Chlorophyll-a and Sea Surface Temperature Changes in Relation to Paralytic Shellfish Toxin Production off the East Coast of Tasmania, Australia
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Area
2.2. Datasets
2.3. Temporal and Spatial Analyses
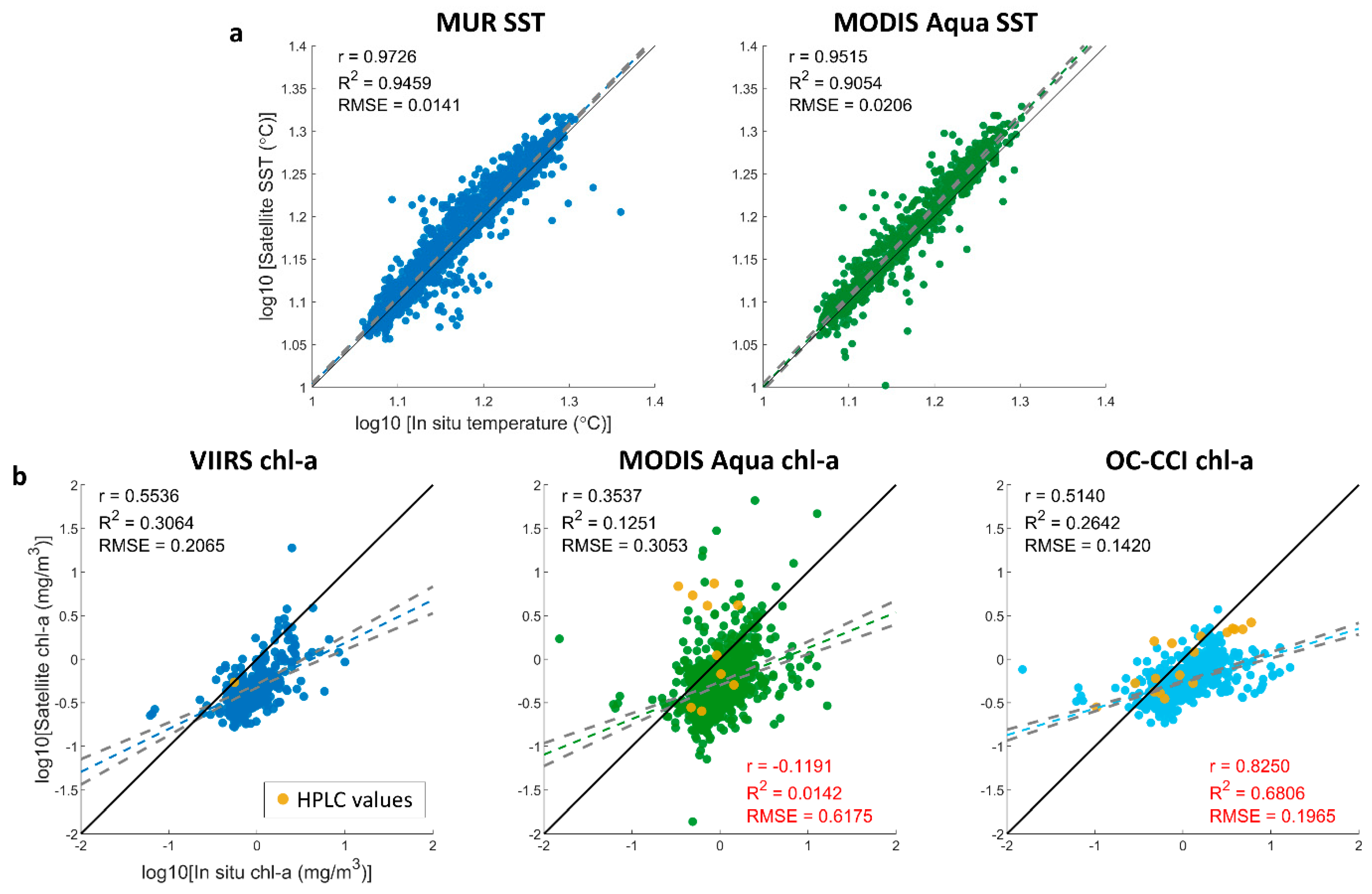
2.4. Chl-a and SST Satellite Validation
3. Results
3.1. Paralytic Shellfish Toxins
3.2. Sea Surface Temperature Spatial and Temporal Analyses
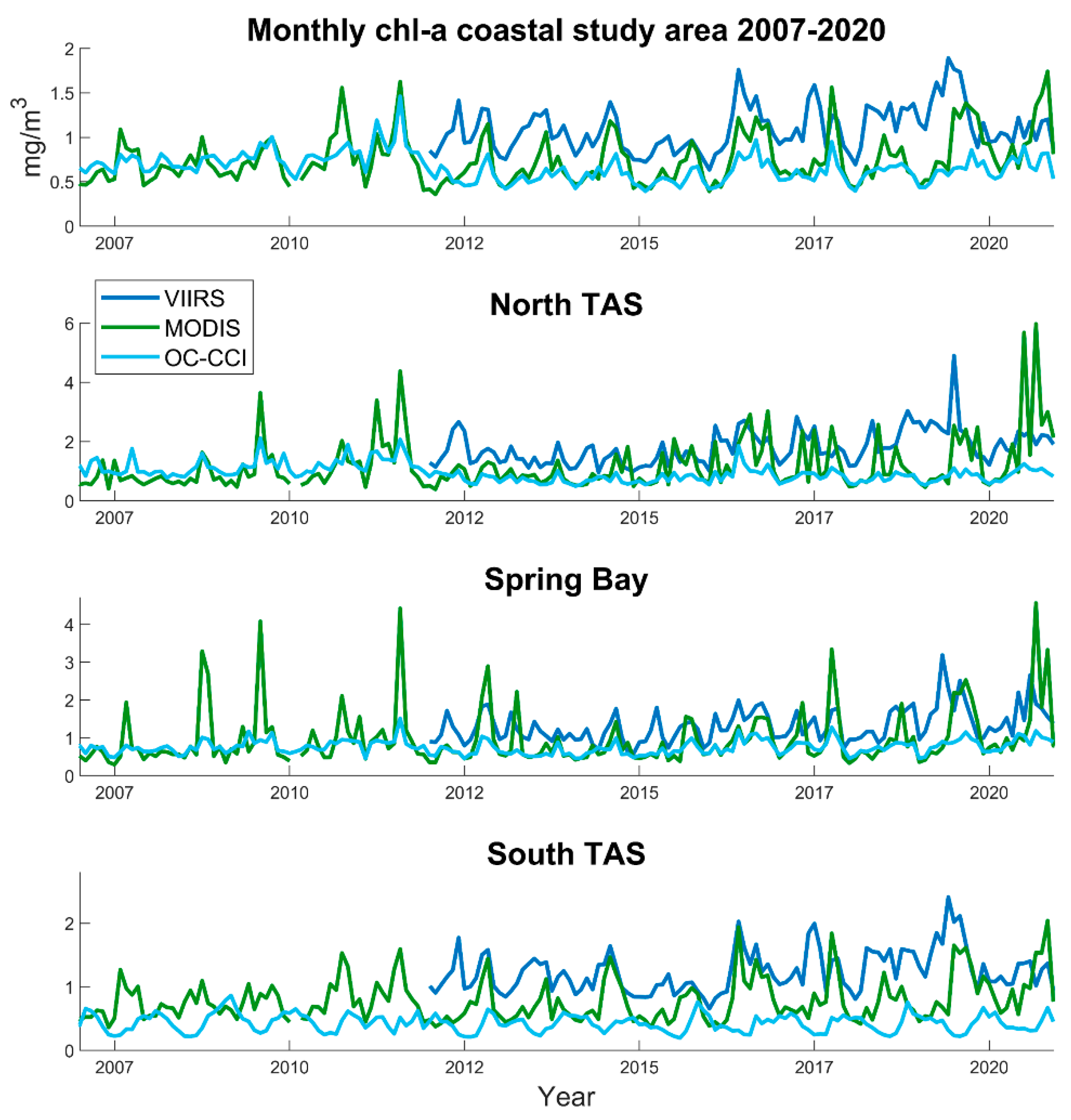
3.3. Chlorophyll-a Spatial Anomalies and Temporal Analyses
3.4. Relationships between SST, Chl-a, and Toxin Data
3.5. Sensor Validation
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Hallegraeff, G.M.; Bolch, C.; Campbell, K.; Condie, S.; Dorantes-Aranda, J.; Murray, S.; Turnbull, A.; Ugalde, S. Improved Understanding of Tasmanian Harmful Algal Blooms and Biotoxin Events to Support Seafood Risk Management; Fisheries Research and Development Corporation: Deakin, ACT, Australia, 2018; ISBN 1925646084. [Google Scholar]
- Condie, S.A.; Oliver, E.C.J.; Hallegraeff, G.M. Environmental drivers of unprecedented Alexandrium catenella dinoflagellate blooms off eastern Tasmania, 2012–2018. Harmful Algae 2019, 87, 101628. [Google Scholar] [CrossRef] [PubMed]
- Hallegraeff, G.M.; Schweibold, L.; Jaffrezic, E.; Rhodes, L.; MacKenzie, L.; Hay, B.; Farrell, H. Overview of Australian and New Zealand harmful algal species occurrences and their societal impacts in the period 1985 to 2018, including a compilation of historic records. Harmful Algae 2020, 102, 101848. [Google Scholar] [CrossRef] [PubMed]
- Xie, H.; Fischer, A.M.; Strutton, P.G. Generalized linear models to assess environmental drivers of paralytic shellfish toxin blooms (Southeast Tasmania, Australia). Cont. Shelf Res. 2021, 223, 104439. [Google Scholar] [CrossRef]
- Oliver, E.C.J.; Lago, V.; Hobday, A.J.; Holbrook, N.J.; Ling, S.D.; Mundy, C.N. Marine heatwaves off eastern Tasmania: Trends, interannual variability, and predictability. Prog. Oceanogr. 2018, 161, 116–130. [Google Scholar] [CrossRef]
- Grebner, W.; Berglund, E.C.; Berggren, F.; Eklund, J.; Harðadóttir, S.; Andersson, M.X.; Selander, E. Induction of defensive traits in marine plankton—New copepodamide structures. Limnol. Oceanogr. 2019, 64, 820–831. [Google Scholar] [CrossRef]
- Lundholm, N.; Krock, B.; John, U.; Skov, J.; Cheng, J.; Pančić, M.; Wohlrab, S.; Rigby, K.; Nielsen, T.G.; Selander, E.; et al. Induction of domoic acid production in diatoms—Types of grazers and diatoms are important. Harmful Algae 2018, 79, 64–73. [Google Scholar] [CrossRef] [PubMed]
- Selander, E.; Thor, P.; Toth, G.; Pavia, H. Copepods induce paralytic shellfish toxin production in marine dinoflagellates. Proc. R. Soc. B Biol. Sci. 2006, 273, 1673–1680. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Selander, E.; Fagerberg, T.; Wohlrab, S.; Pavia, H. Fight and flight in dinoflagellates? Kinetics of simultaneous grazer-induced responses in Alexandrium tamarense. Limnol. Oceanogr. 2011, 57, 58–64. [Google Scholar] [CrossRef]
- Kelly, P.; Clementson, L.; Davies, C.; Corney, S.; Swadling, K. Zooplankton responses to increasing sea surface temperatures in the southeastern Australia global marine hotspot. Estuar. Coast. Shelf Sci. 2016, 180, 242–257. [Google Scholar] [CrossRef]
- Thompson, P.A.; Baird, M.E.; Ingleton, T.; Doblin, M.A. Long-term changes in temperate Australian coastal waters: Implications for phytoplankton. Mar. Ecol. Prog. Ser. 2009, 394, 1–19. [Google Scholar] [CrossRef] [Green Version]
- Thompson, P.A.; Bonham, P.; Thomson, P.; Rochester, W.; Doblin, M.A.; Waite, A.M.; Richardson, A.; Rousseaux, C.S. Climate variability drives plankton community composition changes: The 2010–2011 El Niño to La Niña transition around Australia. J. Plankton Res. 2015, 37, 966–984. [Google Scholar] [CrossRef] [Green Version]
- Evans, R.; Lea, M.A.; Hindell, M.A.; Swadling, K.M. Significant shifts in coastal zooplankton populations through the 2015/16 Tasman Sea marine heatwave. Estuar. Coast. Shelf Sci. 2020, 235, 106538. [Google Scholar] [CrossRef]
- Oliver, E.C.J.; Benthuysen, J.A.; Bindoff, N.L.; Hobday, A.J.; Holbrook, N.J.; Mundy, C.N.; Perkins-Kirkpatrick, S.E. Theunprecedented 2015/16 Tasman Sea marine heatwave. Nat. Commun. 2017, 8, 16101. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sathyendranath, S.; Brewin, B.; Mueller, D.; Doerffer, R.; Krasemann, H.; Melin, F.; Brockmann, C.; Fomferra, N.; Peters, M.; Grant, M.; et al. Ocean Colour Climate Change Initiative—Approach and initial results. Int. Geosci. Remote Sens. Symp. 2012, 2024–2027. [Google Scholar] [CrossRef]
- Chin, T.M.; Vazquez-cuervo, J.; Armstrong, E.M. Remote Sensing of Environment A multi-scale high-resolution analysis of global sea surface temperature. Remote Sens. Environ. 2017, 200, 154–169. [Google Scholar] [CrossRef]
- Davies, C.; Sommerville, E.; Hidas, M.; Suthers, I.; Lara-Lopez, A.; Matis, P.; Van Derk Kamp, J.; Tibben, S.; Abell, G.; Allen, S.; et al. National Reference Stations Biogeochemical Operations Manual—Version 3.3.1; Integrated Marine Observing System: Hobart, Australia, 2020. [Google Scholar]
- Burgoyne, M.; Mills, A. Tasmanian Shellfish Market Access Program (ShellMAP) Biotoxin Management Plan Version 5.1; Department of Primary Industries, Parks, Water and Environment, Tasmanian Government: Hobart, Australia, 2019. [Google Scholar]
- Krasemann, H.; Müller, D.; Mélin, F.; Valente, A.; Grant, M. Product Validation and Inter-Comparison Report; Plymouth Marine Laboratory: Plymouth, UK, 2017. [Google Scholar]
- Aguilera-Belmonte, A.; Inostroza, I.; Franco, J.M.; Riobó, P.; Gómez, P.I. The growth, toxicity and genetic characterization of seven strains of Alexandrium catenella (Whedon and Kofoid) Balech 1985 (Dinophyceae) isolated during the 2009 summer outbreak in southern Chile. Harmful Algae 2011, 12, 105–112. [Google Scholar] [CrossRef]
- Navarro, J.M.; Muñoz, M.G.; Contreras, A.M. Temperature as a factor regulating growth and toxin content in the dinoflagellate Alexandrium catenella. Harmful Algae 2006, 5, 762–769. [Google Scholar] [CrossRef]
- Etheridge, S.M.; Roesler, C.S. Effects of temperature, irradiance, and salinity on photosynthesis, growth rates, total toxicity, and toxin composition for Alexandrium fundyense isolates from the Gulf of Maine and Bay of Fundy. Deep Sea Res. Part II Top. Stud. Oceanogr. 2005, 52, 2491–2500. [Google Scholar] [CrossRef]
- Bracher, A.; Bouman, H.A.; Brewin, R.J.W.; Bricaud, A.; Brotas, V.; Ciotti, A.M.; Clementson, L.; Devred, E.; Di Cicco, A.; Dutkiewicz, S.; et al. Obtaining phytoplankton diversity from ocean color: A scientific roadmap for future development. Front. Mar. Sci. 2017, 4, 55. [Google Scholar] [CrossRef] [Green Version]
- Hirata, T.; Hardman-Mountford, N.J.; Brewin, R.J.W.; Aiken, J.; Barlow, R.; Suzuki, K.; Isada, T.; Howell, E.; Hashioka, T.; Noguchi-Aita, M.; et al. Synoptic relationships between surface Chlorophyll-a and diagnostic pigments specific to phytoplankton functional types. Biogeosciences 2011, 8, 311–327. [Google Scholar] [CrossRef] [Green Version]
- Ajani, P.A.; Hallegraeff, G.M.; Allen, D.; Coughlan, A.; Richardson, A.J.; Armand, L.K.; Ingleton, T.; Murray, S.A. Establishing Baselines: Eighty Years of Phytoplankton Diversity and Biomass in South-Eastern Australia. Oceanogr. Mar. Biol. Annu. Rev. 2016, 54, 395–420. [Google Scholar] [CrossRef]
- Smith, R.C. Remote Sensing and Depth Distribution of Ocean Chlorophyll on JSTOR. Mar. Ecol. Prog. Ser. 1981, 5, 359–361. [Google Scholar] [CrossRef]
- Chekalyuk, A.; Hafez, M. Photo-physiological variability in phytoplankton chlorophyll fluorescence and assessment of chlorophyll concentration. Opt. Express 2011, 19, 22643. [Google Scholar] [CrossRef] [PubMed]
- Herraiz-Borreguero, L.; Rintoul, S.R. Regional circulation and its impact on upper ocean variability south of Tasmania. Deep. Res. Part II Top. Stud. Oceanogr. 2011, 58, 2071–2081. [Google Scholar] [CrossRef]
- Westwood, K.J.; Brian Griffiths, F.; Webb, J.P.; Wright, S.W. Primary production in the Sub-Antarctic and Polar Frontal Zones south of Tasmania, Australia; SAZ-Sense survey, 2007. Deep Sea Res. Part II Top. Stud. Oceanogr. 2011, 58, 2162–2178. [Google Scholar] [CrossRef] [Green Version]
- Herzfeld, M.; Andrewartha, J.; Sakov, P. Modelling the physical oceanography of the D’Entrecasteaux Channel and the Huon Estuary, south-eastern Tasmania. Mar. Freshw. Res. 2010, 61, 568–586. [Google Scholar] [CrossRef]
- Cherukuru, N.; Brando, V.E.; Schroeder, T.; Clementson, L.A.; Dekker, A.G. Influence of river discharge and ocean currents on coastal optical properties. Cont. Shelf Res. 2014, 84, 188–203. [Google Scholar] [CrossRef]
- Kahru, M.; Anderson, C.; Barton, A.D.; Carter, M.L.; Catlett, D.; Send, U.; Sosik, H.M.; Weiss, E.L.; Mitchell, B.G. Satellite detection of dinoflagellate blooms off California by UV reflectance ratios. Elem. Sci. Anthr. 2021, 9, 00157. [Google Scholar] [CrossRef]
- Werdell, P.J.; Behrenfeld, M.J.; Bontempi, P.S.; Boss, E.; Cairns, B.; Davis, G.T.; Franz, B.A.; Gliese, U.B.; Gorman, E.T.; Hasekamp, O.; et al. The Plankton, Aerosol, Cloud, Ocean Ecosystem Mission: Status, Science, Advances. Bull. Am. Meteorol. Soc. 2019, 100, 1775–1794. [Google Scholar] [CrossRef]
- Trapp, A.; Heuschele, J.; Selander, E. Eavesdropping on plankton—Can zooplankton monitoring improve forecasting of biotoxins from harmful algae blooms? Limnol. Oceanogr. 2021, 66, 3455–3471. [Google Scholar] [CrossRef]

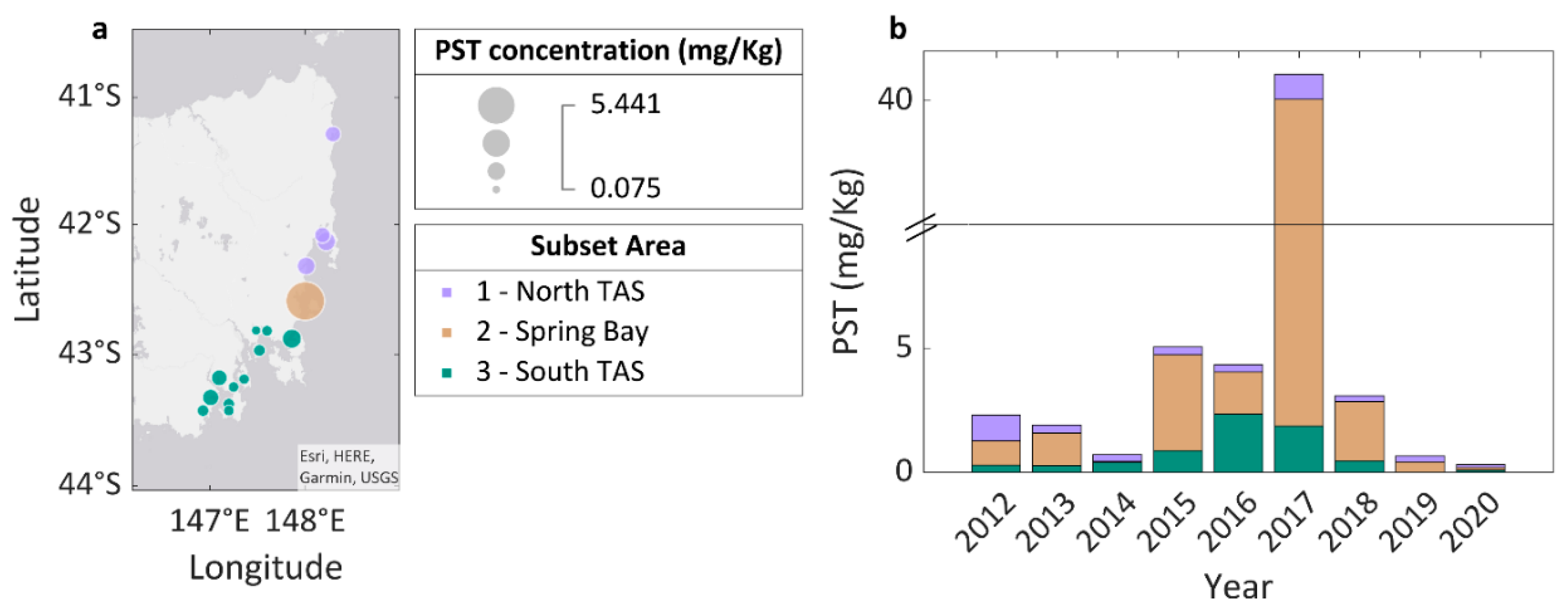
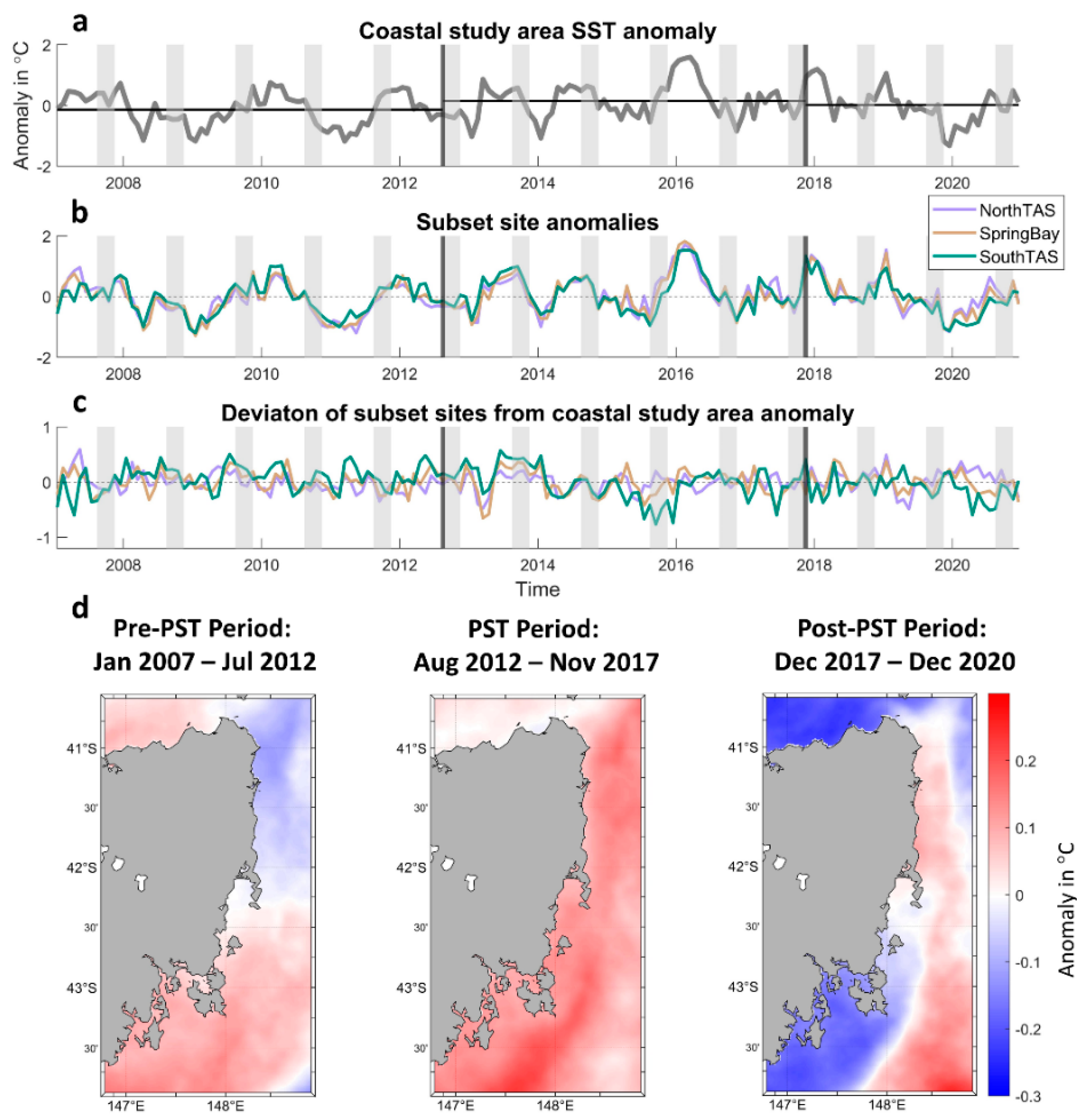
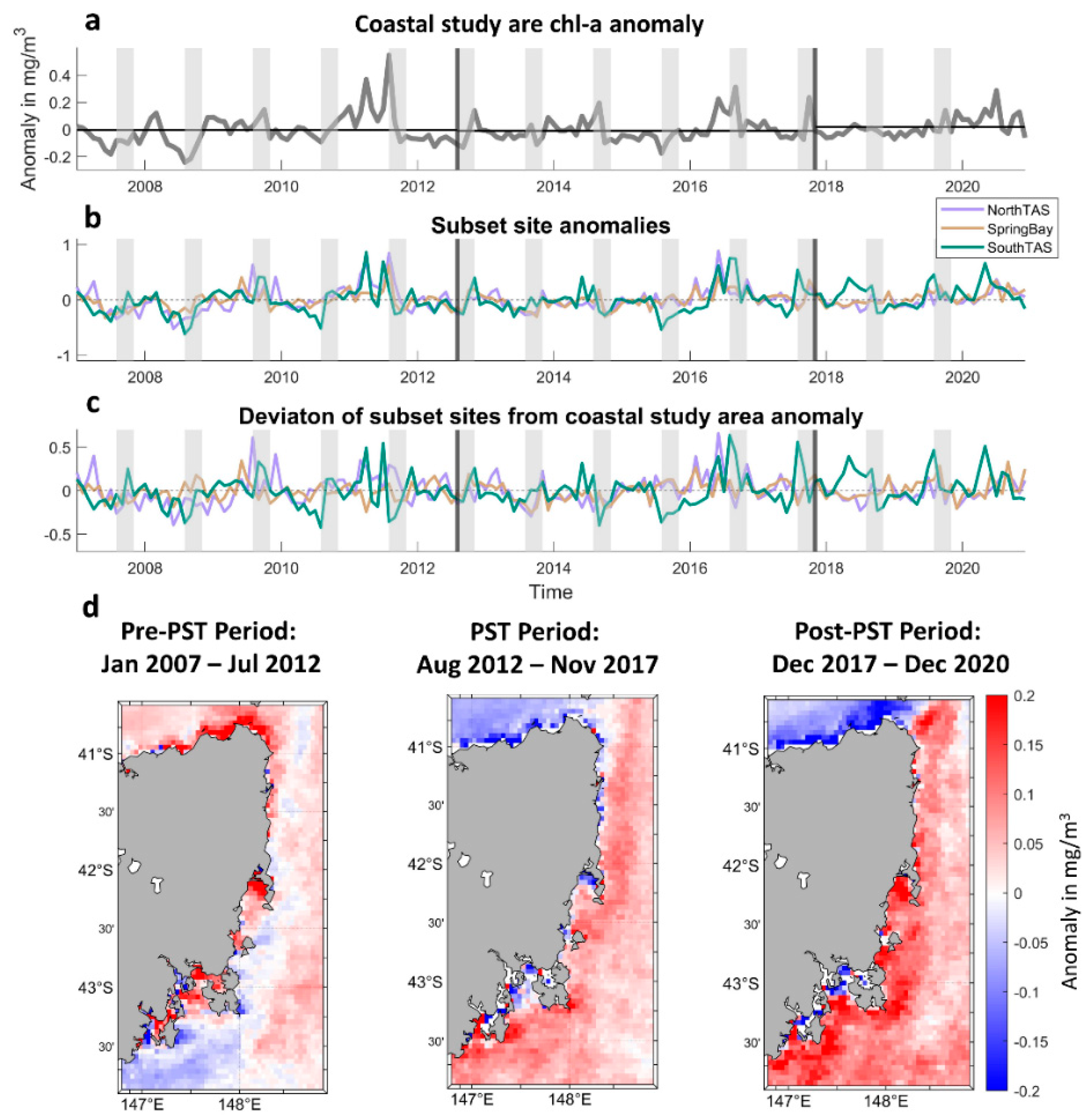
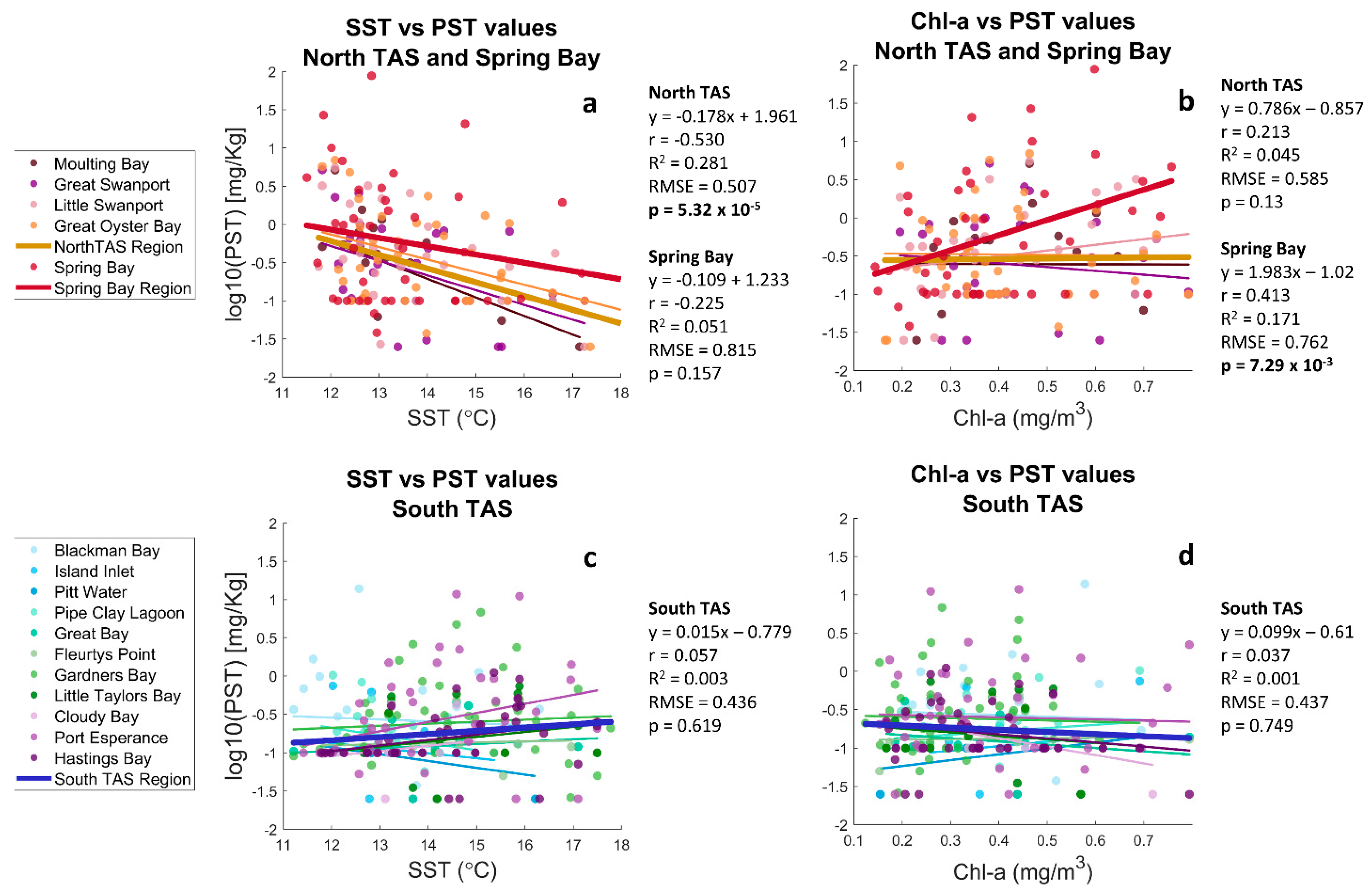
| Category | North TAS | Spring Bay | South TAS |
|---|---|---|---|
| Marine Farms | Moulting Bay, Great Swanport, Little Swanport, Great Oyster Bay | Spring Bay | Blackman Bay, Pitt Water, Island Inlet, Pipe Clay Lagoon, Great Bay, Fleurty’s Point, Gardner’s Bay, Little Taylor’s Bay, Cloudy Bay, Port Esperance, Hastings Bay |
| Total | 4 | 1 | 11 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wakamatsu, L.; Britten, G.L.; Styles, E.J.; Fischer, A.M. Chlorophyll-a and Sea Surface Temperature Changes in Relation to Paralytic Shellfish Toxin Production off the East Coast of Tasmania, Australia. Remote Sens. 2022, 14, 665. https://doi.org/10.3390/rs14030665
Wakamatsu L, Britten GL, Styles EJ, Fischer AM. Chlorophyll-a and Sea Surface Temperature Changes in Relation to Paralytic Shellfish Toxin Production off the East Coast of Tasmania, Australia. Remote Sensing. 2022; 14(3):665. https://doi.org/10.3390/rs14030665
Chicago/Turabian StyleWakamatsu, Lael, Gregory L. Britten, Elliot J. Styles, and Andrew M. Fischer. 2022. "Chlorophyll-a and Sea Surface Temperature Changes in Relation to Paralytic Shellfish Toxin Production off the East Coast of Tasmania, Australia" Remote Sensing 14, no. 3: 665. https://doi.org/10.3390/rs14030665







