Ground-Based Hyperspectral Characterization of Alaska Tundra Vegetation along Environmental Gradients
Abstract
:1. Introduction
2. Material & Methods
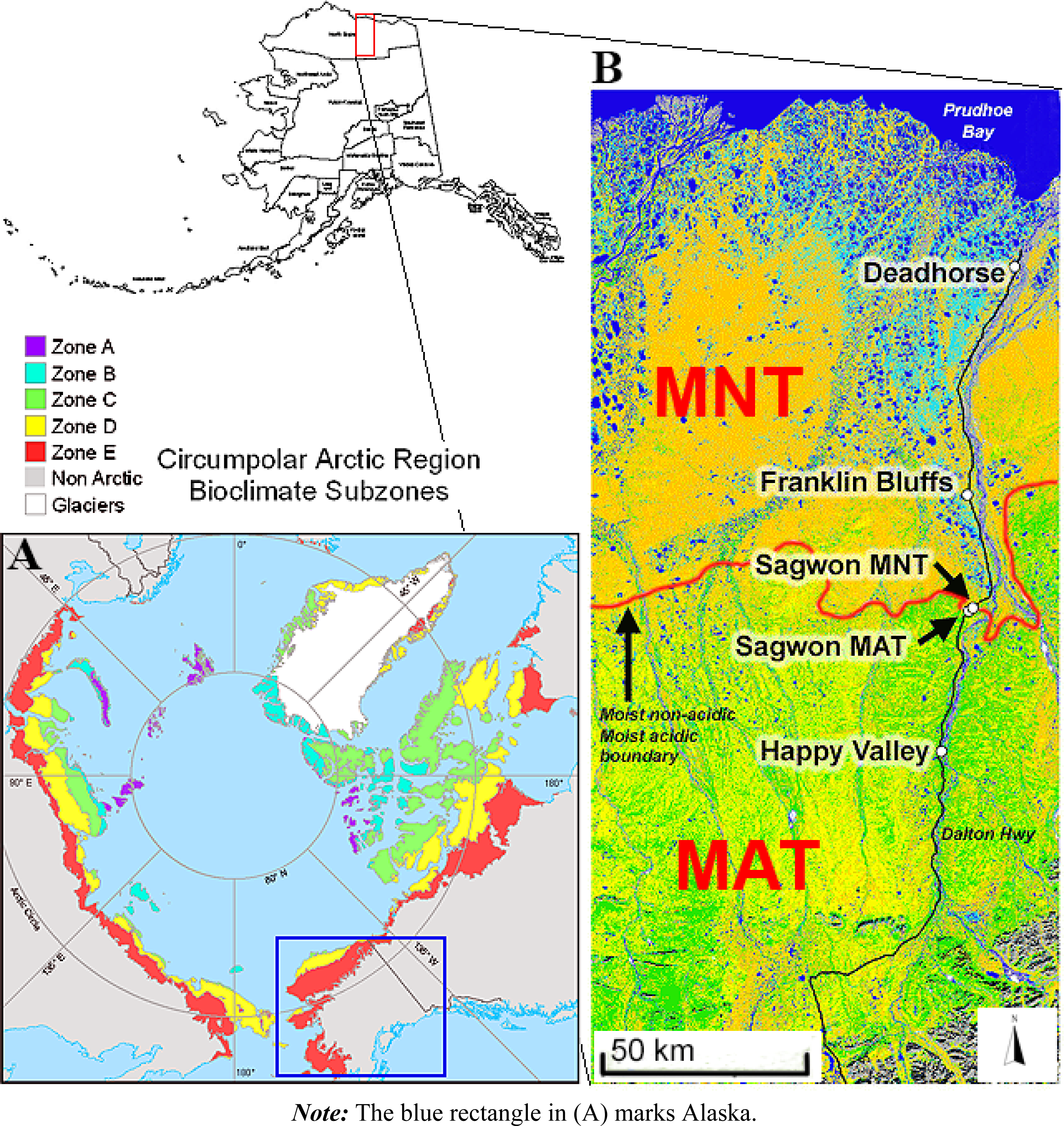
2.1. Study Area
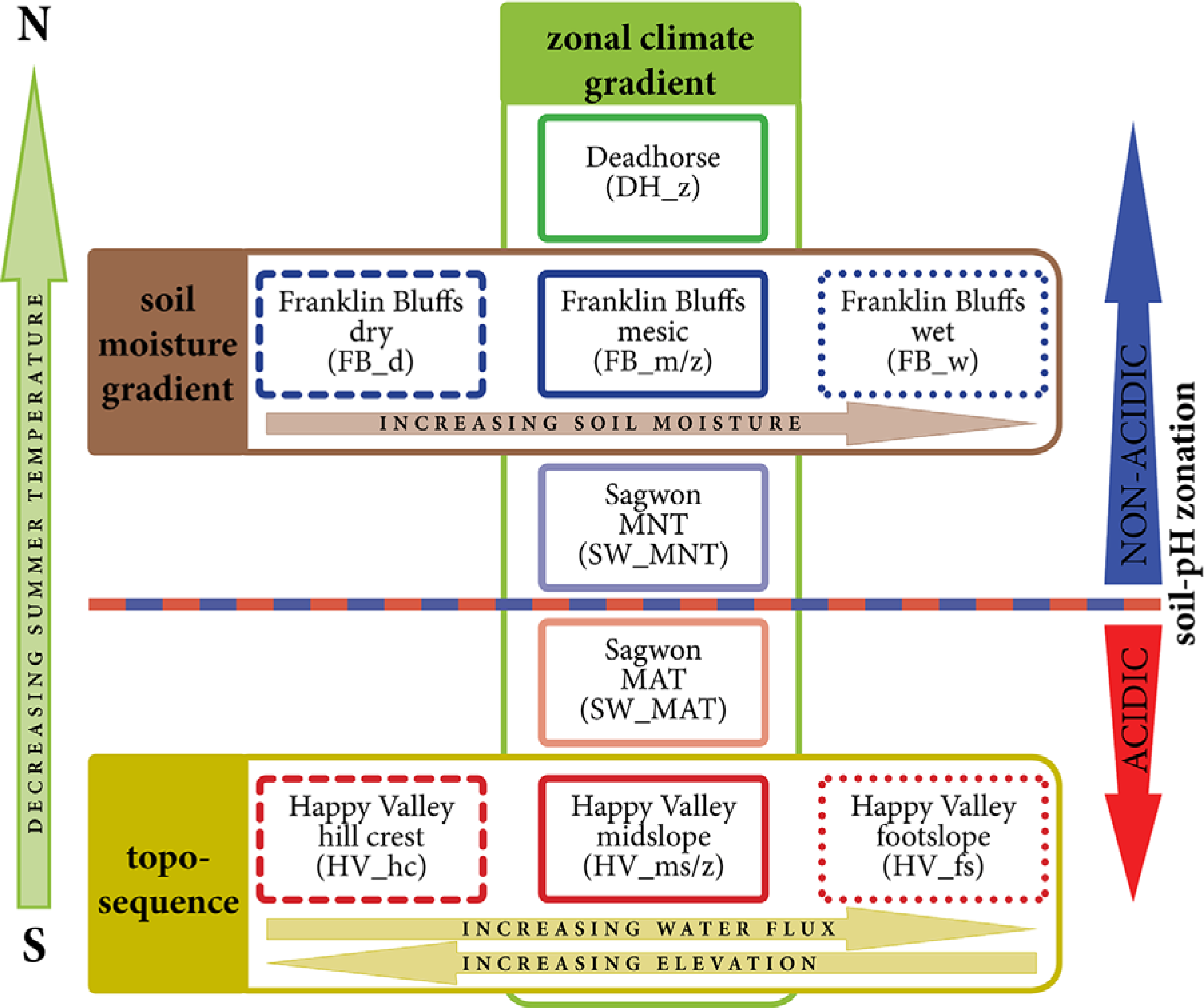
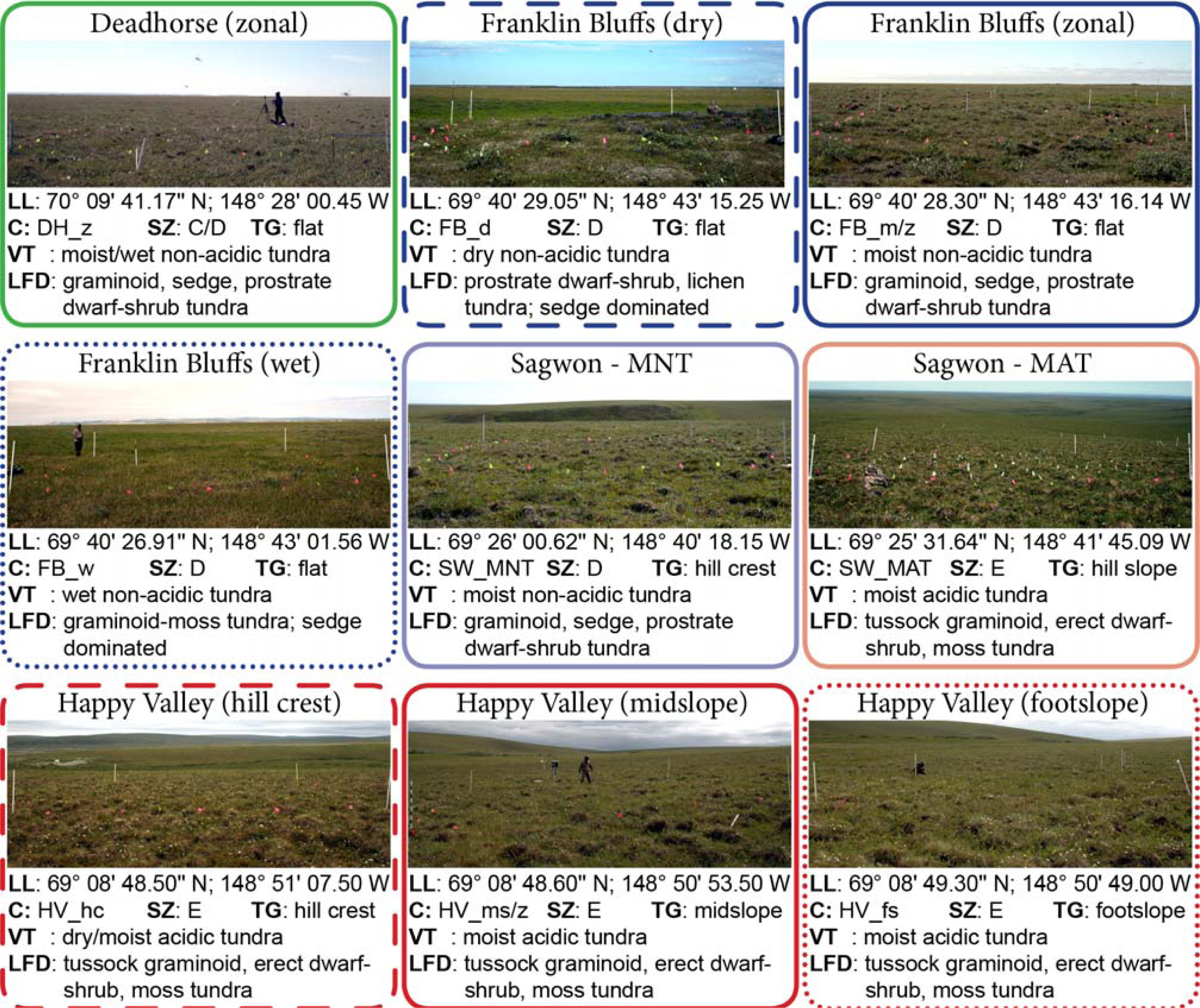
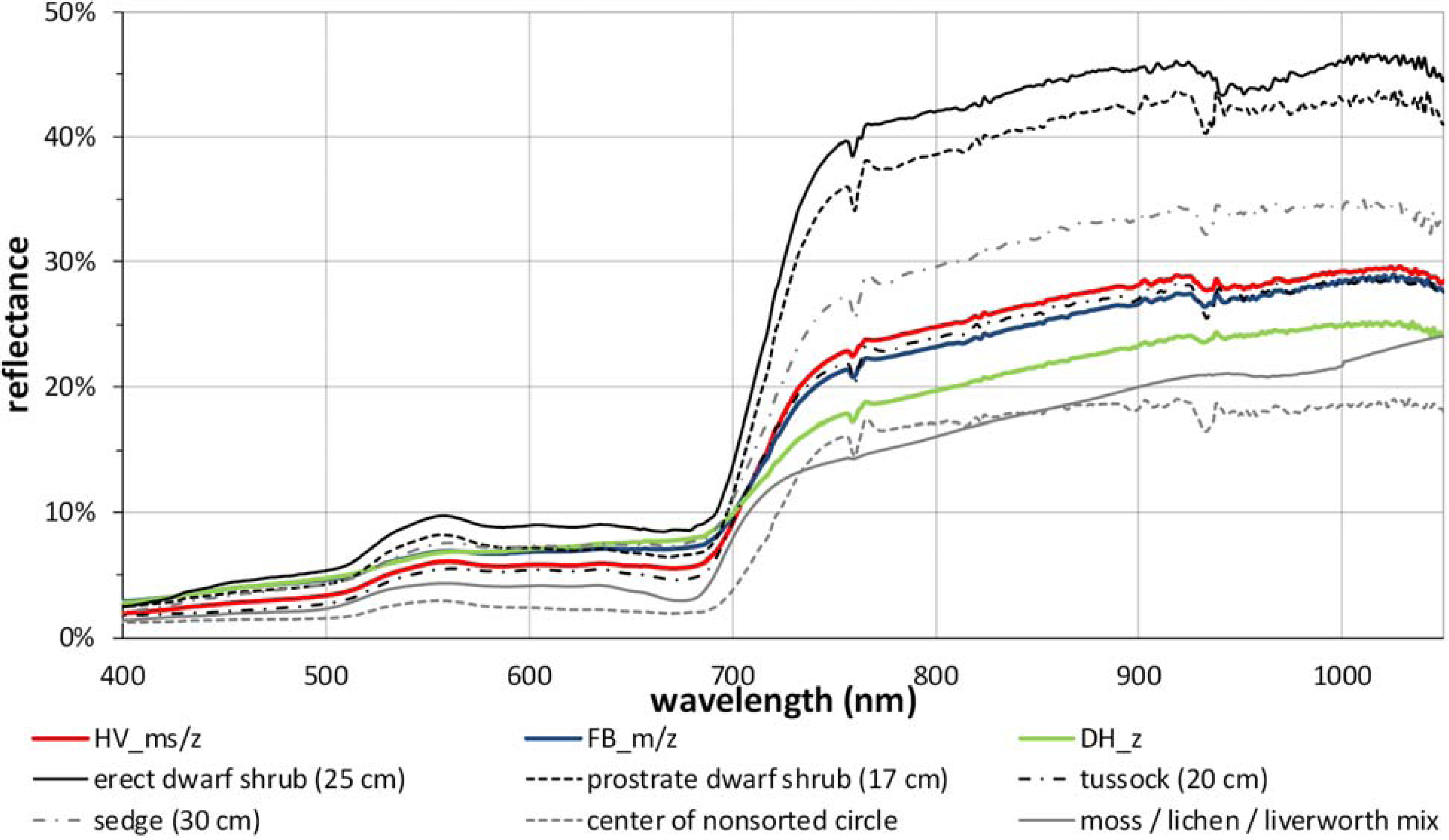
2.2. Environmental Gradients/Zones and Vegetation Description
2.3. Data Acquisition and Pre-Processing
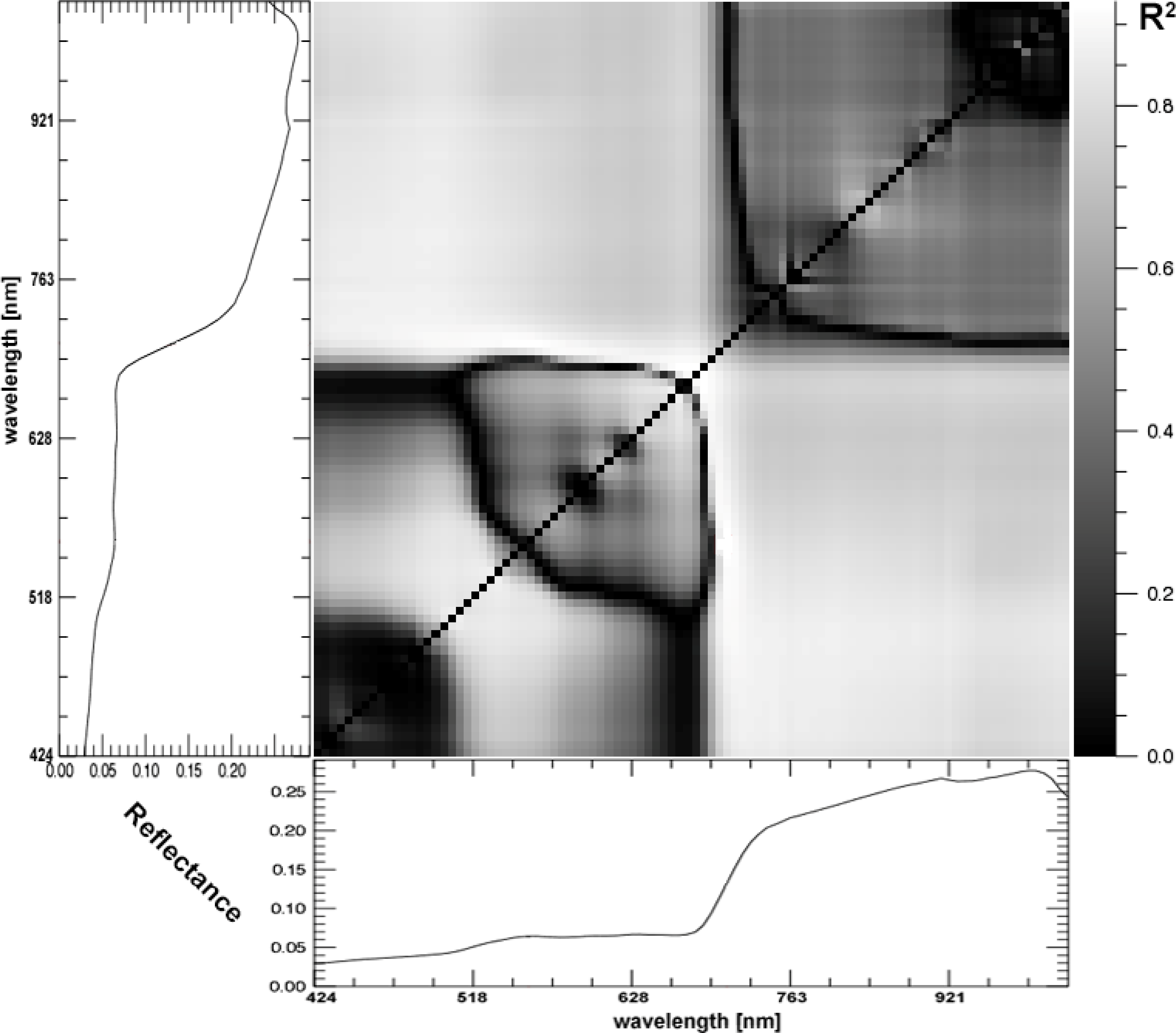
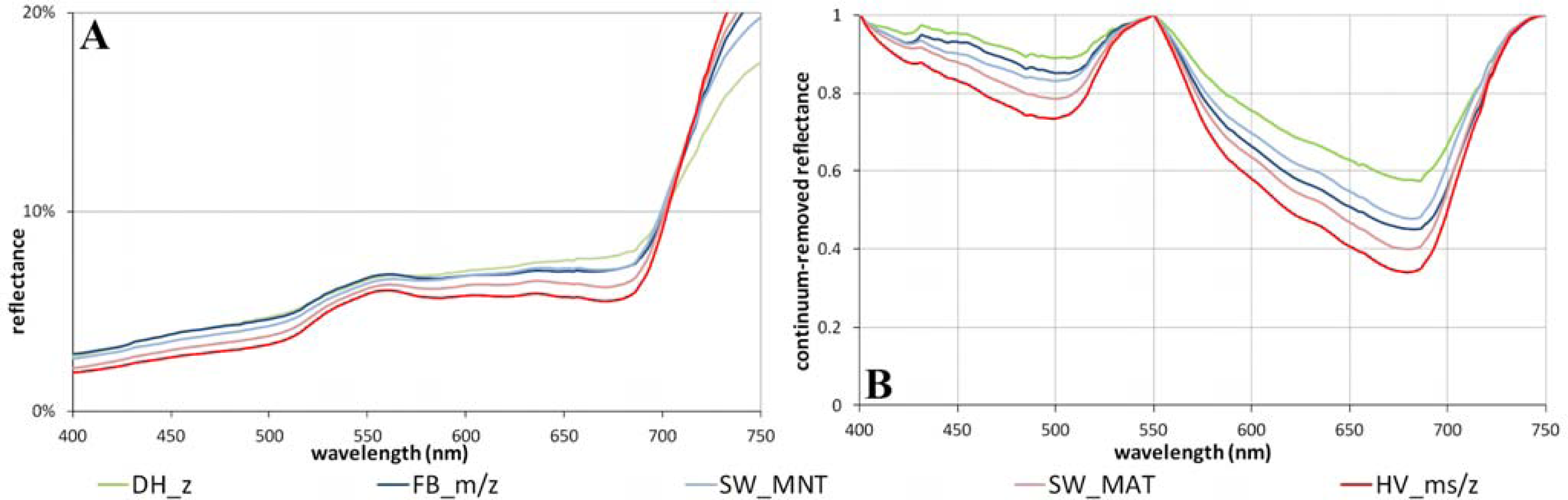
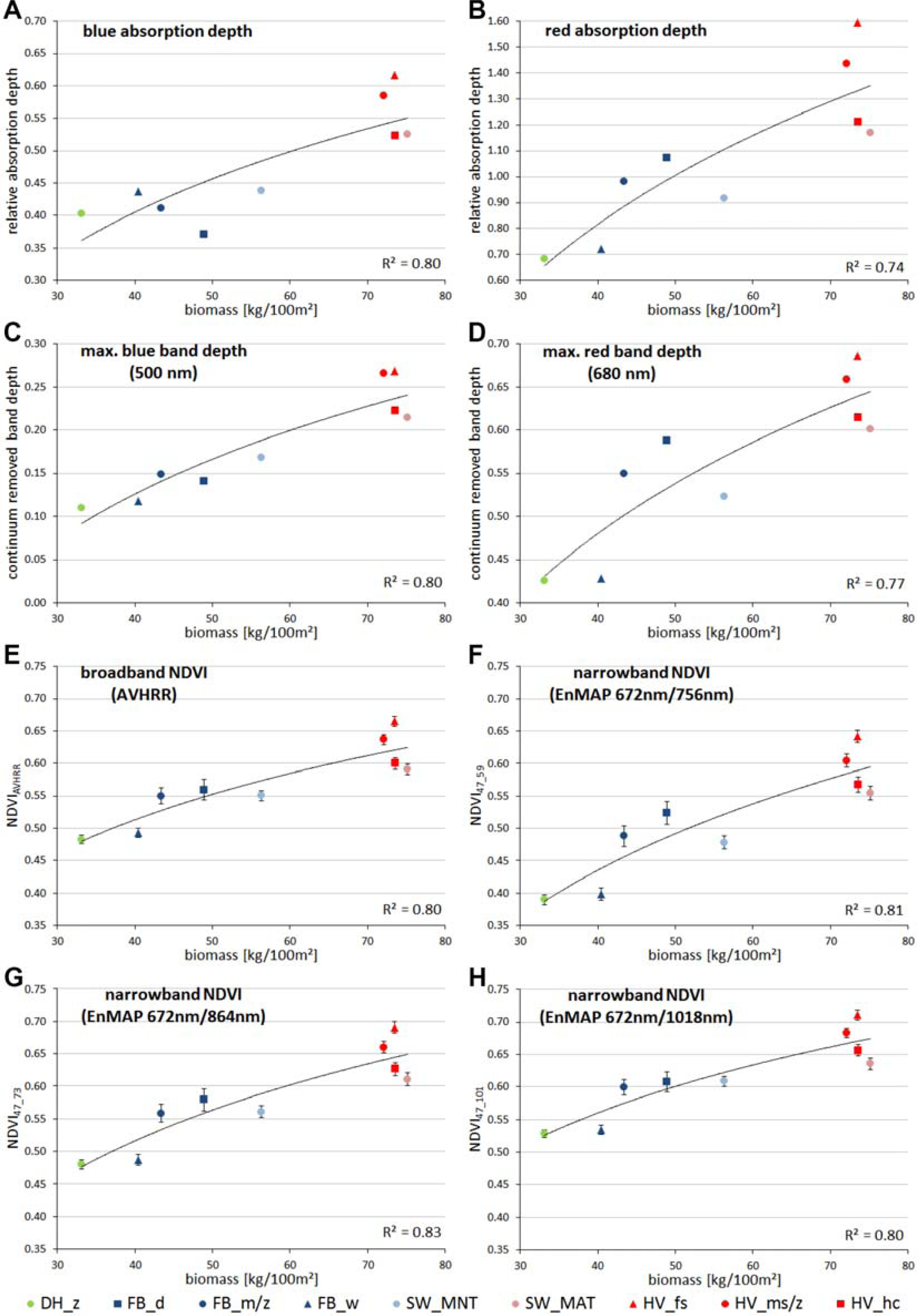
2.4. Data Analysis
3. Results
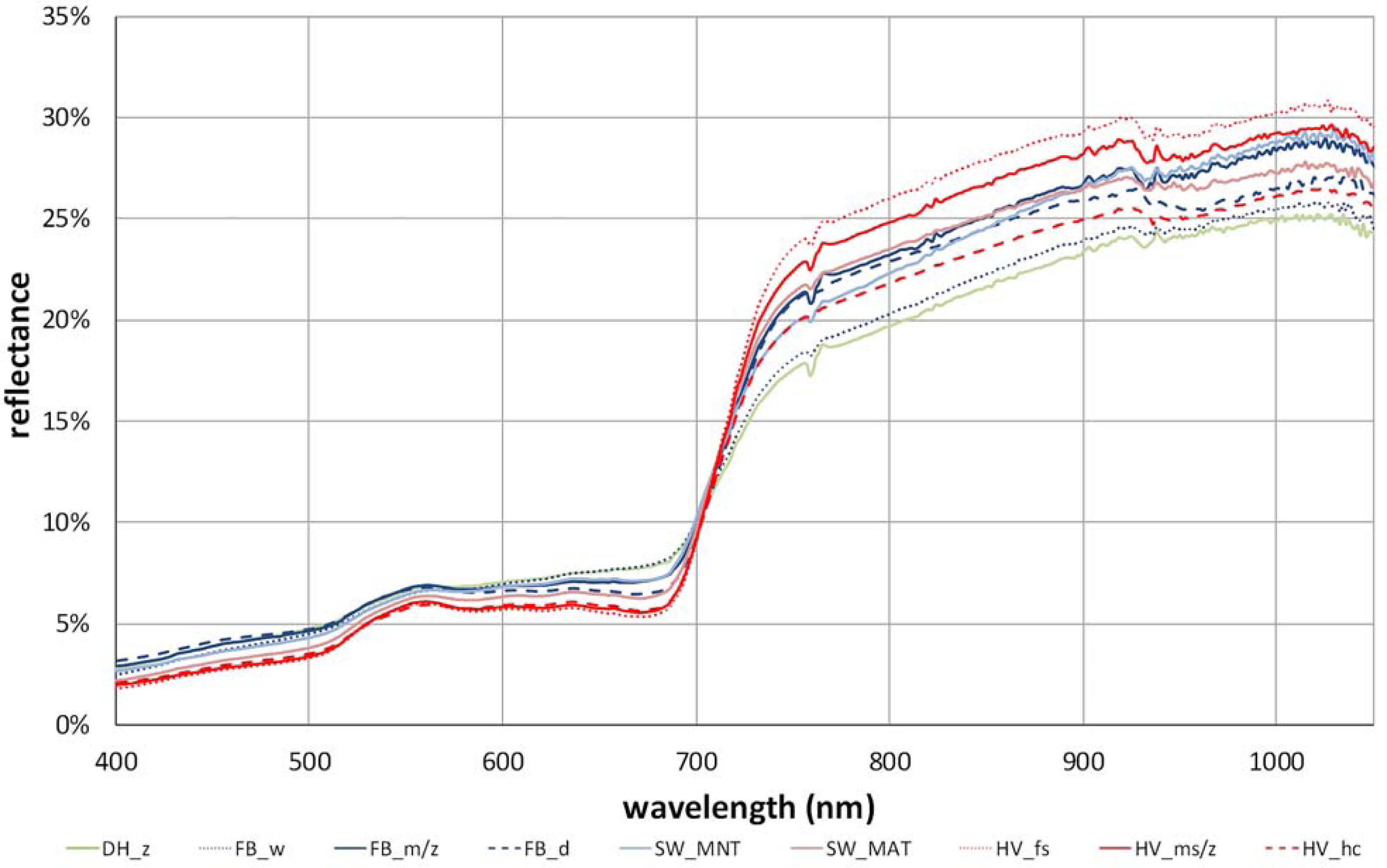
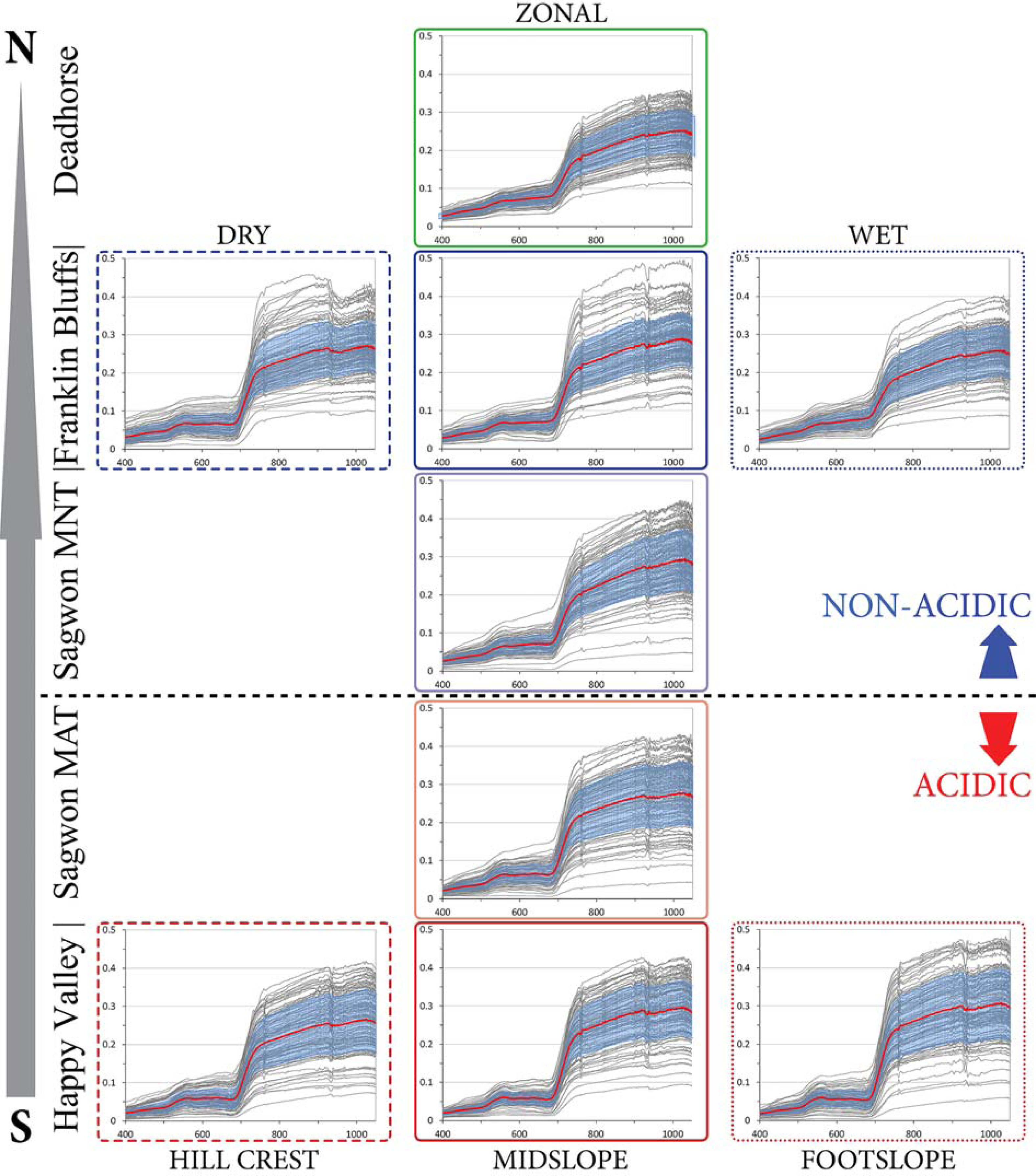
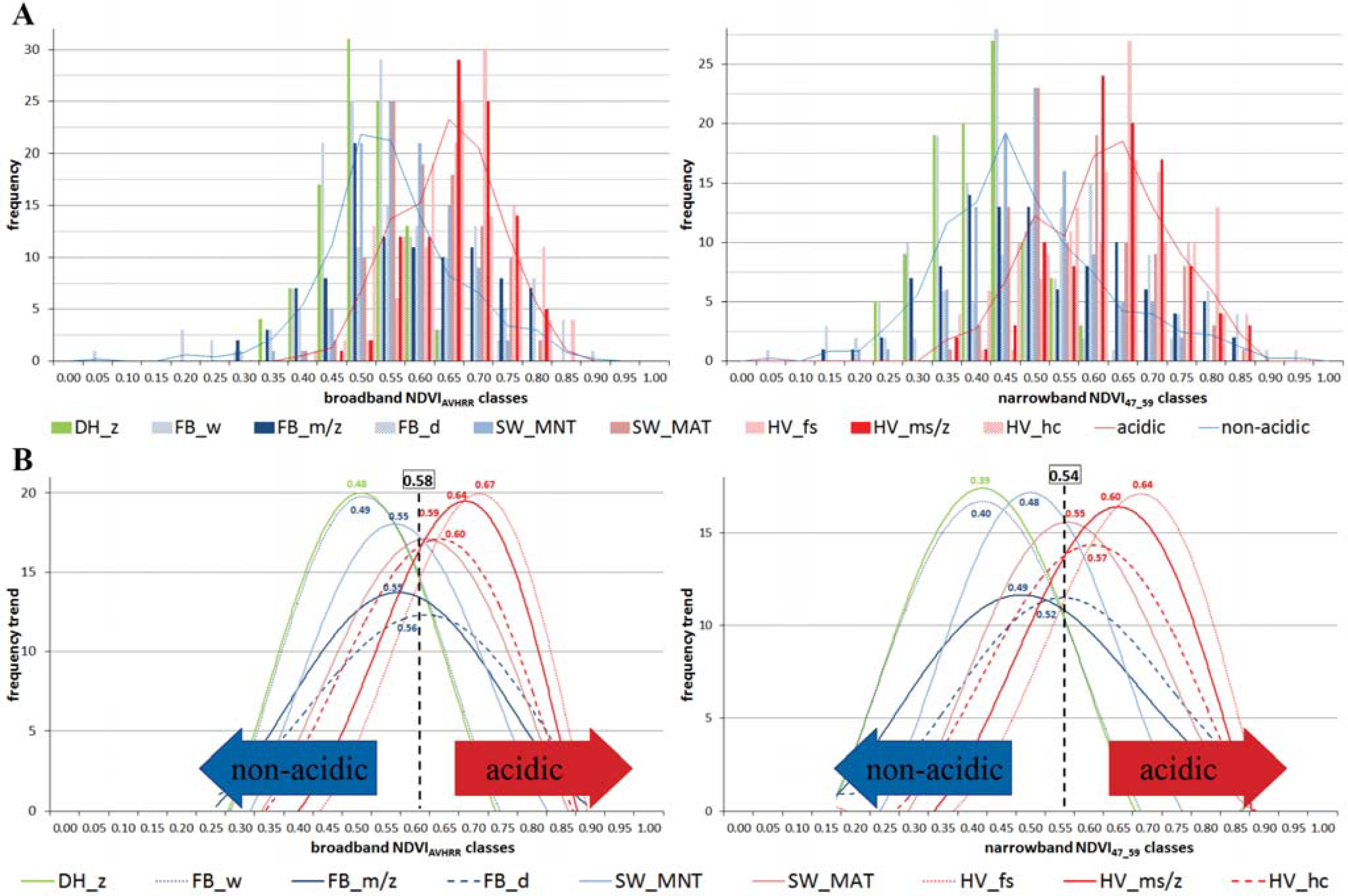
3.1. The Zonal Climate Gradient
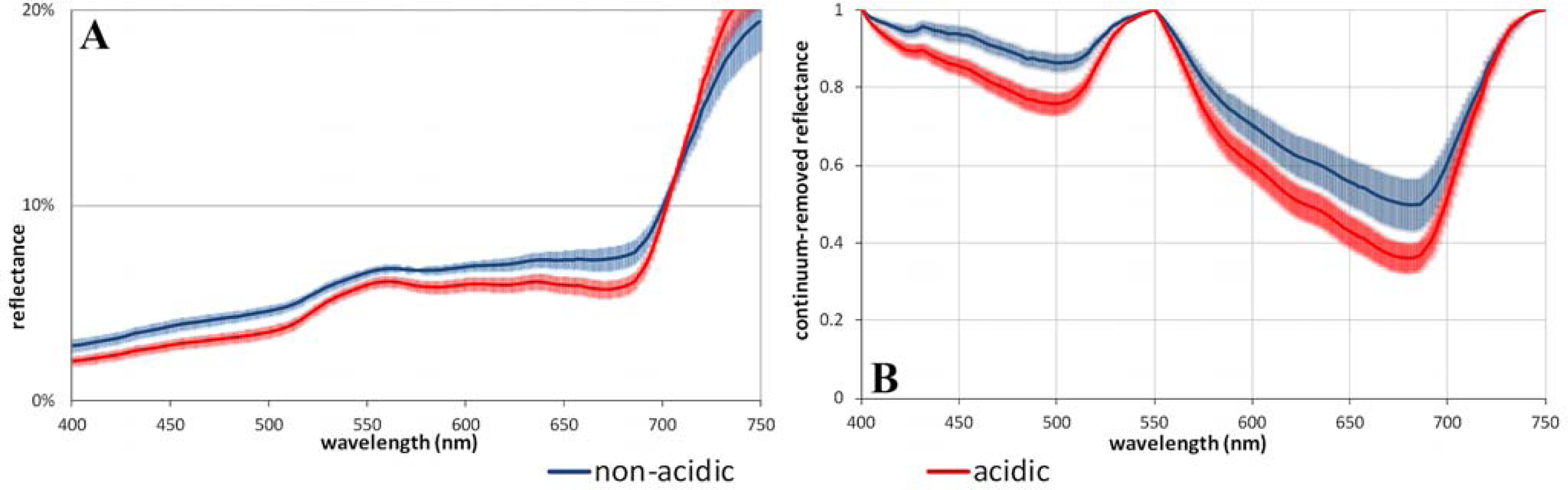
3.2. Acidic vs. Non-Acidic Tundra (Soil pH Zones)
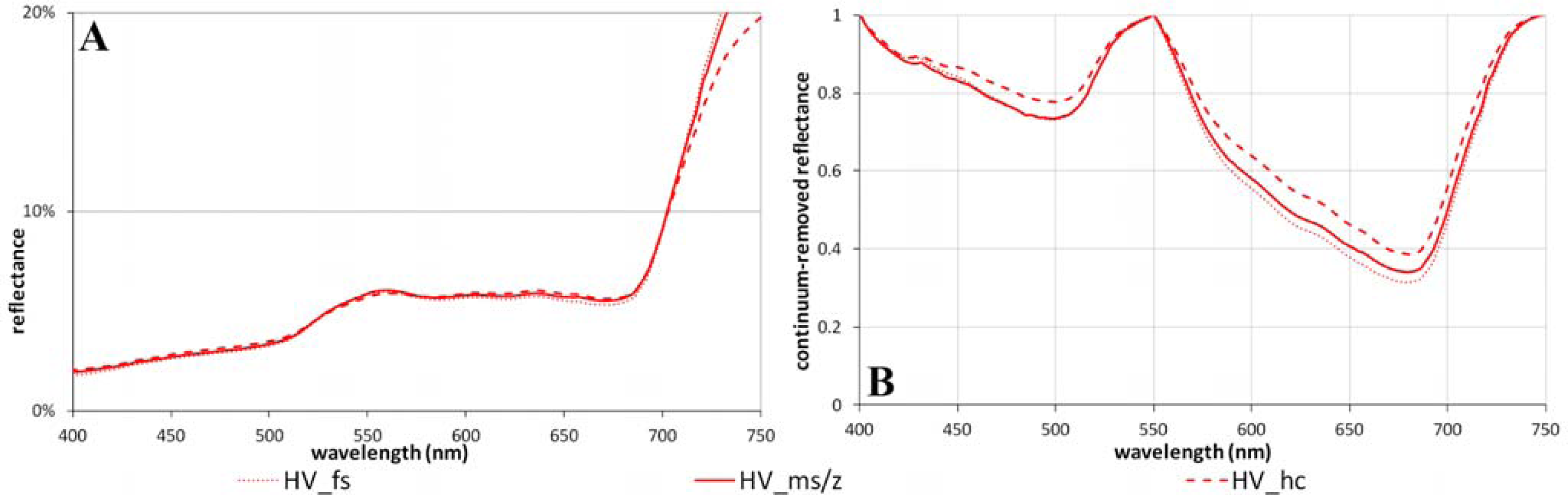
3.3. The Toposequence at Happy Valley (Subzone E)
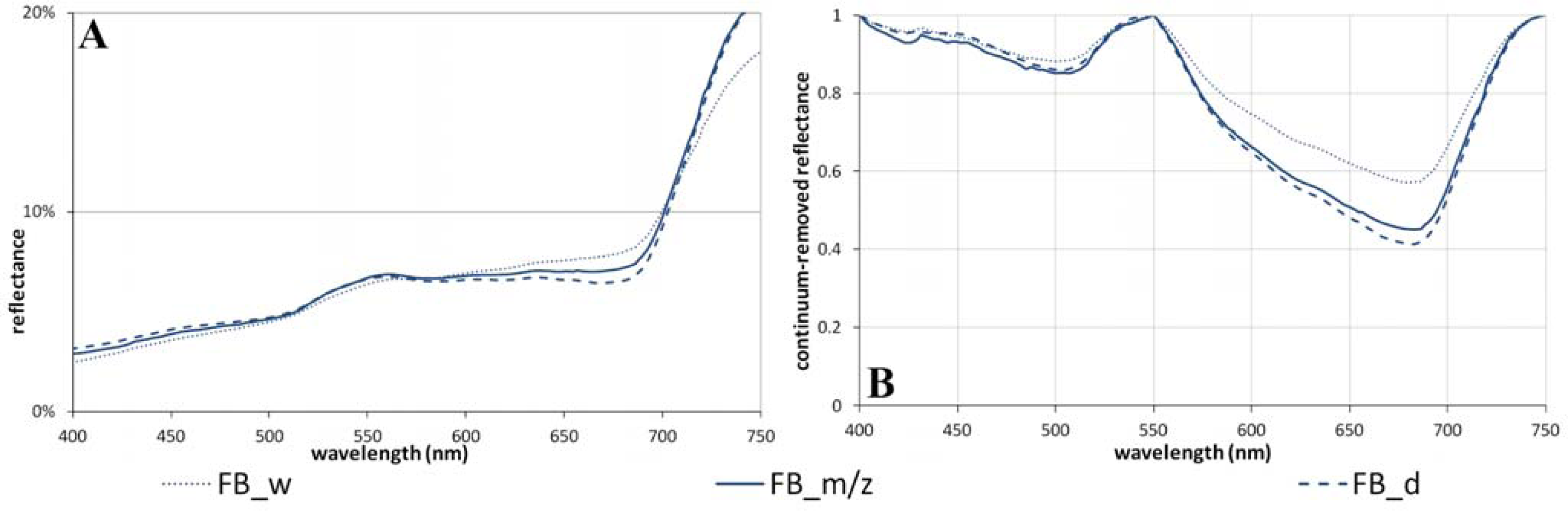
3.4. The Soil Moisture Gradient at Franklin Bluffs (Subzone D)
4. Discussion
4.1. Overview of Field Characterization and Spectral Properties along the Gradients
4.2. Performance of Spectral Metrics and Vegetation Indices
5. Conclusions
Acknowledgments
Conflicts of Interest
References
- Bhatt, U.S.; Walker, D.A.; Raynolds, M.K.; Comiso, J.C.; Epstein, H.E.; Jia, G.; Gens, R.; Pinzon, J.E.; Tucker, C.J.; Tweedie, C.E.; et al. Circumpolar arctic tundra vegetation change is linked to sea ice decline. Earth Interact 2010, 14, 1–20. [Google Scholar]
- Lawrence, D.M.; Slater, A.G.; Tomas, R.A.; Holland, M.M.; Deser, C. Accelerated Arctic land warming and permafrost degradation during rapid sea ice loss. Geophys. Res. Lett. 2008. [Google Scholar] [CrossRef]
- Callaghan, T.V.; Tweedie, C.E.; Webber, P.J. Multi-decadal changes in tundra environments and ecosystems: The international polar year-back to the future project (IPY-BTF). AMBIO 2011, 40, 555–557. [Google Scholar]
- Epstein, H.E.; Raynolds, M.K.; Walker, D.A.; Bhatt, U.S.; Tucker, C.J.; Pinzon, J.E. Dynamics of aboveground phytomass of the circumpolar Arctic tundra during the past three decades. Environ. Res. Lett 2012, 7, 15506. [Google Scholar]
- Walker, M.D. From The Cover: Plant community responses to experimental warming across the tundra biome. Proc. Natl. Acad. Sci. USA 2006, 103, 1342–1346. [Google Scholar]
- Winton, M. Amplified Arctic climate change: What does surface albedo feedback have to do with it? Geophys. Res. Lett 2006. [Google Scholar] [CrossRef]
- Walker, D.A.; Bhatt, U.S.; Epstein, H.E.; Bieniek, P.; Comiso, J.; Frost, G.V.; Pinzon, J.; Raynolds, M.K.; Tucker, C.J. Changing arctic tundra vegetation biomass and greenness—State of the climate in 2011. Bull. Amer. Meteor. Soc 2012, 93, S138–S139. [Google Scholar]
- Ulrich, M.; Grosse, G.; Chabrillat, S.; Schirrmeister, L. Spectral characterization of periglacial surfaces and geomorphological units in the Arctic Lena Delta using field spectrometry and remote sensing. Remote Sens. Environ 2009, 113, 1220–1235. [Google Scholar] [Green Version]
- Olthof, I.; Fraser, R. Mapping northern land cover fractions using Landsat ETM+. Remote Sens. Environ 2007, 107, 496–509. [Google Scholar]
- Stow, D.A.; Hope, A.; McGuire, D.; Verbyla, D.; Gamon, J.; Huemmrich, F.; Houston, S.; Racine, C.; Sturm, M.; Tape, K.; et al. Remote sensing of vegetation and land-cover change in arctic tundra ecosystems. Remote Sens. Environ 2004, 89, 281–308. [Google Scholar]
- Raynolds, M.K.; Walker, D.A.; Epstein, H.E.; Pinzon, J.E.; Tucker, C.J. A new estimate of tundra-biome phytomass from trans-Arctic field data and AVHRR NDVI. Remote Sens. Lett 2012, 3, 403–411. [Google Scholar]
- Vierling, L.A.; Deering, D.W.; Eck, T.F. Differences in arctic tundra vegetation type and phenology as seen using bidirectional radiometry in the early growing season. Remote Sens. Environ 1997, 60, 71–82. [Google Scholar]
- Kääb, A. Remote sensing of permafrost-related problems and hazards. Permafrost. Periglacial. Pro 2008, 19, 107–136. [Google Scholar]
- Laidler, G.; Treitz, P.; Atkinson, D. Remote sensing of Arctic vegetation: Relations between the NDVI, spatial resolution and vegetation cover on Boothia Peninsula, Nunavut. Arctic 2008, 61, 1–13. [Google Scholar]
- Riedel, S.M.; Epstein, H.E.; Walker, D.A. Biotic controls over spectral reflectance of arctic tundra vegetation. Int. J. Remote Sens 2005, 26, 2391–2405. [Google Scholar]
- Huemmrich, F.; Gamon, J.A.; Tweedie, C.; Oberbauer, S.F.; Kinoshita, G.; Houston, S.; Kuchy, A.; Hollister, R.D.; Kwon, H.; Mano, M.; et al. Remote sensing of tundra gross ecosystem productivity and light use efficiency under varying temperature and moisture conditions. Remote Sens. Environ 2010, 114, 481–489. [Google Scholar]
- Hope, A.; Kimball, J.S.; Stow, D.A. The relationship between tussock tundra spectral reflectance properties and biomass and vegetation composition. Int. J. Remote Sens 1993, 14, 1861–1874. [Google Scholar]
- Olthof, I.; Latifovic, R. Short-term response of arctic vegetation NDVI to temperature anomalies. Int. J. Remote Sens 2007, 28, 4823–4840. [Google Scholar]
- Walker, D.A.; Epstein, H.E.; Jia, G.J.; Balser, A.; Copass, C.; Edwards, W.J.; Gould, W.A.; Hollingsworth, J.; Knudson, J.; Maier, H.A.; et al. Phytomass, LAI, and NDVI in northern Alaska: Relationships to summer warmth, soil pH, plant functional types, and extrapolation to the circumpolar Arctic. J. Geophys. Res. 2003. [Google Scholar] [CrossRef]
- Horler, D.N.H.; Dockray, M.; Barber, J. The red edge of plant leaf reflectance. Int. J. Remote Sens 1983, 4, 273–288. [Google Scholar]
- Elvidge, C.D. Vegetation Reflectance Features in AVIRIS Data. Proceedings of the Sixth Thematic Conference on Remote Sensing for Exploration Geology: Applications, Technology, Economics, Houston, TX, USA, 16–19 May 1988; pp. 169–182.
- Barnsley, M.; Settle, J.; Cutter, M.; Lobb, D.; Teston, F. The PROBA/CHRIS mission: A low-cost smallsat for hyperspectral multiangle observations of the Earth surface and atmosphere. IEEE Trans. Geosci. Remote Sens 2004, 42, 1512–1520. [Google Scholar]
- Pearlman, J.; Barry, P.; Segal, C.; Shepanski, J.; Beiso, D.; Carman, S. Hyperion, a space-based imaging spectrometer. IEEE Trans. Geosci. Remote Sens 2003, 41, 1160–1173. [Google Scholar]
- Stuffler, T.; Kaufmann, C.; Hofer, S.; Förster, K.P.; Schreier, G.; Mueller, A.; Eckardt, A.; Bach, H.; Penné, B.; Benz, U.; et al. The EnMAP Hyperspectral Imager—An Advanced Optical Payload for Future Applications in Earth Observation Programmes: Bringing Space Closer to People. Proceedings of the 57th International AstronauticalFederation (IAF) Congress, Valencia, Spain, 2–6 October 2006; pp. 115–120.
- Schaepman, M.E.; Ustin, S.L.; Plaza, A.J.; Painter, T.H.; Verrelst, J.; Liang, S. Earth system science related imaging spectroscopy—An assessment. Remote Sens. Environ 2009, 113, S123. [Google Scholar]
- Asner, G.P. Biophysical and biochemical sources of variability in canopy reflectance. Remote Sens. Environ 1998, 64, 234–253. [Google Scholar]
- Jakomulska, A.; Zagajewski, B.; Sobczak, M. Field Remote Sensing Techniques for Mountains Vegetation Investigation. Proceedings of the 3rd EARSeL workshop on Imaging Spectroscopy, Herrsching, Germany, 13–16 May 2003; pp. 580–596.
- Gitelson, A.A.; Gritz, Y.; Merzlyak, M.N. Relationships between leaf chlorophyll content and spectral reflectance and algorithms for non-destructive chlorophyll assessment in higher plant leaves. J. Plant. Physiol 2003, 160, 271–282. [Google Scholar]
- Gausman, H.W. Leaf reflectance of near-infrared. Photogramm. Eng. Remote Sensing 1974, 40, 183–191. [Google Scholar]
- Richardson, A.J.; Wiegand, C.L. Distinguishing vegetation from soil background information: A gray mapping technique allows delineation of any Landsat scene into vegetative cover stages, degrees of soil brightness, and water. Photogramm. Eng. Remote Sensing 1977, 43, 1541–1552. [Google Scholar]
- Thenkabail, P.S.; Smith, R.B.; de Pauw, E. Hyperspectral vegetation indices and their relationships with agricultural crop characteristics. Remote Sens. Environ 2000, 71, 158–182. [Google Scholar]
- Roberts, D.; Roth, K.; Perroy, R. Hyperspectral Vegetation Indices. In Hyperspectral Remote Sensing of Vegetation; Thenkabail, P.S., Lyon, J.G., Huete, A., Eds.; CRC Press/Taylor and Francis Group: Boca Raton, FL, USA/London, UK/New York, NY, USA, 2011; pp. 309–328. [Google Scholar]
- Elvidge, C.D.; Chen, Z. Comparison of broad-band and narrow-band red and near-infrared vegetation indices. Remote Sens. Environ 1995, 54, 38–48. [Google Scholar]
- Thenkabail, P.; Lyon, J.; Huete, A. Advances in Hyperspectral Remote Sensing of Vegetation and Agricultural Croplands. In Hyperspectral Remote Sensing of Vegetation; Thenkabail, P.S., Lyon, J.G., Huete, A., Eds.; CRC Press/Taylor and Francis Group: Boca Raton, FL, USA/London, UK/New York, NY, USA, 2011; pp. 3–36. [Google Scholar]
- Walker, D.A.; Raynolds, M.K.; Daniëls, F.J.A.; Einarsson, E.; Elvebakk, A.; Gould, W.A.; Katenin, A.E.; Kholod, S.S.; Markon, C.J.; Melnikov, E.S.; et al. The Circumpolar arctic vegetation map. J. Veg. Sci 2005, 16, 267–282. [Google Scholar]
- Kuparuk River Basin Vegetation: Vegetation Classification for the Kuparuk River Basin Vegetation Mapping Project; Alaska Geobotany Center: Fairbanks, AK, USA, 2010.
- Walker, D.A.; Epstein, H.E.; Romanovsky, V.E.; Ping, C.L.; Michaelson, G.J.; Daanen, R.P.; Shur, Y.; Peterson, R.A.; Krantz, W.B.; Raynolds, M.K.; et al. Arctic patterned-ground ecosystems: A synthesis of field studies and models along a North American Arctic Transect. J. Geophys. Res. 2008. [Google Scholar] [CrossRef]
- Buchhorn, M.; Schwieder, M. Expedition “EyeSight-NAAT-Alaska” 2012. In Expeditions to Permafrost 2012: “Alaskan North Slope/Itkillik”,“Thermokarst in Central Yakutia”, “EyeSight-NAAT-Alaska”; Alfred-Wegener-Inst. für Polar- und Meeresforschung: Bremerhaven, Germany, 2012; pp. 41–65. [Google Scholar]
- Kade, A.; Walker, D.A.; Raynolds, M.K. Plant communities and soils in cryoturbated tundra along a bioclimate gradient in the Low Arctic, Alaska. Phytocoenologia 2005, 35, 761–820. [Google Scholar]
- Vonlanthen, C.M.; Walker, D.A.; Raynolds, M.K.; Kade, A.; Kuss, P.; Daniëls, F.J.A.; Matveyeva, N.V. Patterned-ground plant communities along a bioclimate gradient in the high arctic, Canada. Phytocoenologia 2008, 38, 23–63. [Google Scholar]
- Walker, D.A.; Epstein, H.E.; Welker, J.M. Introduction to special section on biocomplexity of arctic tundra ecosystems. J. Geophys. Res 2008. [Google Scholar] [CrossRef]
- Walker, D.A.; Kuss, P.; Epstein, H.E.; Kade, A.N.; Vonlanthen, C.M.; Raynolds, M.K.; Daniëls, F.J. Vegetation of zonal patterned-ground ecosystems along the North America Arctic bioclimate gradient. Appl. Veg. Sci 2011, 14, 440–463. [Google Scholar]
- Alaska Geobotany Center. North American Arctic Transect, Data Reports. 2013. Available online: http://www.geobotany.uaf.edu/naat/data (accessed on 6 February 2013).
- Walker, D.A. Hierarchical subdivision of Arctic tundra based on vegetation response to climate, parent material and topography. Glob. Change. Biol 2000, 6, 19–34. [Google Scholar]
- Razzhivin, V.Y. Zonation of Vegetation in the Russian Arctic. In The Species Concept in the High North: A Panarctic Flora Initiative; Nordal, I., Razzhivin, V.Y., Eds.; Norwegian Academy of Science and Letters: Oslo, Norway, 1999; pp. 113–130. [Google Scholar]
- Walker, D.A.; Auerbach, N.A.; Bockheim, J.G.; Chapin, F.S.; Eugster, W.; King, J.Y.; McFadden, J.P.; Michaelson, G.J.; Nelson, F.E.; Oechel, W.C.; et al. Energy and trace-gas fluxes across a soil pH boundary in the Arctic. Nature 1998, 394, 469–472. [Google Scholar]
- Zhang, T.; Osterkamp, T.E.; Stamnes, K. Some characteristics of the climate in Northern Alaska, USA. Arctic Alp. Res 1996, 28, 509–518. [Google Scholar]
- Walker, M.D.; Walker, D.A.; Auerbach, N.A. Plant communities of a tussock tundra landscape in the brooks range Foothills, Alaska. J. Veg. Sci 1994, 5, 843–866. [Google Scholar]
- Washburn, A.L. Geocryology: A Survey of Periglacial Processes and Environments; Wiley: New York, NY, USA, 1980. [Google Scholar]
- De Molenaar, J.G. An Ecohydrological approach to floral and vegetational patterns in arctic landscape ecology. Arctic Alp. Res 1987, 19, 414–424. [Google Scholar]
- Billings, W.D. Arctic and alpine vegetations: Similarities, differences, and susceptibility to disturbance. BioScience 1973, 23, 697–704. [Google Scholar]
- Walker, D.A.; Auerbach, N.A.; Nettleton, T.K.; Gallant, A.; Murphy, S.M. Happy Valley Permanent Vegetation Plots; Institute of Arctic and Alpine Research: Boulder, CO, USA, 1997. [Google Scholar]
- Epstein, H.E.; Walker, D.A.; Raynolds, M.K.; Jia, G.J.; Kelley, A.M. Phytomass patterns across a temperature gradient of the North American arctic tundra. J. Geophys. Res. 2008. [Google Scholar] [CrossRef]
- Raynolds, M.K.; Walker, D.A.; Munger, C.A.; Vonlanthen, C.M.; Kade, A.N. A map analysis of patterned-ground along a North American arctic transect. J. Geophys. Res 2008, 113, 1–18. [Google Scholar]
- Walker, D.A.; Epstein, H.E.; Raynolds, M.K.; Kuss, P.; Kopecky, M.A.; Frost, G.V.; Daniëls, F.J.A.; Leibman, M.O.; Moskalenko, N.G.; Matyshak, G.V.; et al. Environment, vegetation and greenness (NDVI) along the North America and Eurasia arctic transects. Environ. Res. Lett 2012, 7, 15504. [Google Scholar]
- Dierschke, H. Pflanzensoziologie: Grundlagen und Methoden: 55 Tabellen; Ulmer: Stuttgart, Germany, 1994. [Google Scholar]
- Kuehni, R.G. The early development of the Munsell system. Color Res. Appl 2002, 27, 20–27. [Google Scholar]
- Spectra Vista Corporation. GER 1500—User manual: Revision 3.8; Spectra Vista Corporation: Poughkeepsie, NY, USA, 2009. [Google Scholar]
- Milton, E.J. Review Article Principles of field spectroscopy. Int. J. Remote Sens 1987, 8, 1807–1827. [Google Scholar]
- Milton, E.J.; Schaepman, M.E.; Anderson, K.; Kneubühler, M.; Fox, N. Progress in field spectroscopy: Imaging spectroscopy special issue. Remote Sens. Environ 2009, 113, S92–S109. [Google Scholar]
- Schopfer, J.; Dangel, S.; Kneubühler, M.; Itten, K.I. The improved dual-view field goniometer system FIGOS. Sensors 2008, 8, 5120–5140. [Google Scholar]
- Suomalainen, J.; Hakala, T.; Peltoniemi, J.; Puttonen, E. Polarised multiangular reflectance measurements using the finnish geodetic institute field goniospectrometer. Sensors 2009, 9, 3891–3907. [Google Scholar]
- Crowley, J.K.; Brickey, D.W.; Rowan, L.C. Airborne imaging spectrometer data of the Ruby Mountains, Montana: Mineral discrimination using relative absorption band-depth images. Remote Sens. Environ 1989, 29, 121–134. [Google Scholar]
- Clark, R.N.; Roush, T.L. Reflectance spectroscopy: Quantitative analysis techniques for remote sensing applications. J. Geophys. Res 1984, 89, 6329–6340. [Google Scholar]
- Kokaly, R.F.; Clark, R.N. Spectroscopic determination of leaf biochemistry using band-depth analysis of absorption features and stepwise multiple linear regression. Remote Sens. Environ 1999, 67, 267–287. [Google Scholar]
- Mutanga, O.; Skidmore, A.; Prins, H. Predicting in situ pasture quality in the Kruger National Park, South Africa, using continuum-removed absorption features. Remote Sens. Environ 2004, 89, 393–408. [Google Scholar]
- Schmidt, K.S.; Skidmore, A. Spectral discrimination of vegetation types in a coastal wetland. Remote Sens. Environ 2003, 85, 92–108. [Google Scholar]
- Romney, A.K.; Fulton, J.T. Transforming reflectance spectra into Munsell color space by using prime colors. Proc. Natl. Acad. Sci. USA 2006, 103, 15698–15703. [Google Scholar]
- Braun, A.C.; Weidner, U.; Hinz, S. Support vector machines for vegetation classification—A revision (in German). Photogramm. Fernerkund. Geoinf 2010, 2010, 273–281. [Google Scholar]
- Thenkabail, P.S.; Mariotto, I.; Gumma, M.K.; Middleton, E.M.; Landis, D.R.; Huemmrich, K.F. Selection of Hyperspectral Narrowbands (HNBs) and Composition of Hyperspectral Twoband Vegetation Indices (HVIs) for biophysical characterization and discrimination of crop types using field reflectance and hyperion/EO-1 data. IEEE J. Sel. Top. Appl. Earth Obs. Remote Sens 2013, 6, 427–439. [Google Scholar]
- Buddenbaum, H.; Stern, O.; Stellmes, M.; Stoffels, J.; Pueschel, P.; Hill, J.; Werner, W. Field imaging spectroscopy of beech seedlings under dryness stress. Remote Sens 2012, 4, 3721–3740. [Google Scholar]
- Schlerf, M.; Atzberger, C.; Hill, J. Remote sensing of forest biophysical variables using HyMap imaging spectrometer data. Remote Sens. Environ 2005, 95, 177–194. [Google Scholar]
- Buddenbaum, H.; Püschel, P. SpInMine (Spectral Index Data Mining Tool): Manual for Application: SpInMine (1.0); University of Trier: Trier, Germany, 2012. [Google Scholar]
- Held, M.; Jakimow, B.; Rabe, A.; van der Linden, S.; Wirth, F.; Hostert, P. EnMAP-Box Manual: Version 1.4; Humboldt-Universität zu Berlin: Berlin, Germany, 2012. [Google Scholar]
- Rouse, J.W.; Haas, R.H.; Schell, J.A.; Deering, D.W. Monitoring Vegetation Systems in the Great Plains with ERTS. Proceedings of the Third ERTS Symposium, Washington, DC, USA, 10–14 December 1973; pp. 309–317.
- Tucker, C.J. Red and photographic infrared linear combinations for monitoring vegetation. Remote Sens. Environ 1979, 8, 127–150. [Google Scholar]
- Stow, D.A.; Burns, B.H.; Hope, A. Spectral, spatial and temporal characteristics of Arctic tundra reflectance. Int. J. Remote Sens 1993, 14, 2445–2462. [Google Scholar]
- Thenkabail, P.S.; Smith, R.B.; de Pauw, E. Evaluation of narrowband and broadband vegetation indices for determining optimal hyperspectral wavebands for agricultural crop characterization. Photogramm. Eng. Remote Sensing 2002, 68, 607–621. [Google Scholar]
- Weidong, L.; Baret, F.; Xingfa, G.; Qingxi, T.; Lanfen, Z.; Bing, Z. Relating soil surface moisture to reflectance. Remote Sens. Environ 2002, 81, 238–246. [Google Scholar]
- McGuffie, K.; Henderson-Sellers, A. Technical note. Illustration of the influence of shadowing on high latitude information derived from satellite imagery. Int. J. Remote Sens 1986, 7, 1359–1365. [Google Scholar]
- Ranson, K.; T. Daughtry, C. Scene Shadow Effects on Multispectral Response. IEEE Trans. Geosci. Remote Sens. 1987, GE-25, 502–509. [Google Scholar]
- Tucker, C.J. Spectral estimation of grass canopy variables. Remote Sens. Environ 1977, 6, 11–26. [Google Scholar]
- Clark, R.N.; Swayze, G.A.; Wise, R.; Livo, E.; Hoefen, T.; Kokaly, R.; Sutley, S.J. USGS Digital Spectral Library Splib06a. 2007. Available online: http://speclab.cr.usgs.gov/spectral.lib06 (accessed on 6 February 2013).
- Walker, D.A.; Lederer, N.D. Toposequence Study: Site Factors, Soil Physical and Chemical Properties and Plant Species Cover; Department of Energy, R4D Program Report, Institute of Arctic and Alpine Research: Boulder, CO, USA, 1987. [Google Scholar]
- Muster, S.; Heim, B.; Abnizova, A.; Boike, J. Water body distributions across scales: A remote sensing based comparison of three arctic tundrawetlands. Remote Sens 2013, 5, 1498–1523. [Google Scholar]
| NDVI | Sensor | Sensor Band | Center Wavelength (nm) | Band Width (nm) |
|---|---|---|---|---|
| NDVIAVHRR (broadband) | AVHRR/3 | red: band 1 | 630 | 100 |
| NIR: band 2 | 865 | 275 | ||
| NDVI47_59 (narrowband) | EnMAP | red: band 47 | 672 | 6.5 |
| NIR: band 59 | 756 | 6.5 | ||
| NDVI47_73 (narrowband) | EnMAP | red: band 47 | 672 | 6.5 |
| NIR: band 73 | 864 | 8 | ||
| NDVI47_101 (narrowband) | EnMAP | red: band 47 | 672 | 6.5 |
| NIR: band 101 | 1,018 | 11 |
| Site Code | SWI (°C) | Soil Parameters | Vegetation Parameters | Munsell Color Information+ | Detailed Vegetation Parameters (Nadir Cover) | ||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| pH-Value | Moisture (Vol %) | Average Height (cm)* | Average Top Height (cm)Δ | Biomass (kg/100 m2) | Overall | L1 | L2 | L3 | Soil Crust (%)□ | L1 Cover (%) | L1 Height (cm) | L1 Description | L2 Cover (%) | L2 Height (cm) | L2 Description | L3 Cover (%) | L3 Height (cm) | L3 Description | |||
| Regional climate | DH_z | 17.3 | 7.9 | 70 | 23 | 25 | 33.17 | 10Y (5/4) | 5Y (6/8) | 5GY (5/4) | 10 | 20 | <2 | Moss | 70 | 2–30 | sedge & shrub | 0 | – | – | |
| FB_m/z | 24.2 | 8.0 | 48 | 15 | 35 | 43.40 | 2.5GY (6/4) | 5Y (6/8) | 5GY (6/4) | 5GY (5/6) | 5 | 8 | <2 | Moss | 82 | 2–15 | sedge & shrub | 5 | 15–40 | shrub | |
| SW_MNT | 26.5 | 7.7 | 39 | 8 | 45 | 56.30 | 7.5Y (7/8) | 5Y (8/10) | 5GY (6/6) | 5GY (5/6) | 3 | 64 | <2 | Moss | 30 | 2–15 | sedge & shrub | 3 | 15–50 | shrub | |
| SW_MAT | 26.5 | 5.4 | 35 | 12 | 30 | 75.10 | 2.5GY (7/8) | 2.5GY (8/10) | 5GY (5/6) | 5GY (5/4) | 2 | 57 | <2 | Moss | 40 | 2–25 | tussock & shrub | 1 | 25–35 | shrub | |
| HV_ms/z | 29.5 | 5.1 | 38 | 14 | 45 | 72.08 | 2.5GY (6/6) | 2.5GY (8/10) | 5GY (5/4) | 5GY (5/4) | 4 | 43 | <2 | Moss | 48 | 2–20 | tussock & shrub | 5 | 20–50 | shrub | |
| Soil-pH (Sagwon) | SW_MNT | 26.5 | 7.7 | 39 | 8 | 45 | 56.30 | 7.5Y (7/8) | 5Y (8/10) | 5GY (6/6) | 5GY (5/6) | 3 | 64 | <2 | Moss | 30 | 2–15 | sedge & shrub | 3 | 15–50 | shrub |
| SW_MAT | 26.5 | 5.4 | 35 | 12 | 30 | 75.10 | 2.5GY (7/8) | 2.5GY (8/10) | 5GY (5/6) | 5GY (5/4) | 2 | 57 | <2 | Moss | 40 | 2–25 | tussock & shrub | 1 | 25–35 | shrub | |
| Topo-sequence | HV_hc | 29.5 | 5.1 | 27 | 12 | 40 | 73.54 | 2.5GY (6/8) | 2.5GY (8/10) | 5GY (5/6) | 5GY (5/6) | 10 | 43 | <2 | Moss | 45 | 2–20 | tussock & shrub | 2 | 20–45 | shrub |
| HV_ms/z | 29.5 | 5.1 | 38 | 14 | 45 | 72.08 | 2.5GY (6/6) | 2.5GY (8/10) | 5GY (5/4) | 5GY (5/4) | 4 | 43 | <2 | Moss | 48 | 2–20 | tussock & shrub | 5 | 20–50 | shrub | |
| HV_fs | 29.5 | 5.1 | 45 | 25 | 55 | 73.44 | 5GY (6/6) | 2.5GY (8/10) | 5GY (6/4) | 5GY (5/4) | 2 | 28 | <2 | Moss | 50 | 2–25 | tussock & shrub | 20 | 25–60 | shrub | |
| Soil-moisture | FB_d | 24.2 | 8.1 | 36 | 14 | 30 | 48.96 | 2.5GY (5/6) | 5Y (6/8) | 5GY (5/4) | 5GY (5/6) | 10 | 30 | <2 | Moss | 40 | 2–15 | sedge & shrub | 20 | 15–35 | shrub |
| FB_m/z | 24.2 | 8.0 | 48 | 15 | 35 | 43.40 | 2.5GY (6/4) | 5Y (6/8) | 5GY (6/4) | 5GY (5/6) | 5 | 8 | <2 | Moss | 82 | 2–15 | sedge & shrub | 5 | 15–40 | shrub | |
| FB_w | 24.2 | 7.8 | 74 | 30 | 35 | 40.39 | 2.5GY (6/4) | 5Y (6/8) | 5GY (6/4) | 10 | 10 | <2 | Moss | 80 | 2–40 | sedge & shrub | 0 | – | – | ||
| Average R (Broad Bands) | Max. R in NIR* | Relative Absorption Depth | Continuum Removed | NDVI's with SE | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Site Code | VIS (%) (400–700) | NIR (%) (700–1,050) | 750 (%) | 1,020 (%) | Delta R (1,020-750) | 400–550 (blue) | 550–750 (red) | Area 400–550 | Area 550–750 | Max. Blue Band Depth | Max. Red Band Depth | Broad NDVIAVHRR | Narrow 1 NDVI47_59 | Narrow 2 NDVI47_73 | Narrow 3 NDVI47_101 | |
| Regional climate | DH_z | 5.9 | 21.4 | 17.8 | 25.2 | 7.4 | 0.40 | 0.68 | 8.7 | 48.4 | 0.11 | 0.43 | 0.48 (±0.01) | 0.39 (±0.01) | 0.48 (±0.01) | 0.53 (±0.01) |
| FB_m/z | 5.8 | 24.6 | 21.3 | 28.9 | 7.6 | 0.41 | 0.98 | 11.9 | 64.5 | 0.15 | 0.55 | 0.55 (±0.01) | 0.49 (±0.02) | 0.56 (±0.01) | 0.60 (±0.01) | |
| SW_MNT | 5.6 | 24.4 | 20.2 | 28.9 | 8.7 | 0.44 | 0.92 | 14.2 | 58.3 | 0.17 | 0.52 | 0.55 (±0.01) | 0.48 (±0.01) | 0.56 (±0.01) | 0.61 (±0.01) | |
| SW_MAT | 5.1 | 24.4 | 21.7 | 27.5 | 5.8 | 0.53 | 1.17 | 17.6 | 68.9 | 0.21 | 0.60 | 0.59 (±0.01) | 0.55 (±0.01) | 0.61 (±0.01) | 0.64 (±0.01) | |
| HV_ms/z | 4.7 | 25.8 | 22.8 | 29.5 | 6.7 | 0.58 | 1.43 | 23.0 | 77.5 | 0.27 | 0.66 | 0.64 (±0.01) | 0.60 (±0.01) | 0.66 (±0.01) | 0.68 (±0.01) | |
| Soil-pH (all) | non acidic | 5.8 | 23.2 | 19.8 | 27.1 | 7.3 | 0.41 | 0.86 | 10.8 | 57.8 | 0.14 | 0.50 | 0.53 (±0.01) | 0.46 (±0.01) | 0.53 (±0.01) | 0.58 (±0.01) |
| acidic | 4.6 | 25.0 | 22.2 | 28.5 | 6.3 | 0.56 | 1.34 | 20.6 | 74.2 | 0.24 | 0.64 | 0.62 (±0.01) | 0.59 (±0.01) | 0.65 (±0.01) | 0.67 (±0.01) | |
| Soil-pH (Sagwon) | SW_MNT | 5.6 | 24.4 | 20.2 | 28.9 | 8.7 | 0.44 | 0.92 | 14.2 | 58.3 | 0.17 | 0.52 | 0.55 (±0.01) | 0.48 (±0.01) | 0.56 (±0.01) | 0.61 (±0.01) |
| SW_MAT | 5.1 | 24.4 | 21.7 | 27.5 | 5.8 | 0.53 | 1.17 | 17.6 | 68.9 | 0.21 | 0.60 | 0.59 (±0.01) | 0.55 (±0.01) | 0.61 (±0.01) | 0.64 (±0.01) | |
| Topo-sequence | HV_hc | 4.7 | 23.0 | 20.1 | 26.4 | 6.3 | 0.52 | 1.21 | 19.1 | 68.7 | 0.22 | 0.61 | 0.60 (±0.01) | 0.57 (±0.01) | 0.63 (±0.01) | 0.66 (±0.01) |
| HV_ms/z | 4.7 | 25.8 | 22.8 | 29.5 | 6.7 | 0.58 | 1.43 | 23.0 | 77.5 | 0.27 | 0.66 | 0.64 (±0.01) | 0.60 (±0.01) | 0.66 (±0.01) | 0.68 (±0.01) | |
| HV_fs | 4.5 | 26.9 | 24.0 | 30.5 | 6.5 | 0.62 | 1.60 | 22.5 | 81.8 | 0.27 | 0.69 | 0.67 (±0.01) | 0.64 (±0.01) | 0.69 (±0.01) | 0.71 (±0.01) | |
| Soil-moisture | FB_d | 5.7 | 23.7 | 21.3 | 26.9 | 5.6 | 0.37 | 1.07 | 9.9 | 68.2 | 0.14 | 0.59 | 0.56 (±0.02) | 0.52 (±0.02) | 0.58 (±0.02) | 0.61 (±0.02) |
| FB_m/z | 5.8 | 24.6 | 21.3 | 28.9 | 7.6 | 0.41 | 0.98 | 11.9 | 64.5 | 0.15 | 0.55 | 0.55 (±0.01) | 0.49 (±0.02) | 0.56 (±0.01) | 0.60 (±0.01) | |
| FB_w | 5.8 | 21.9 | 18.4 | 25.7 | 7.3 | 0.44 | 0.72 | 9.2 | 49.4 | 0.12 | 0.43 | 0.49 (±0.01) | 0.40 (±0.01) | 0.49 (±0.01) | 0.53 (±0.01) | |
© 2013 by the authors; licensee MDPI, Basel, Switzerland This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license ( http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Buchhorn, M.; Walker, D.A.; Heim, B.; Raynolds, M.K.; Epstein, H.E.; Schwieder, M. Ground-Based Hyperspectral Characterization of Alaska Tundra Vegetation along Environmental Gradients. Remote Sens. 2013, 5, 3971-4005. https://doi.org/10.3390/rs5083971
Buchhorn M, Walker DA, Heim B, Raynolds MK, Epstein HE, Schwieder M. Ground-Based Hyperspectral Characterization of Alaska Tundra Vegetation along Environmental Gradients. Remote Sensing. 2013; 5(8):3971-4005. https://doi.org/10.3390/rs5083971
Chicago/Turabian StyleBuchhorn, Marcel, Donald A. Walker, Birgit Heim, Martha K. Raynolds, Howard E. Epstein, and Marcel Schwieder. 2013. "Ground-Based Hyperspectral Characterization of Alaska Tundra Vegetation along Environmental Gradients" Remote Sensing 5, no. 8: 3971-4005. https://doi.org/10.3390/rs5083971





