Version]
- Stevens, A.; Nocita, M.; Toth, G.; Montanarella, L.; van Wesemael, B. Prediction of soil organic carbon at the european scale by visible and near infrared reflectance spectroscopy. PLoS ONE 2013, 8, 13. [Google Scholar] [CrossRef]
- Huang, C.Y.; Xu, J.M. Pedology, 3rd ed.; China Agriculture Press: Beijing, China, 2011; pp. 80–107, 293–308. [Google Scholar]
- Chen, L.M.; Zhang, G.L. Soil chronosequences and the significance in the study of pedogenesis. Acta Pedol. Sin. 2011, 48, 421–428. [Google Scholar]








| Soil Order | Soil Suborder | Numbers of Samples |
|---|---|---|
| Anthrosol | Stagnic Anthrosol | 54 |
| Orthic Anthrosol | 2 | |
| Halosol | Orthic Halosol | 2 |
| Gleyosol | Stagnic Gleyosol | 1 |
| Isohumosol | Udic Isohumosol | 2 |
| Ferrosol | Udic Ferrosol | 4 |
| Argosol | Udic Argosol | 21 |
| Cambosol | Aquic Cambosol | 16 |
| Perudic Cambosol | 6 | |
| Udic Cambosol | 24 | |
| Primosol | Alluvic Primosol | 6 |
| Orthic Primosol | 6 | |
| Anthric Primosol | 1 |
| Chinese Soil Taxonomy | World Reference Base |
|---|---|
| Cambosols | Cambisols |
| Argosols | Luvisols, Alisols |
| Gleyosols | Gleysols, Cryosols |
| Primosols | Fluvisols, Leptisols, Arenosols, Regosols, Cryosols |
| Halosols | Solonchaks, Solonetz |
| Isohumosols | Chernozems, Kastanozems, Phaeozems |
| Anthrosols | Anthrosols |
| Ferrosols | Acrisols, Lixisols, Plinthosols, Nitisols |
| Method | PCs Selection | Contribution Rate/% | Optimal Parameters | Total Accuracy | ||
|---|---|---|---|---|---|---|
| Gamma | Cost | Calibration | Validation | |||
| Vis-NIR | PC1 to PC20 | 99.8 | 0.025 | 1 | 0.94 | 0.642 |
| MIR | PC1 to PC40 | 99.76 | 0.0125 | 1.5 | 0.951 | 0.645 |
| Combination | PC1 to PC50 | 99.5 | 0.025 | 2.5 | 1 | 0.611 |
| OPA | PC1 to PC20 | 98.99 | 0.0625 | 0.5 | 0.889 | 0.684 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xu, H.; Xu, D.; Chen, S.; Ma, W.; Shi, Z. Rapid Determination of Soil Class Based on Visible-Near Infrared, Mid-Infrared Spectroscopy and Data Fusion. Remote Sens. 2020, 12, 1512. https://doi.org/10.3390/rs12091512
Xu H, Xu D, Chen S, Ma W, Shi Z. Rapid Determination of Soil Class Based on Visible-Near Infrared, Mid-Infrared Spectroscopy and Data Fusion. Remote Sensing. 2020; 12(9):1512. https://doi.org/10.3390/rs12091512
Chicago/Turabian StyleXu, Hanyi, Dongyun Xu, Songchao Chen, Wanzhu Ma, and Zhou Shi. 2020. "Rapid Determination of Soil Class Based on Visible-Near Infrared, Mid-Infrared Spectroscopy and Data Fusion" Remote Sensing 12, no. 9: 1512. https://doi.org/10.3390/rs12091512