Estimating Canopy Nitrogen Concentration in Sugarcane Using Field Imaging Spectroscopy
Abstract
:1. Introduction
2. Materials and Methods
2.1. Field Experimental Design
2.2. Measurements of Hyperspectral Reflectance
2.3. Determination of Nitrogen Concentration
2.4. Spectral Transformations
2.4.1. First-Derivative Transformation
2.4.2. Calculation of Absorption Features
- Continuum-Removed Derivative Reflectance (CRDR) was calculated by applying a first-derivative transformation to the continuum-removed reflectance spectrum R′.
- The band depth (BD) was calculated by subtracting the continuum-removed reflectance at wavelength i from 1:
2.5. Univariate Approach: Narrow Vegetation Indices
2.6. Multivariate Approach: Stepwise Multiple Linear Regression
2.7. Model Validation
3. Results and Discussion
3.1. Variations in Nitrogen Concentration and Hyperspectral Canopy Reflectance
3.1.1. Variations in Nitrogen Concentration
3.1.2. Hyperspectral Canopy Reflectance
3.2. Relationships between the Nitrogen Concentration and Narrow Vegetation Index
3.3. Relationships between the Nitrogen Concentration and Spectral Wavelength Determined by a SMLR Technique
3.4. Discussion
3.4.1. Utility of the Methods Used in this Study in Estimating the Nitrogen Concentration
3.4.2. Wavelength Selection
3.4.3. Effects of Plant Morphology and Structure on the Spectral Response
3.4.4. Performance Comparison of Proposed Models with Previous Models
4. Conclusions
- Stepwise multiple linear regression could explain the nitrogen variations in sugarcane canopy better than a narrow vegetation index. This technique utilizes more than two wavelengths from the entire spectral region (400–2,500 nm) to estimate the dependent variable.
- First Derivative Spectrum (FDS) showed a better relationship with canopy nitrogen concentration than Continuum-Removed Derivative Reflectance (CRDR) and Band Depth (BD) when the models were developed with multivariate approaches and validated with a combined cultivar data set.
- It was concluded that a First Derivative Spectrum (FDS) has potential when used to estimate the nitrogen content in sugarcane with combined cultivars at the maturity stage (9–12 months).
- Visible, red edge and far near-infrared regions contain more information on canopy nitrogen concentration of combined sugarcane cultivars compared to other parts of the electromagnetic spectrum.
- Canopy architecture directly influences the spectral response and the predictive precision. Canopy structure, therefore, should be taken into consideration when mapping sugarcane nutrient quality in rangelands with combined cultivars.
- In the case of a known cultivar, partitioning the data into cultivars could increase the estimation capability of the method applied in this research.
- The modified estimation model, generated by SMLR technique from FDS centered at 410, 426, 720, 754, and 1,216 nm, yields the highest correlation coefficient value of 0.86 and RMSE value of 0.033%N (n = 90) with nitrogen concentration in sugarcane. This result is much better than those of the previous studies.
Acknowledgments
References
- Xavier, A.C.; Rudorff, B.F.T.; Shimabukuro, Y.E.; Berka, L.M.S.; Moreira, M.A. Multi-temporal analysis of MODIS data to classify sugarcane crop. Int. J. Remote Sens 2006, 27, 755–768. [Google Scholar]
- Abdel-Rahman, E.M.; Ahmed, F.B. The application of remote sensing techniques to sugarcane (Saccharum spp. hybrid) production: A review of the literature. Int. J. Remote Sens 2008, 29, 3753–3767. [Google Scholar]
- Fageria, N.K. The Use of Nutrients in Crop Plants; Taylor and Francis Group: London, UK, 2009; p. 430. [Google Scholar]
- Thompson, G.D. The Growth of Sugarcane Variety N14 at Pongola, Mount Edgecombe Research Report No. 7; South African Sugar Association Experiment Station: Mount Edgecombe, South Africa, 1991.
- Wiedenfeld, R.P. Effects of irrigation and N fertilizer application on sugarcane yield and quality. Field Crops Res 1995, 43, 101–108. [Google Scholar]
- Spaner, D.; Todd, A.; Navabi, A.; Mckenzie, D.; Goonewardene, L. Cane leaf chlorophyll measures at differing growth stages be used as an indicator of winter wheat and spring barley nitrogen requirements in eastern Canada. J. Agron. Crop. Sci 2005, 91, 393–399. [Google Scholar]
- Roth, G.W.; Fox, R.H.; Marshall, H.G. Plant tissue tests for predicting nitrogen fertilizer requirements of winter Wheat. Agron. J 1989, 81, 50. [Google Scholar]
- Turner, F.T.; Jund, M.F. Assessing the nitrogen requirements of rice crops with a chlorophyll meter. Aust. J. Exp. Agric 1994, 34, 1001–1005. [Google Scholar]
- Wang, S.; Zhu, Y.; Jiang, H.; Cao, W. Positional differences in nitrogen and sugar concentrations of upper leaves relate to plant N status in rice under different N rates. Field Crops Res 2006, 96, 224–234. [Google Scholar]
- Mutanga, O.; Skidmore, A.K.; Prins, H.H.T. Predicting in situ pasture quality in the Kruger National Park, South Africa, using continuum removed absorption features. Remote Sens. Environ 2004, 89, 393–408. [Google Scholar]
- Clevers, J.G.P.W.; Kooistra, L.; Schaepman, M.E. Using spectral information from the NIR water absorption features for the retrieval of canopy water content. Int. J. Appl. Earth Obs. Geoinf 2008, 10, 388–397. [Google Scholar]
- Stroppiana, D.; Boschetti, M.; Brivio, P.A.; Bocchi, S. Plant nitrogen concentration in paddy rice from field canopy hyperspectral radiometry. Field Crops Res 2009, 111, 119–129. [Google Scholar]
- Delegido, J.; Alonso, L.; Gonzalez, G.; Moreno, J. Estimating chlorophyll content of crops from hyperspectral data using a normalized area over reflectance curve (NAOC). Int. J. Appl. Earth Obs. Geoinf 2010, 12, 165–174. [Google Scholar]
- Jain, N.; Ray, S.S.; Sinph, J.P.; Panigrahy, S. Use of hyperspectral data to assess the effects of different nitrogen application on a potato crop. Prec. Agr 2007, 8, 225–239. [Google Scholar]
- Yao, X.; Zhu, Y.; Tian, Y.C.; Feng, W.; Cao, W. Exploring hyperspectral bands and estimation indices for leaf nitrogen accumulation in wheat. Int. J. Appl. Earth Obs. Geoinf 2010, 12, 89–100. [Google Scholar]
- Galvao, L.S.; Formaggio, A.R.; Tisot, D.A. Discrimination of sugarcane varieties in Southeastern Brazil with EO-1 Hyperion data. Remote Sens. Environ 2005, 94, 523–534. [Google Scholar]
- Vaiphasa, C.; Skidmore, A.K.; De Boer, W.F.; Vaiphasa, T. A hyperspectral band selector for plant species discrimination. ISPRS J. Photogramm 2007, 62, 225–235. [Google Scholar]
- Kamal, M.; Phinn, S. Hyperspectral data for mangrove species mapping: A comparison of pixel-based and object-based approach. Remote Sens 2011, 3, 2222–2242. [Google Scholar]
- Apan, A.; Held, A.; Phinn, S.; Markley, J. Detecting sugarcane ‘Orange Rust’ disease using EO-1 hyperion hyperspectral imagery. Int. J. Remote Sens 2004, 25, 489–498. [Google Scholar]
- Liu, Z.; Huang, J.; Tao, R.; Zhou, W.; Zhang, L. Characterizing and estimating fungal disease severity of rice brown spot with hyperspectral reflectance data. Rice Sci 2008, 15, 232–242. [Google Scholar]
- Vigneau, N.; Ecarnot, M.; Rabatel, G.; Roumet, P. Potential of field hyperspectral imaging as a non destructive method to assess leaf nitrogen content in Wheat. Field Crops Res 2011, 122, 25–31. [Google Scholar]
- Kumar, L.; Schmidt, K.; Dury, S.; Skidmore, A. Imaging Spectrometry and Vegetation Science. In Image Spectrometry; van der Meer, F.D., de Jong, S.M., Eds.; Kluwer Academic Publishers: London, UK, 2001; Volume 4, pp. 111–155. [Google Scholar]
- Curran, P.J.; Dungan, J.L.; Peterson, D.L. Estimating the foliar biochemical concentration of leaves with reflectance spectrometry: Testing the Kokaly and Clark methodologies. Remote Sens. Environ 2001, 76, 349–359. [Google Scholar]
- Read, J.J.; Tarpley, L.; Mc Kinion, J.M.; Reddy, K.R. Narrow waveband reflectance ratios for remote estimation of nitrogen status in cotton. J. Environ. Qual 2002, 31, 1442–1452. [Google Scholar]
- Zarco-Tejada, P.J.; Miller, J.R.; Mohammed, G.H.; Noland, T.L.; Sampson, P.H. Vegetation stress detection through chlorophyll a + b estimation and fluorescence effects on hyperspectral imagery. J. Environ. Qual 2002, 31, 1433–1441. [Google Scholar]
- Abdel-Rahman, E.M.; Ahmed, F.B.; Van den berg, M. Estimation of sugarcane leaf nitrogen concentration using in situ spectroscopy. Int. J. Appl. Earth Obs. Geoinf 2010, 12, S52–S57. [Google Scholar]
- Johnson, R.M.; Richard, E.P., Jr. Prediction of sugarcane sucrose content with high resolution, hyperspectral leaf reflectance measurements. Int. Sugar J 2011, 113, 48–55. [Google Scholar]
- Mokhele, A.; Ahmed, F.B. Estimation of leaf nitrogen and silicon using hyperspectral remote sensing. J. Appl. Remote Sens 2010, 4, 1–18. [Google Scholar]
- Begue, A.; Lebourgeois, V.; Bappel, E.; Todoroff, P.; Pellegrino, A.; Baillarin, F.; Siegmund, B. Spatio-temporal variability of sugarcane fields and recommendations for yield forecast using NDVI. Int. J. Remote Sens 2010, 21, 5391–5407. [Google Scholar]
- Roder, A.; Kuemmerle, T.; Hill, J.; Papanastasis, V.P.; Tsiourlis, G.M. Adaptaetion of a grazing gradient concept to heterogeneous Mediterranean rangelands using cost surface modelling. Ecol. Model 2007, 204, 387–398. [Google Scholar]
- ASD. Field Spec Pro spectrometer; Analytical Spectral Devices: Boulder, CO, USA, 1995. [Google Scholar]
- Mutanga, O.; Skidmore, A.K. Narrow band vegetation indices overcome the saturation problem in biomass estimation. Int. J. Remote Sens 2004, 25, 1–16. [Google Scholar]
- Darvishzadeh, R.; Skidmore, A.; Schlerf, M.; Atzberger, C.; Corsi, F.; Cho, M. LAI and chlorophyll estimation for a heterogeneous grassland using hyperspectral measurements. ISPRS J. Photogramm 2008, 63, 409–426. [Google Scholar]
- Muchovej, R.M.; Newman, P.R. Nitrogen fertilization of Sugarcane on sandy soil: Yield and leaf nutrient composition. J. Am. Soc. Sugar Cane Tech 2004, 24, 210–224. [Google Scholar]
- SASRI. Leaf Sampling. Information Sheet. South African Sugarcane Research Institute: Mount Edgecombe, South Africa, 2003. [Google Scholar]
- Muchovej, R.M.; Newman, P.R.; Luo, Y. Sugarcane leaf nutrient concentrations: With or without midribs tissue. J. Plant Nutr 2005, 28, 1271–1286. [Google Scholar]
- FOSS. Kjeltec™ 2200Auto Distillation; FOSS Analytical: Hilleroed, Denmark, 2005. [Google Scholar]
- Dawson, T.P.; Curran, P.J. A new technique for interpolating the reflectance red edge position. Int. J. Remote Sens 1998, 19, 2133–2139. [Google Scholar]
- de Jong, S.M. Imaging spectrometry for monitoring tree damage caused by volcanic activity in the long valley caldera, California. Int. J. Appl. Earth Obs. Geoinf 1998, 1, 1–10. [Google Scholar]
- Mutanga, O.; Skidmore, A.K. Continuum-Removed Absorption Features Estimate Tropical Savanna Grass Quality in situ. Proceedings of 3rd EARSeL Workshop on Imaging Spectroscopy, Hersching, Germany, 13–16 May 2003.
- Kokaly, R.F.; Clark, R.N. Spectroscopic determination of leaf biochemistry using band-depth analysis of absorption features and stepwise multiple linear regression. Remote Sens. Environ 1999, 67, 267–287. [Google Scholar]
- Thenkabail, P.S.; Smith, R.B.; De Pauw, E. Hyperspectral vegetation indices and their relationships with agricultural crop characteristics. Remote Sens. Environ 2000, 71, 158–182. [Google Scholar]
- Thenkabail, P.S.; Smith, R.B.; De Pauw, E. Evaluation of narrowband and broadband vegetation indices for determining optimal hyperspectral wavebands for agricultural crop characterization. Photogramm. Eng. Remote Sensing 2002, 68, 607–621. [Google Scholar]
- Motohka, T.; Nasahara, K.N.; Oguma, H.; Tsuchida, S. Applicability of green-red vegetation index for remote sensing of vegetation phenology. Remote Sens 2010, 2, 2369–2387. [Google Scholar]
- Martin, M.E.; Aber, J.D. Estimation of forest canopy lignin and nitrogen concentration and ecosystem processes by high spectral resolution remote sensing. Ecol. Appl 1997, 7, 431–443. [Google Scholar]
- Serrano, L.; Penuelas, J.; Ustin, S.L. Remote sensing of nitrogen and lignin in Mediterranean vegetation for AVIRIS data: decomposing biochemical from structural signals. Remote Sens. Environ 2002, 81, 355–364. [Google Scholar]
- Yi, Q.X.; Huang, J.F.; Wang, F.M.; Wang, X.Z.; Liu, Z.Y. Monitoring rice nitrogen status using hyperspectral reflectance and artificial neural network. Environ. Sci. Tech 2007, 41, 6770–6775. [Google Scholar]
- Yoder, B.J.; Pettigrew-Crosby, R.E. Predicting nitrogen and chlorophyll content and concentrations from reflectance spectra (400–2500 nm) at the leaf and canopy scale. Remote Sens. Environ 1995, 53, 199–211. [Google Scholar]
- Elvidge, C.D.; Chen, Z. Comparison of broadband and narrow-band red and near-infrared vegetation indices. Remote Sens. Environ 1995, 54, 38–48. [Google Scholar]
- Thenkabail, P.S.; Enclona, E.A.; Ashton, M.S.; van der Meer, V. Accuracy assessments of hyperspectral waveband performance for vegetation analysis applications. Remote Sens. Environ 2004, 91, 354–376. [Google Scholar]
- Thenkabail, P.S.; Enclona, E.A.; Ashton, M.S.; Legg, C.; Jean De Dieu, M. Hyperion, KONOS, ALI, and ETM+ sensors in the study of African rainforests. Remote Sens. Environ 2004, 90, 23–43. [Google Scholar]
- Tejera, N.A.; Rodes, R.; Ortega, E. Comparative analysis of physiological characteristics and yield components in sugarcane cultivars. Field Crops Res 2007, 102, 67–72. [Google Scholar]
- Belward, A.S. Spectral Characteristics of Vegetation, Soil and Water in the Visible, Near-Infrared and Middle-Infrared Wavelengths. In Remote Sensing and Geographical Information Systems for Resource Management in Developing Countries; Belward, A.S., Valenzuela, C.R., Eds.; Kluwer: Dordrecht, The Netherlands, 1991. [Google Scholar]
- Hoffer, R.M. Biological and Physical Considerations in Applying Computer Aided Analysis Techniques to Remote Sensor Data. In Remote Sensing: The Quantitative Approach; Swain, P.H., Davis, S.M., Eds.; McGraw Hill: New York, NY, USA, 1978; pp. 227–289. [Google Scholar]





| Plot 1 | Plot 2 | |
|---|---|---|
| Location | 13.866°N 102.287°E | 13.710°N 102.214°E |
| Soil texture | loamy sand | loamy clay |
| Annual precipitation, mm (2010) | 1,268.2 | 1,296.6 |
| Annual air temperature, °C (2010) | 22.91/35.14 (Min/Max) | 22.8/34.08 (Min/Max) |
| Plot size | 36 m × 76 m | 36 m × 76 m |
| Sensor | Wavelength (nm) | Spectral Resolution (nm) | Number of Bands |
|---|---|---|---|
| Fieldspec® 3 spectroradiometer | 400–1,050 | 1.4 | 465 |
| 1,050–2,500 * | 2 | 560 |
| Data Set | Sample | Min (%N) | Max (%N) | Mean (%N) | Std Deviation (%N) |
|---|---|---|---|---|---|
| Calibration | 90 | 1.142 | 1.483 | 1.313 | 0.098 |
| Validation | 90 | 1.148 | 1.457 | 1.319 | 0.084 |
| Pooled | 180 | 1.142 | 1.483 | 1.316 | 0.091 |
| By Cultivar | |||||
| LK92-11 | 60 | 1.251 | 1.415 | 1.333 | 0.046 |
| KK-3 | 60 | 1.275 | 1.483 | 1.395 | 0.062 |
| K84-200 | 60 | 1.142 | 1.322 | 1.221 | 0.051 |
| VI | Variable | λ1/λ2 (nm) | Calibration Data Set (N = 90) | Validation Data Set (N = 90) | Pooled Data Set (N = 180) | |||
|---|---|---|---|---|---|---|---|---|
| R2c | RMSEc | R2v | RMSEv | R2cv | RMSEcv | |||
| NDI | FDS | 750/700 | 0.82 | 0.041 | 0.73 | 0.044 | 0.78 | 0.043 |
| CRDR | 748/690 | 0.82 | 0.041 | 0.73 | 0.043 | 0.78 | 0.043 | |
| BD | 748/680 | 0.81 | 0.042 | 0.70 | 0.047 | 0.76 | 0.045 | |
| RVI | FDS | 724/700 | 0.80 | 0.043 | 0.73 | 0.044 | 0.78 | 0.043 |
| CRDR | 748/630 | 0.83 | 0.041 | 0.73 | 0.044 | 0.78 | 0.043 | |
| BD | 748/670 | 0.82 | 0.042 | 0.70 | 0.048 | 0.76 | 0.045 | |
| VI | Variable | LK92-11 (N = 60) | KK-3 (N = 60) | K84-200 (N = 60) | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| λ1 | λ2 | R2v | λ1 | λ2 | R2v | λ1 | λ2 | R2v | ||
| NDI | FDS | 748 | 728 | 0.81 | 718 | 710 | 0.91 | 712 | 706 | 0.80 |
| CRDR | 570 | 748 | 0.80 | 736 | 712 | 0.90 | 712 | 700 | 0.79 | |
| BD | 748 | 584 | 0.78 | 716 | 710 | 0.92 | 746 | 680 | 0.75 | |
| RVI | FDS | 748 | 728 | 0.79 | 718 | 710 | 0.90 | 724 | 692 | 0.82 |
| CRDR | 748 | 510 | 0.78 | 712 | 574 | 0.91 | 712 | 700 | 0.82 | |
| BD | 748 | 586 | 0.78 | 716 | 710 | 0.91 | 712 | 678 | 0.76 | |
| Data set | VI | Variablewavelength | Regression Model | R2v |
|---|---|---|---|---|
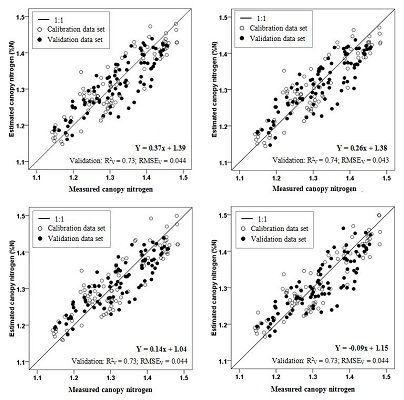
| Combined cultivars | NDI | FDS750,700 | Y = 0.37x + 1.39 | 0.73 |
| CRDR748,690 | Y = 0.26x + 1.38 | 0.73 | ||
| BD748,680 | Y = 9.64× + 10.77 | 0.70 | ||
| RVI | FDS724,700 | Y = 0.14x + 1.04 | 0.73 | |
| CRDR748,630 | Y = −0.09x + 1.15 | 0.73 | ||
| BD748,670 | Y = 18.79x + 1.12 | 0.70 | ||
| Separated cultivar - LK92-11 | NDI | FDS748,728 | Y = 0.95x + 1.71 | 0.79 |
| CRDR570,748 | Y = 0.11x + 1.12 | 0.80 | ||
| BD748,584 | Y = 4.58x + 5.72 | 0.78 | ||
| RVI | FDS748,728 | Y = 0.93x + 0.93 | 0.79 | |
| CRDR748,510 | Y = 0.24x + 1.21 | 0.78 | ||
| BD748,586 | Y = 9.05x + 1.14 | 0.78 | ||
| Separated cultivar - KK-3 | NDI | FDS718,710 | Y = 1.59x + 1.09 | 0.91 |
| CRDR736,712 | Y = 0.44x + 1.39 | 0.90 | ||
| BD716,710 | Y = 4.18x + 1.73 | 0.92 | ||
| RVI | FDS718,710 | Y = 0.53x + 0.61 | 0.90 | |
| CRDR712,574 | Y = 0.61x + 2.05 | 0.91 | ||
| BD716,710 | Y = 2.45x – 0.7 | 0.91 | ||
| Separated cultivar - K84-200 | NDI | FDS712,706 | Y = 1.45x + 1.18 | 0.80 |
| CRDR712,700 | Y = 0.75x + 1.23 | 0.79 | ||
| BD746,680 | Y = 3.47x + 4.59 | 0.75 | ||
| RVI | FDS724,692 | Y = 0.08x + 1.07 | 0.82 | |
| CRDR712,700 | Y = 0.39x + 0.84 | 0.82 | ||
| BD712,678 | Y = 0.63x + 0.86 | 0.76 | ||
| Variable | Wavelength (nm) | Calibration Data set (N = 90) | Validation Data set (N = 90) | Pooled Data Set (N = 180) | |||
|---|---|---|---|---|---|---|---|
| R2c | RMSEc | R2v | RMSEv | R2cv | RMSEcv | ||
| FDS | 410, 430, 720, 754,1216 | 0.90 | 0.030 | 0.80 | 0.038 | 0.86 | 0.033 |
| CRDR | 748, 1158, 1184, 1216, 1276 | 0.89 | 0.033 | 0.64 | 0.053 | 0.78 | 0.043 |
| BD | 748, 1262 | 0.83 | 0.040 | 0.70 | 0.047 | 0.77 | 0.044 |
| Variable | LK92-11 (N = 60) | KK-3 (N = 60) | K84-200 (N = 60) | |||
|---|---|---|---|---|---|---|
| Wavelength (nm) | R2cv | Wavelength (nm) | R2cv | Wavelength (nm) | R2cv | |
| FDS | 670, 754, 1,266, 1,494, 2,313 | 0.86 | 750, 1,104, 1,572, 1,586, 2,153 | 0.93 | 552, 1,032, 1,284, 1,604, 2,359 | 0.80 |
| CRDR | 656, 704, 1,266 | 0.79 | 598, 674, 740, 1,228 | 0.92 | 678, 746, 1,128 | 0.80 |
| BD | 684, 748, 1,228 | 0.82 | 552, 736 | 0.92 | 746 | 0.78 |
| Data set | Variable | Regression Model | R2v |
|---|---|---|---|
| Combined cultivars | FDS | Y = 212.76x1 – 471.9x2 – 31.98x3 + 133.78x 4 – 115.86x5 + 1.4 | 0.80 |
| CRDR | Y = 55.77x1 + 19.0x2 + 61.92x3 – 73.5x 4 – 34.25x5 + 1.26 | 0.64 | |
| BD | Y = 25.26x1 – 3.7x2 + 1.19 | 0.70 | |
| Separated cultivar -LK92-11 | FDS | Y = 520.59x1 + 14.794x2 – 97.004x3 + 95.08x 4 – 63.49x5 + 1.347 | 0.86 |
| CRDR | Y = 33.04x1 – 18.84x2 – 32.51x3 + 1.72 | 0.79 | |
| BD | Y = −0.47x1 + 17.86x2 – 0.7845x3 + 1.62 | 0.82 | |
| Separated cultivar-KK-3 | FDS | Y = 67.4x1 – 24.64x2 – 136.2x3 – 168.34x4 – 42.007x 5 + 1.4153 | 0.93 |
| CRDR | Y = −14.27x1 – 105.25x2 + 23.31x3 + 8.84x4 + 1.004 | 0.92 | |
| BD | Y = −3.31x1 + 2.26x2 + 1.17 | 0.92 | |
| Separated cultivar - K84-200 | FDS | Y = −194.8x1 + 138.92x2 – 94.52x3 – 213.97x 4 – 22.31x5 + 1.29 | 0.80 |
| CRDR | Y = −64.57x1 + 25.36x2 + 6.34x3 + 28.04x4 + 1.08 | 0.80 | |
| BD | Y = 7.86x1 + 1.12 | 0.78 | |
| Spectral Parameter | Regression Equation | Validation | No. of Samples | Study Level | Reference | |
|---|---|---|---|---|---|---|
| R2 | RMSE | |||||
| RVI (FDS741, FDS1323) | Y = −0.237x + 0.587 | 0.74 | 0.084 | 25 | Leaf | [26] |
| RVI (R740, R720) | Y = 0.73x + 0.753 | 0.81 | 0.103 | 37 | Leaf | [28] |
| NDI (FDS750, FDS700) | Y = 0.37x + 1.39 | 0.78 | 0.043 | 90 | Canopy | This paper |
| RVI (FDS724, FDS700) | Y = 0.14x + 1.04 | 0.78 | 0.043 | 90 | Canopy | This paper |
| FDS (410, 430, 720, 754,1216) * | Y = 212.76x1 – 471.9x2 – 31.98x3 + 133.78x 4 – 115.86x5 + 1.4 | 0.86 | 0.033 | 90 | Canopy | This paper |
Share and Cite
Miphokasap, P.; Honda, K.; Vaiphasa, C.; Souris, M.; Nagai, M. Estimating Canopy Nitrogen Concentration in Sugarcane Using Field Imaging Spectroscopy. Remote Sens. 2012, 4, 1651-1670. https://doi.org/10.3390/rs4061651
Miphokasap P, Honda K, Vaiphasa C, Souris M, Nagai M. Estimating Canopy Nitrogen Concentration in Sugarcane Using Field Imaging Spectroscopy. Remote Sensing. 2012; 4(6):1651-1670. https://doi.org/10.3390/rs4061651
Chicago/Turabian StyleMiphokasap, Poonsak, Kiyoshi Honda, Chaichoke Vaiphasa, Marc Souris, and Masahiko Nagai. 2012. "Estimating Canopy Nitrogen Concentration in Sugarcane Using Field Imaging Spectroscopy" Remote Sensing 4, no. 6: 1651-1670. https://doi.org/10.3390/rs4061651
APA StyleMiphokasap, P., Honda, K., Vaiphasa, C., Souris, M., & Nagai, M. (2012). Estimating Canopy Nitrogen Concentration in Sugarcane Using Field Imaging Spectroscopy. Remote Sensing, 4(6), 1651-1670. https://doi.org/10.3390/rs4061651