Mapping Leaf Area Index at Various Rice Growth Stages in Southern India Using Airborne Hyperspectral Remote Sensing
Abstract
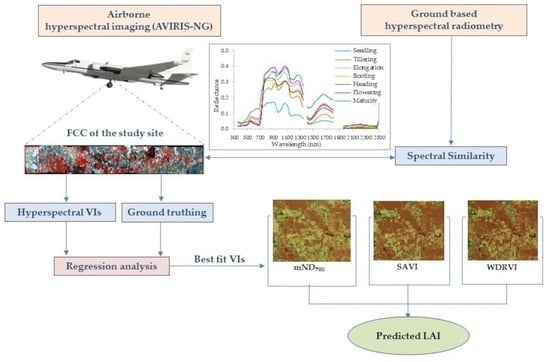
:1. Introduction
2. Materials and Methods
2.1. Study Area
2.2. Sampling Sites and Field Data Collection
2.3. Sampling of LAI
2.4. Canopy Spectral Reflectance Measurements
2.5. AVIRIS-NG Airborne Data Acquisition
2.6. Data Processing
2.7. Data Analysis
3. Results
3.1. Reflectance Spectra of Rice Canopy from Hand Held Hyperspectral Radiometry
3.2. Reflectance Spectra of Rice Canopy from AVIRIS-NG
3.3. Rice LAI at Different Phenological Stages
3.4. Relationship of Vegetation Indices to Rice Phenological Stages
3.5. Evaluation of Vegetation Indices for Rice LAI Estimation
3.6. Validation of VIs for Estimation of LAI
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A

| VI | Seedling | Tillering | Elongation | Booting | Heading | Flowering | Maturity |
|---|---|---|---|---|---|---|---|
| WI | 1.80 ± 0.93 | 1.63 ± 0.69 | 1.24 ± 0.19 | 1.22 ± 0.18 | 1.18 ± 0.02 | 1.13 ± 0.03 | 1.07 ± 0.07 |
| NDWI | 0.26 ± 0.28 | 0.26 ± 0.20 | 0.14 ± 0.11 | 0.14 ± 0.08 | 0.15 ± 0.01 | 0.11 ± 0.03 | 0.04 ± 0.07 |
| NDII | 0.56 ± 0.20 | 0.54 ± 0.17 | 0.43 ± 0.11 | 0.44 ± 0.06 | 0.51 ± 0.01 | 0.46 ± 0.04 | 0.29 ± 0.14 |
| SLAIDI | 0.01 ± 0.00 | 0.01 ± 0.00 | 0.01 ± 0.00 | 0.01 ± 0.00 | 0.01 ± 0.00 | 0.01 ± 0.00 | 0.01 ± 0.00 |
| NDVI | 0.54 ± 0.14 | 0.64 ± 0.08 | 0.80 ± 0.08 | 0.82 ± 0.08 | 0.91 ± 0.02 | 0.84 ± 0.03 | 0.53 ± 0.21 |
| OSAVI | 0.38 ± 0.10 | 0.44 ± 0.07 | 0.58 ± 0.07 | 0.62 ± 0.08 | 0.74 ± 0.04 | 0.67 ± 0.04 | 0.43 ± 0.15 |
| GNDVI | 0.41 ± 0.09 | 0.49 ± 0.07 | 0.64 ± 0.08 | 0.67 ± 0.09 | 0.78 ± 0.02 | 0.71 ± 0.03 | 0.53 ± 0.11 |
| RVSI | −0.01 ± 0.00 | −0.01 ± 0.00 | −0.01 ± 0.00 | −0.01 ± 0.01 | 0.01 ± 0.00 | −0.00 ± 0.00 | −0.01 ± 0.00 |
| REP | 735.60 ± 64.56 | 728.59 ± 8.77 | 725.35 ± 1.64 | 725.43 ± 2.45 | 729.34 ± 0.54 | 727.22 ± 1.09 | 742.50 ± 16.60 |
| SR | 3.78 ± 1.43 | 4.89 ± 1.31 | 10.64 ± 4.44 | 12.01 ± 4.70 | 20.79 ± 3.97 | 11.82 ± 2.88 | 4.28 ± 2.68 |
| RDVI | 0.27 ± 0.07 | 0.31 ± 0.05 | 0.42 ± 0.06 | 0.45 ± 0.06 | 0.56 ± 0.04 | 0.50 ± 0.03 | 0.33 ± 0.11 |
| SAVI | 0.82 ± 0.21 | 0.97 ± 0.13 | 1.20 ± 0.12 | 1.23 ± 0.12 | 1.36 ± 0.03 | 1.26 ± 0.05 | 0.80 ± 0.32 |
| MSR | 0.91 ± 0.37 | 1.19 ± 0.30 | 2.19 ± 0.70 | 2.40 ± 0.70 | 3.54 ± 0.43 | 2.42 ± 0.41 | 0.98 ± 0.65 |
| TVI | 7.15 ± 2.86 | 8.00 ± 2.18 | 12.01 ± 2.47 | 13.69 ± 2.33 | 17.95 ± 2.07 | 15.94 ± 1.54 | 9.75 ± 4.18 |
| MNLI | −0.41 ± 0.05 | −0.40 ± 0.05 | −0.47 ± 0.05 | −0.50 ± 0.04 | −0.60 ± 0.04 | −0.57 ± 0.03 | −0.51 ± 0.04 |
| MTCI | 0.58 ± 0.32 | 1.27 ± 0.49 | 2.80 ± 0.84 | 3.32 ± 1.33 | 6.26 ± 0.54 | 3.80 ± 0.83 | 1.69 ± 0.76 |
| MTVI2 | 0.17 ± 0.06 | 0.20 ± 0.05 | 0.30 ± 0.06 | 0.34 ± 0.06 | 0.47 ± 0.06 | 0.40 ± 0.04 | 0.21 ± 0.11 |
| PLS | 1.40 ± 0.42 | 1.53 ± 0.33 | 2.07 ± 0.49 | 2.41 ± 0.38 | 3.26 ± 0.31 | 2.74 ± 0.37 | 1.99 ± 0.46 |
| RVI | 3.83 ± 1.49 | 4.84 ± 1.22 | 10.83 ± 4.54 | 12.71 ± 5.72 | 21.75 ± 3.91 | 11.95 ± 2.90 | 4.30 ± 2.73 |
| DVI | 0.14 ± 0.04 | 0.15 ± 0.03 | 0.22 ± 0.05 | 0.25 ± 0.04 | 0.35 ± 0.04 | 0.31 ± 0.03 | 0.21 ± 0.06 |
| PRI | 0.13 ± 0.03 | 0.09 ± 0.02 | 0.05 ± 0.03 | 0.04 ± 0.03 | 0.00 ± 0.01 | 0.04 ± 0.02 | 0.09 ± 0.02 |
| WDRVI | 0.50 ± 0.19 | 0.64 ± 0.14 | 0.98 ± 0.20 | 1.03 ± 0.20 | 1.27 ± 0.06 | 1.06 ± 0.10 | 0.51 ± 0.31 |
| VOG1 | 1.06 ± 0.09 | 1.19 ± 0.12 | 1.53 ± 0.19 | 1.62 ± 0.25 | 2.11 ± 0.09 | 1.72 ± 0.15 | 1.26 ± 0.19 |
| mND705 | 0.16 ± 0.10 | 0.31 ± 0.10 | 0.52 ± 0.09 | 0.56 ± 0.11 | 0.74 ± 0.02 | 0.60 ± 0.07 | 0.29 ± 0.16 |
| MSR705 | 0.71 ± 0.09 | 0.74 ± 0.05 | 0.86 ± 0.05 | 0.86 ± 0.06 | 0.91 ± 0.01 | 0.88 ± 0.02 | 0.79 ± 0.10 |
| SIPI | 1.27 ± 0.21 | 1.13 ± 0.08 | 1.05 ± 0.03 | 1.04 ± 0.05 | 1.01 ± 0.01 | 1.04 ± 0.02 | 1.41 ± 0.30 |
| DDN | −0.20 ± 0.04 | −0.21 ± 0.05 | −0.32 ± 0.07 | −0.37 ± 0.07 | −0.54 ± 0.06 | −0.46 ± 0.05 | −0.41 ± 0.02 |
| DD | −0.05 ± 0.01 | −0.01 ± 0.02 | 0.04 ± 0.03 | 0.06 ± 0.04 | 0.13 ± 0.02 | 0.08 ± 0.02 | 0.00 ± 0.04 |
| VOG2 | −0.02 ± 0.01 | −0.04 ± 0.02 | −0.11 ± 0.04 | −0.13 ± 0.07 | −0.28 ± 0.03 | −0.16 ± 0.04 | −0.05 ± 0.04 |
| VI | Seedling | VI | Tillering | VI | Elongation | VI | Booting | VI | Heading | VI | Flowering | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| R2 | RMSE | R2 | RMSE | R2 | RMSE | R2 | RMSE | R2 | RMSE | R2 | RMSE | ||||||
| SR | 0.66 *** | 0.28 | VOG2 | 0.52 *** | 0.38 | REP | 0.74 ** | 0.29 | WDRVI | 0.67 ** | 0.49 | NDII | 0.10 ns | 0.47 | PRI | 0.44 ns | 0.37 |
| MSR | 0.65 *** | 0.28 | PRI | 0.47 ** | 0.41 | MSR705 | 0.70 ** | 0.31 | NDVI | 0.65 ** | 0.52 | WI | 0.08 ns | 0.48 | REP | 0.36 ns | 0.39 |
| RVI | 0.65 *** | 0.28 | mND705 | 0.44 ** | 0.42 | NDVI | 0.69 ** | 0.31 | SAVI | 0.65 ** | 0.51 | SIPI | 0.08 ns | 0.48 | mND705 | 0.36 ns | 0.39 |
| WDRVI | 0.64 *** | 0.28 | NDVI | 0.42 ** | 0.42 | SAVI | 0.69 ** | 0.31 | MSR | 0.64 ** | 0.52 | MSR705 | 0.07 ns | 0.48 | SIPI | 0.35 ns | 0.39 |
| SAVI | 0.60 ** | 0.29 | SAVI | 0.42 ** | 0.42 | WDRVI | 0.68 ** | 0.32 | SIPI | 0.62 ** | 0.53 | RVSI | 0.05 ns | 0.48 | MTCI | 0.33 ns | 0.40 |
| NDVI | 0.59 ** | 0.30 | WDRVI | 0.42 ** | 0.42 | MSR | 0.66 ** | 0.33 | MSR705 | 0.60 ** | 0.55 | REP | 0.03 ns | 0.49 | VOG2 | 0.32 ns | 0.40 |
| RVSI | 0.59 ** | 0.30 | MSR | 0.41 ** | 0.42 | SR | 0.65 ** | 0.33 | SR | 0.59 ** | 0.55 | SR | 0.02 ns | 0.49 | RVSI | 0.26 ns | 0.42 |
| OSAVI | 0.57 ** | 0.31 | RVI | 0.41 ** | 0.43 | SIPI | 0.64 * | 0.34 | mND705 | 0.59 ** | 0.55 | MSR | 0.01 ns | 0.50 | VOG | 0.25 ns | 0.42 |
| RDVI | 0.56 ** | 0.31 | SIPI | 0.41 ** | 0.43 | RVI | 0.60 * | 0.36 | OSAVI | 0.58 ** | 0.56 | TVI | 0.01 ns | 0.50 | NDVI | 0.24 ns | 0.42 |
| GNDVI | 0.55 ** | 0.32 | SR | 0.40 ** | 0.43 | GNDVI | 0.58 * | 0.37 | GNDVI | 0.54 * | 0.58 | MNLI | 0.01 ns | 0.50 | SR | 0.24 ns | 0.42 |
| MTVI2 | 0.55 ** | 0.32 | VOG | 0.40 ** | 0.43 | mND705 | 0.57 * | 0.37 | RDVI | 0.54 * | 0.58 | PLS | 0.01 ns | 0.50 | SAVI | 0.24 ns | 0.42 |
| TVI | 0.53 ** | 0.32 | MTCI | 0.38 ** | 0.44 | PRI | 0.55 * | 0.38 | MTVI2 | 0.53 * | 0.59 | RVI | 0.01 ns | 0.49 | MSR | 0.24 ns | 0.42 |
| mND705 | 0.52 ** | 0.32 | GNDVI | 0.32 * | 0.46 | VOG | 0.54 * | 0.38 | RVI | 0.53 * | 0.59 | DVI | 0.01 ns | 0.50 | WDRVI | 0.24 ns | 0.42 |
| PLS | 0.48 ** | 0.34 | DD | 0.30 * | 0.46 | MTCI | 0.51 * | 0.39 | DD | 0.52 * | 0.60 | mND705 | 0.01 ns | 0.50 | DD | 0.24 ns | 0.43 |
References
- World Rice Production 2022/2023. Available online: http://www.worldagriculturalproduction.com/crops/rice.aspx/ (accessed on 21 September 2022).
- Thenkabail, P.S.; Smith, R.B.; Pauw, E.D. Hyperspectral vegetation indices and their relationship with agricultural crop characteristics. Remote Sens. Environ. 2000, 71, 152–182. [Google Scholar] [CrossRef]
- Atzberger, C.; Darvishzadeh, R.; Immitzer, M.; Schlerf, M.; Skidmore, A.; Le Maire, G. Comparative analysis of different retrieval methods for mapping grassland leaf area index using airborne imaging spectroscopy. Int. J. Appl. Earth Obs. Geoinf. 2015, 43, 19–31. [Google Scholar] [CrossRef]
- Prabhakar, M.; Gopinath, K.A.; Kumar, N.R.; Thirupathi, M.; Sravan, U.S.; Kumar, G.S.; Siva, G.S.; Meghalakshmi, G.; Vennila, S. Detecting the invasive fall armyworm pest incidence in farm fields of southern India using Sentinel-2A satellite data. Geocarto Int. 2021, 37, 3801–3816. [Google Scholar] [CrossRef]
- Lillesand, T.; Kiefer, R.W.; Chipman, J. Remote Sensing and Image Interpretation, 7th ed.; John Wiley & Sons: Hoboken, NJ, USA, 2015. [Google Scholar]
- Datt, B. Remote sensing of Chlorophyll a, Chlorophyll b, Chlorophyll a+b, and total Carotenoid content in Eucalyptus leaves. Remote Sens. Environ. 1998, 66, 111–121. [Google Scholar] [CrossRef]
- Chen, J.M.; Black, T. Defining leaf area index for non-flat leaves. Plant Cell Environ. 1992, 15, 421–429. [Google Scholar] [CrossRef]
- Jonckheere, I.; Fleck, S.; Nackaerts, K.; Muys, B.; Coppin, P.; Weiss, M.; Baret, F. Review of methods for in-situ leaf area index determination: Part, I. Theories, sensors and hemispherical photography. Agric. For. Meteorol. 2004, 121, 19–35. [Google Scholar] [CrossRef]
- Tao, H.; Feng, H.; Xu, L.; Miao, M.; Long, H.; Yue, J.; Li, Z.; Yang, G.; Yang, X.; Fan, L. Estimation of crop growth parameters using UAV-based hyperspectral remote sensing data. Sensors 2020, 20, 1296. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Zhang, Z.; Tao, F. Improving regional winter wheat yield estimation through assimilation of phenology and leaf area index from remote sensing data. Eur. J. Agron. 2018, 101, 163–173. [Google Scholar] [CrossRef]
- Srinet, R.; Nandy, S.; Patel, N. Estimating leaf area index and light extinction coefficient using Random Forest regression algorithm in a tropical moist deciduous forest, India. Ecol. Inform. 2019, 52, 94–102. [Google Scholar] [CrossRef]
- Qiao, K.; Zhu, W.; Xie, Z.; Li, P. Estimating the seasonal dynamics of the leaf area index using piecewise LAI-VI relationships based on phenophases. Remote Sens. 2019, 11, 689. [Google Scholar] [CrossRef]
- Duan, B.; Liu, Y.; Gong, Y.; Peng, Y.; Wu, X.; Zhu, R.; Fang, S. Remote estimation of rice LAI based on Fourier spectrum texture from UAV image. Plant Methods 2019, 15, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Weiss, M.; Jacob, F.; Duveiller, G. Remote sensing for agricultural applications: A meta-review. Remote Sens. Environ. 2020, 236, 111402. [Google Scholar] [CrossRef]
- Gong, Y.; Yang, K.; Lin, Z.; Fang, S.; Wu, X.; Zhu, R.; Peng, Y. Remote estimation of leaf area index (LAI) with unmanned aerial vehicle (UAV) imaging for different rice cultivars throughout the entire growing season. Plant Methods 2021, 17, 88. [Google Scholar] [CrossRef] [PubMed]
- Torre, D.M.G.; Gao, J.; Macinnis-Ng, C. Remote sensing-based estimation of rice yields using various models: A critical review. Geo-Spat. Inf. Sci. 2021, 24, 580–603. [Google Scholar] [CrossRef]
- Luo, S.; Jiang, X.; Jiao, W.; Yang, K.; Li, Y.; Fang, S. Remotely sensed prediction of rice yield at different growth durations using UAV multispectral imagery. Agriculture 2022, 12, 1447. [Google Scholar] [CrossRef]
- Walthall, C.; Dulaney, W.; Anderson, M.; Norman, J.; Fang, H.; Liang, S. A comparison of empirical and neural network approaches for estimating corn and soybean leaf area index from Landsat ETM+ imagery. Remote Sens. Environ. 2004, 92, 465–474. [Google Scholar] [CrossRef]
- Haboudane, D.; Miller, J.R.; Pattey, E.; Zarco-Tejada, P.J.; Strachan, I.B. Hyperspectral vegetation indices and novel algorithms for predicting green LAI of crop canopies: Modeling and validation in the context of precision agriculture. Remote Sens. Environ. 2004, 90, 337–352. [Google Scholar] [CrossRef]
- He, L.; Ren, X.; Wang, Y.; Liu, B.; Zhang, H.; Liu, W.; Feng, W.; Guo, T. Comparing methods for estimating leaf area index by multi-angular remote sensing in winter wheat. Sci. Rep. 2020, 10, 13943. [Google Scholar] [CrossRef]
- Huete, A.R. A soil-adjusted vegetation index (SAVI). Remote Sens. Environ. 1988, 25, 295–309. [Google Scholar] [CrossRef]
- Broge, N.H.; Leblanc, E. Comparing prediction power and stability of broadband and hyperspectral vegetation indices for estimation of green leaf area index and canopy chlorophyll density. Remote Sens. Environ. 2001, 76, 156–172. [Google Scholar] [CrossRef]
- Roujean, J.; Breon, F. Estimating PAR absorbed by vegetation from bidirectional reflectance measurements. Remote Sens. Environ. 1995, 51, 375–384. [Google Scholar] [CrossRef]
- Ma, B.L.; Dwyer, L.M.; Costa, C.; Cober, E.R.; Morrison, M.J. Early prediction of soybean yield from canopy reflectance measurements. Agron. J. 2001, 93, 1227–1234. [Google Scholar] [CrossRef]
- Clevers, J.; de Jong, S.M.; Epema, G.F.; van der Meer, F.D.; Bakker, W.H.; Skidmore, A.K.; Scholte, K.H. Derivation of the red edge index using the MERIS standard band setting. Int. J. Remote Sens. 2002, 23, 3169–3184. [Google Scholar] [CrossRef]
- Vina, A.; Gitelson, A.A.; Nguy-Robertson, A.L.; Peng, Y. Comparison of different vegetation indices for the remote assessment of green leaf area index of crops. Remote Sens. Environ. 2011, 115, 3468–3478. [Google Scholar] [CrossRef]
- Berger, K.; Atzberger, C.; Danner, M.; D’Urso, G.; Mauser, W.; Vuolo, F.; Hank, T. Evaluation of the PROSAIL model capabilities for future hyperspectral model environments: A review study. Remote Sens. 2018, 10, 85. [Google Scholar] [CrossRef]
- Jensen, J.R. Remote Sensing of the Environment: An Earth Resource Perspective, 2nd ed.; Pearson Education India: Noida, India, 2009. [Google Scholar]
- Xin, M.-Y.; Yin, H.; Chen, L.; Zhang, M.-l.; Ren, Z.-Y.; Miao, J. Estimation of rice canopy LAI with different growth stages based on hyperspectral remote sensing data. Chin. J. Agrometeorol. 2015, 36, 762. [Google Scholar]
- Yang, K.; Gong, Y.; Fang, S.; Duan, B.; Yuan, N.; Peng, Y.; Wu, X.; Zhu, R. Combining spectral and texture features of UAV images for the remote estimation of rice LAI throughout the entire growing season. Remote Sens. 2021, 13, 3001. [Google Scholar] [CrossRef]
- Zhang, Y.; Yang, J.; Du, L. Analyzing the effects of hyperspectral ZhuHai-1 band combinations on LAI estimation based on the PROSAIL model. Sensors 2021, 21, 1869. [Google Scholar] [CrossRef]
- Gopinath, G.; Surendran, U.; Vishak, J.; Sasidharan, N.; Muhamed Fasil, C.T. Hyperspectral data and vegetative indices for paddy: A case study in Kerala, India. Remote Sens. Appl. Soc. Environ. 2024, 33, 101109. [Google Scholar] [CrossRef]
- Du, L.; Gong, W.; Shi, S.; Yang, J.; Sun, J.; Zhu, B.; Song, S. Estimation of rice leaf nitrogen contents based on hyperspectral LIDAR. Int. J. Appl. Earth. Obs. Geoinf. 2016, 44, 136–143. [Google Scholar] [CrossRef]
- Din, M.; Zheng, W.; Rashid, M.; Wang, S.; Shi, Z. Evaluating hyperspectral vegetation indices for leaf area index estimation of Oryza sativa L. at diverse phenological stages. Front. Plant Sci. 2017, 8, 820. [Google Scholar] [CrossRef] [PubMed]
- Duan, D.D.; Zhao, C.J.; Li, Z.H.; Yang, G.J.; Yang, W.D. Estimating total leaf nitrogen concentration in winter wheat by canopy hyperspectral data and nitrogen vertical distribution. J. Integr. Agric. 2019, 18, 1562–1570. [Google Scholar] [CrossRef]
- Yue, J.; Yang, G.; Li, C.; Li, Z.; Wang, Y.; Feng, H.; Xu, B. Estimation of winter wheat above-ground biomass using unmanned aerial vehicle-based snapshot hyperspectral sensor and crop height improved models. Remote Sens. 2017, 9, 708. [Google Scholar] [CrossRef]
- Duan, B.; Fang, S.; Zhu, R.; Wu, X.; Wang, S.; Gong, Y.; Peng, Y. Remote estimation of rice yield with unmanned aerial vehicle (UAV) data and spectral mixture analysis. Front. Plant Sci. 2019, 10, 204. [Google Scholar] [CrossRef] [PubMed]
- Singh, P.; Srivastava, P.K.; Mall, R.K.; Bhattacharya, B.K.; Prasad, R. A hyperspectral R based leaf area index estimator: Model development and implementation using AVIRIS-NG. Geocarto Int. 2022, 37, 12792–12809. [Google Scholar] [CrossRef]
- Wang, Y.; Zhang, K.; Tang, C.; Cao, Q.; Tian, Y.; Zhu, Y.; Cao, W.; Liu, X. Estimation of rice growth parameters based on linear mixed-effect model using multispectral images from fixed-wing unmanned aerial vehicles. Remote Sens. 2019, 11, 1371. [Google Scholar] [CrossRef]
- Zheng, H.; Cheng, T.; Zhou, M.; Li, D.; Yao, X.; Tian, Y.; Cao, W.; Zhu, Y. Improved estimation of rice aboveground biomass combining textural and spectral analysis of UAV imagery. Precis. Agric. 2019, 20, 611–629. [Google Scholar] [CrossRef]
- Ryu, C.; Suguri, M.; Umeda, M. Model for predicting the nitrogen content of rice at panicle initiation stage using data from airborne hyperspectral remote sensing. Biosyst. Eng. 2009, 104, 465–475. [Google Scholar] [CrossRef]
- Nakanishi, T.; Imai, Y.; Morita, T.; Akamatsu, Y.; Odagawa, S.; Takeda, T.; Kashimura, O. Evaluation of wheat growth monitoring methods based on hyperspectral data of later grain filling and heading stages in Western Australia. Int. Arch. Photogramm. Remote Sens. Spat. Inf. Sci. 2012, 39, 295–300. [Google Scholar] [CrossRef]
- Sahoo, R.N.; Pradhan, S. Hyperspectral Remote Sensing for Agriculture; Division of Agricultural Physics, Indian Agricultural Research Institute: New Delhi, India, 2017; p. 192. [Google Scholar]
- ASD. Technical Guide, 4th ed.; Analytical Spectral Devices Inc.: Boulder, CO, USA, 1999; p. 136. [Google Scholar]
- Zhang, J.; Zhang, W.; Xiong, S.; Song, Z.; Tian, W.; Shi, L.; Ma, X. Comparison of new hyperspectral index and machine learning models for prediction of winter wheat leaf water content. Plant Methods 2021, 17, 34. [Google Scholar] [CrossRef]
- Kumar, J.; Vashisth, A.; Sehgal, V.K.; Gupta, V.K. Identification of aphid infestation in mustard by hyperspectral remote sensing. J. Agric. Phys. 2010, 10, 53–60. [Google Scholar]
- Bue, B.D.; Thompson, D.R.; Eastwood, M.; Green, R.O.; Gao, B.C.; Keymeulen, D.; Sarture, C.M.; Mazer, A.S.; Luong, H.H. Real-time atmospheric correction of AVIRIS-NG imagery. IEEE Trans. Geosci. Remote Sens. 2015, 53, 6419–6428. [Google Scholar] [CrossRef]
- Bhattacharya, B.K.; Green, R.O.; Rao, S.; Saxena, M.; Sharma, S.; Kumar, K.A.; Srinivasulu, P.; Sharma, S.; Dhar, D.; Bandyopadhyay, S.; et al. An overview of AVIRIS-NG airborne hyperspectral science campaign over India. Curr. Sci. 2019, 116, 1082–1088. [Google Scholar] [CrossRef]
- Delalieux, S.; Somers, B.; Hereijgers, S.; Verstraeten, W.W.; Keulemans, W.; Coppin, P. A near-infrared narrow-waveband ratio to determine Leaf Area Index in orchards. Remote Sens. Environ. 2008, 112, 3762–3772. [Google Scholar] [CrossRef]
- Garg, V.; Kumar, A.S.; Aggarwal, S.P.; Kumar, V.; Dhote, P.R.; Thakur, P.K.; Nikam, B.R.; Sambare, R.S.; Siddiqui, A.; Muduli, P.R.; et al. Spectral similarity approach for mapping turbidity of an inland waterbody. J. Hydrol. 2017, 550, 527–537. [Google Scholar] [CrossRef]
- Govaerts, Y.M.; Verstraete, M.M.; Pinty, B.; Gobron, N. Designing optimal spectral indices: A feasibility and proof of concept study. Int. J. Remote Sens. 1999, 20, 1853–1873. [Google Scholar] [CrossRef]
- SAS Institute Inc. SAS OnlineDocVR 9.2. Cary (NC). 2009. Available online: http://support.sas.com/documentation (accessed on 20 June 2023).
- Liu, Z.; Huang, W.; Mao, G.; Li, C.; Xu, X.; Ding, X.; Shi, J.; Zhou, B. Estimating foliar pigment concentration of rice crop using integrated hyperspectral index. In Computer and Computing Technologies in Agriculture V: CCTA 2011; IFIP Advances in Information and Communication Technology; Li, D., Chen, Y., Eds.; Springer: Berlin/Heidelberg, Germany, 2011; pp. 264–274. [Google Scholar]
- Wu, L.; Yuan, S.; Huang, L.; Sun, F.; Zhu, G.; Li, G.; Fahad, S.; Peng, S.; Wang, F. Physiological mechanisms underlying the high-grain yield and high-nitrogen use efficiency of elite rice varieties under a low rate of nitrogen application in China. Front. Plant Sci. 2016, 7, 1024. [Google Scholar] [CrossRef] [PubMed]
- Carvalho, S.; Van der Putten, W.H.; Hol, W.H.G. The potential of hyperspectral patterns of winter wheat to detect changes in soil microbial community composition. Front. Plant Sci. 2016, 7, 759. [Google Scholar] [CrossRef]
- Lima, I.P.; Jorge, R.G.; de Lima, J.L.M.P. Remote sensing monitoring of rice fields: Towards assessing water saving irrigation management practices. Front. Remote Sens. 2021, 2, 762093. [Google Scholar] [CrossRef]
- Kawamura, K.; Ikeura, H.; Phongchanmaixay, S.; Khanthavong, P. Canopy hyperspectral sensing of paddy fields at the booting stage and PLS regression can assess grain yield. Remote Sens. 2018, 10, 1249. [Google Scholar] [CrossRef]
- Gausman, H.W.; Allen, W.A.; Cardenas, R. Reflectance of cotton leaves and their structure. Remote Sens. Environ. 1969, 1, 19–22. [Google Scholar] [CrossRef]
- Woolley, J.T. Reflectance and transmittance of light by leaves. Plant Physiol. 1970, 47, 656–662. [Google Scholar] [CrossRef] [PubMed]
- Feng, W.; Guo, B.B.; Wang, Z.J.; He, L.; Song, X.; Wang, Y.H.; Guo, T.C. Measuring leaf nitrogen concentration in winter wheat using double-peak spectral reflection remote sensing data. Field Crops Res. 2014, 159, 43–52. [Google Scholar] [CrossRef]
- Gaju, O.; Allard, V.; Martre, P.; Le Gouis, J.; Moreau, D.; Bogard, M.; Hubbart, S.; Foulkes, M.J. Nitrogen partitioning and remobilization in relation to leaf senescence, grain yield and grain nitrogen concentration in wheat cultivars. Field Crops Res. 2014, 155, 213–223. [Google Scholar] [CrossRef]
- Ranjan, A.K.; Parida, B.R. Estimating biochemical parameters of paddy using satellite and near-proximal sensor data in Sahibganj Province, Jharkhand (India). Remote Sens. Appl. Soc. Environ. 2020, 18, 100293. [Google Scholar] [CrossRef]
- Dong, T.; Liu, J.; Shang, J.; Qian, B.; Ma, B.; Kovacs, J.M.; Walters, D.; Jiao, X.; Geng, X.; Shi, Y. Assessment of red-edge vegetation indices for crop leaf area index estimation. Remote Sens. Environ. 2019, 222, 133–143. [Google Scholar] [CrossRef]
- Chen, J.; Gu, S.; Shen, M.; Tang, Y.; Matsushita, B. Estimating aboveground biomass of grassland having a high canopy cover: An exploratory analysis of in-situ hyperspectral data. Int. J. Remote Sens. 2009, 30, 6497–6517. [Google Scholar] [CrossRef]
- Thenkabail, P.S.; Lyon, J.G.; Huete, A. Biophysical and Biochemical Characterization and Plant Species Studies, 2nd ed.; CRC Press: Boca Raton, FL, USA, 2018. [Google Scholar]
- Darvishzadeh, R.; Atzberger, C.; Skidmore, A.K.; Abkar, A.A. Leaf Area Index derivation from hyperspectral vegetation indices and the red edge position. Int. J. Remote Sens. 2009, 30, 6199–6218. [Google Scholar] [CrossRef]
- Herrmann, I.; Pimstein, A.; Karnieli, A.; Cohen, Y.; Alchanatis, V.; Bonfil, D.J. LAI assessment of wheat and potato crops by VENµS and Sentimel-2 bands. Remote Sens Environ. 2011, 115, 2141–2151. [Google Scholar] [CrossRef]
- Li, F.; Miao, Y.; Hennig, S.D.; Gnyp, M.L.; Chen, X.; Jia, L.; Bareth, G. Evaluating hyperspectral vegetation indices for estimating nitrogen concentration of winter wheat at different growth stages. Precis. Agric. 2010, 11, 335–357. [Google Scholar] [CrossRef]
- Motohka, T.; Nasahara, K.N.; Oguma, H.; Tsuchida, S. Applicability of green-red vegetation index for remote sensing of vegetation phenology. Remote Sens. 2010, 2, 2369–2387. [Google Scholar] [CrossRef]
- Inoue, Y.; Sakaiya, E.; Zhu, Y.; Takahashi, W. Diagnostic mapping of canopy nitrogen content in rice based on hyperspectral measurements. Remote Sens. Environ. 2012, 126, 210–221. [Google Scholar] [CrossRef]
- Sims, D.A.; Gamon, J.A. Relationships between leaf pigment content and spectral reflectance across a wide range of species, leaf structures and developmental stages. Remote Sens. Environ. 2002, 81, 331–354. [Google Scholar] [CrossRef]
- He, J.; Zhang, N.; Su, X.; Lu, J.; Yao, X.; Cheng, T.; Zhu, Y.; Cao, W.; Tian, Y. Estimating leaf area index with a new vegetation index considering the influence of rice panicles. Remote Sens. 2019, 11, 1809. [Google Scholar] [CrossRef]
- Gitelson, A.A. Wide dynamic range vegetation index for remote quantification of biophysical characteristics of vegetation. J. Plant Physiol. 2004, 161, 165–173. [Google Scholar] [CrossRef] [PubMed]
- Nguy-Robertson, A.L.; Peng, Y.; Gitelson, A.A.; Arkebauer, T.J.; Pimstein, A.; Herrmann, I.; Karnieli, A.; Rundquist, D.C.; Bonfil, D.J. Estimating green LAI in four crops: Potential of determining optimal spectral bands for a universal algorithm. Agric. For. Meteorol. 2014, 192, 140–148. [Google Scholar] [CrossRef]
- Nguy-Robertson, A.; Gitelson, A.; Peng, Y.; Vina, A.; Arkebauer, T.; Rundquist, D. Green leaf area index estimation in maize and soybean: Combining vegetation indices to achieve maximal sensitivity. Agron. J. 2012, 104, 1336–1347. [Google Scholar] [CrossRef]
- Kattenborn, T.; Fassnacht, F.E.; Schmidtlein, S. Differentiating plant functional types using reflectance: Which traits make the difference? Remote Sens. Ecol. Conserv. 2019, 5, 5–19. [Google Scholar] [CrossRef]








| Crop Growth Stage | Field Samples Considered for Calibration | Field Samples Considered for Validation |
|---|---|---|
| Seedling | 14 | 12 |
| Tillering | 19 | 12 |
| Elongation | 9 | 7 |
| Booting | 11 | 7 |
| Heading | 10 | 6 |
| Flowering | 8 | 5 |
| Maturity | 7 | 5 |
| Total | 78 | 54 |
| Spectral Vegetation Index | Formula |
|---|---|
| Vogelmann Index (VOG 1) | |
| Meris Terrestrial Chlorophyll Index (MTCI) | |
| Vogelmann Index (VOG 2) | |
| Modified simple ratio (MSR) | |
| Modified Red-edge Normalized Difference Vegetation Index (mND705) | |
| Double Difference Index (DD) | |
| Green Normalized Difference Vegetation Index (GNDVI) | |
| Soil Adjusted Vegetation Index (OSAVI) | |
| Renormalized Difference Vegetation Index (RDVI) | |
| Simple Ratio (SR) | |
| Modified Triangular Vegetation Index (MTVI 2) | |
| Soil-Adjusted Vegetation Index (SAVI) | |
| Normalized Difference Vegetation Index (NDVI) | |
| Ratio Vegetation Index (RVI) | |
| Enhanced Vegetation Index (EVI 1) | |
| Difference Vegetation Index (DVI) | |
| Photochemical Refectance Index (PRI) | |
| Transformed Vegetation Index (TVI) | |
| New Double Difference Index (DDn) | |
| Modified Red-edge Simple Ratio Index (MSR705) | |
| Modified Non-Linear Index (MNLI) | |
| Structure Insensitive Pigment Index (SIPI) | |
| Water Index (WI) | |
| Red-Edge Vegetation Stress Index (RVSI) | |
| Standardized LAI Determining Index (SLAIDI) | where S = 5 |
| Normalized Difference Water Index (NDWI) | |
| Normalized Difference Infrared Index (NDII) | |
| Red Edge Position Index (REP) | |
| Wide Dynamic Range Vegetation Index (WDRVI) |
| Crop Growth Stage | Number of Fields Surveyed | LAI | ||||
|---|---|---|---|---|---|---|
| Mean ± SD | Minimum | Maximum | p Value | CV | ||
| Seedling | 14 | 1.21 ± 0.45 | 0.54 | 2.20 | 0.59 | 37.50 |
| Tillering | 19 | 1.71 ± 0.54 | 0.75 | 2.60 | 0.68 | 31.66 |
| Elongation | 9 | 2.41 ± 0.53 | 1.60 | 3.00 | 0.12 | 21.79 |
| Booting | 11 | 3.00 ± 0.82 | 1.70 | 4.00 | 0.04 | 27.24 |
| Heading | 10 | 3.61 ± 0.47 | 3.10 | 4.30 | 0.15 | 13.00 |
| Flowering | 8 | 3.61 ± 0.45 | 2.80 | 4.10 | 0.42 | 12.49 |
| Maturity | 7 | 2.11 ± 0.35 | 1.60 | 2.50 | 0.38 | 16.48 |
| Crop Growth Stage | Number of Fields | LAI | ||||
|---|---|---|---|---|---|---|
| Mean ± SD | Minimum | Maximum | p Value | CV (%) | ||
| Seedling | 12 | 0.99 ± 0.23 | 0.60 | 1.31 | 0.33 | 23.51 |
| Tillering | 12 | 1.78 ± 0.40 | 1.20 | 2.42 | 0.42 | 22.41 |
| Elongation | 7 | 2.89 ± 0.40 | 2.50 | 3.60 | 0.29 | 13.80 |
| Booting | 7 | 3.29 ± 0.58 | 2.50 | 4.10 | 0.88 | 17.70 |
| Heading | 6 | 3.63 ± 0.35 | 3.20 | 4.20 | 0.92 | 9.64 |
| Flowering | 5 | 3.79 ± 0.57 | 2.90 | 4.50 | 0.42 | 15.11 |
| Maturity | 5 | 2.24 ± 0.68 | 1.50 | 3.10 | 0.53 | 30.38 |
| VI | Seedling | Tillering | Elongation | Booting | ||||
|---|---|---|---|---|---|---|---|---|
| R2 | RMSE | R2 | RMSE | R2 | RMSE | R2 | RMSE | |
| mND705 | 0.52 ** | 0.17 | 0.42 * | 0.32 | 0.88 ** | 0.15 | 0.51 ns | 0.45 |
| SR | 0.50 ** | 0.17 | 0.40 * | 0.32 | 0.66 * | 0.26 | 0.10 ns | 0.60 |
| MSR | 0.51 ** | 0.17 | 0.48 * | 0.30 | 0.67 * | 0.25 | 0.42 ns | 0.49 |
| RVI | 0.51 ** | 0.17 | 0.38 * | 0.33 | 0.71 * | 0.24 | 0.10 ns | 0.60 |
| WDRVI | 0.47 * | 0.18 | 0.37 * | 0.33 | 0.76 * | 0.22 | 0.62 * | 0.39 |
| SAVI | 0.49 * | 0.17 | 0.56 ** | 0.28 | 0.63 * | 0.27 | 0.59 * | 0.41 |
| NDVI | 0.45 * | 0.18 | 0.34 * | 0.34 | 0.73 * | 0.23 | 0.54 ns | 0.43 |
| GNDVI | 0.49 * | 0.17 | 0.45 * | 0.31 | 0.83 ** | 0.18 | 0.50 ns | 0.45 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Prabhakar, M.; Gopinath, K.A.; Ravi Kumar, N.; Thirupathi, M.; Sai Sravan, U.; Srasvan Kumar, G.; Samba Siva, G.; Chandana, P.; Singh, V.K. Mapping Leaf Area Index at Various Rice Growth Stages in Southern India Using Airborne Hyperspectral Remote Sensing. Remote Sens. 2024, 16, 954. https://doi.org/10.3390/rs16060954
Prabhakar M, Gopinath KA, Ravi Kumar N, Thirupathi M, Sai Sravan U, Srasvan Kumar G, Samba Siva G, Chandana P, Singh VK. Mapping Leaf Area Index at Various Rice Growth Stages in Southern India Using Airborne Hyperspectral Remote Sensing. Remote Sensing. 2024; 16(6):954. https://doi.org/10.3390/rs16060954
Chicago/Turabian StylePrabhakar, Mathyam, Kodigal A. Gopinath, Nakka Ravi Kumar, Merugu Thirupathi, Uppu Sai Sravan, Golla Srasvan Kumar, Gutti Samba Siva, Pebbeti Chandana, and Vinod Kumar Singh. 2024. "Mapping Leaf Area Index at Various Rice Growth Stages in Southern India Using Airborne Hyperspectral Remote Sensing" Remote Sensing 16, no. 6: 954. https://doi.org/10.3390/rs16060954