Upscaling CH4 Fluxes Using High-Resolution Imagery in Arctic Tundra Ecosystems
Abstract
:1. Introduction
2. Materials and Methods
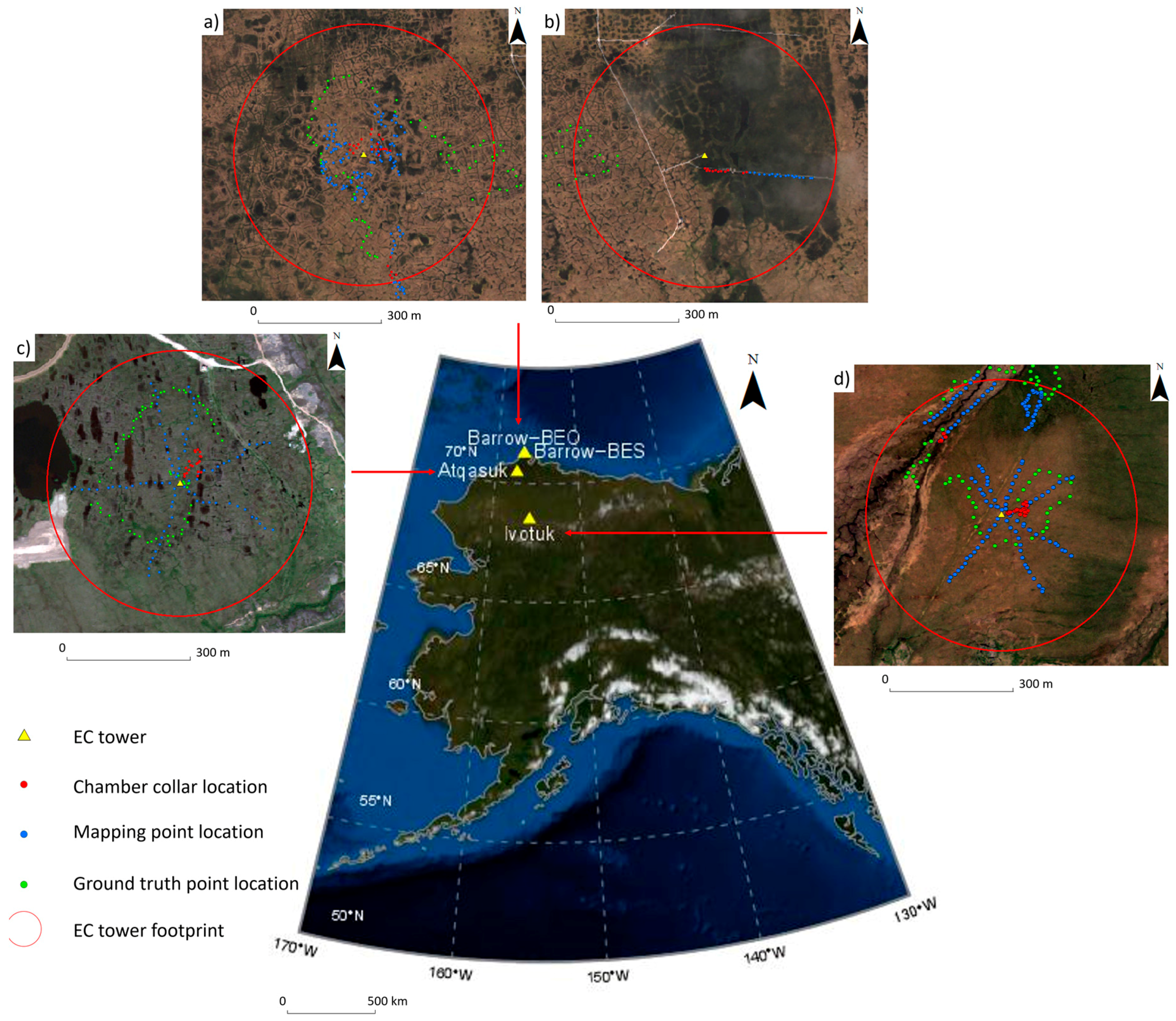
2.1. Site Description
2.2. Remote Sensing Imagery
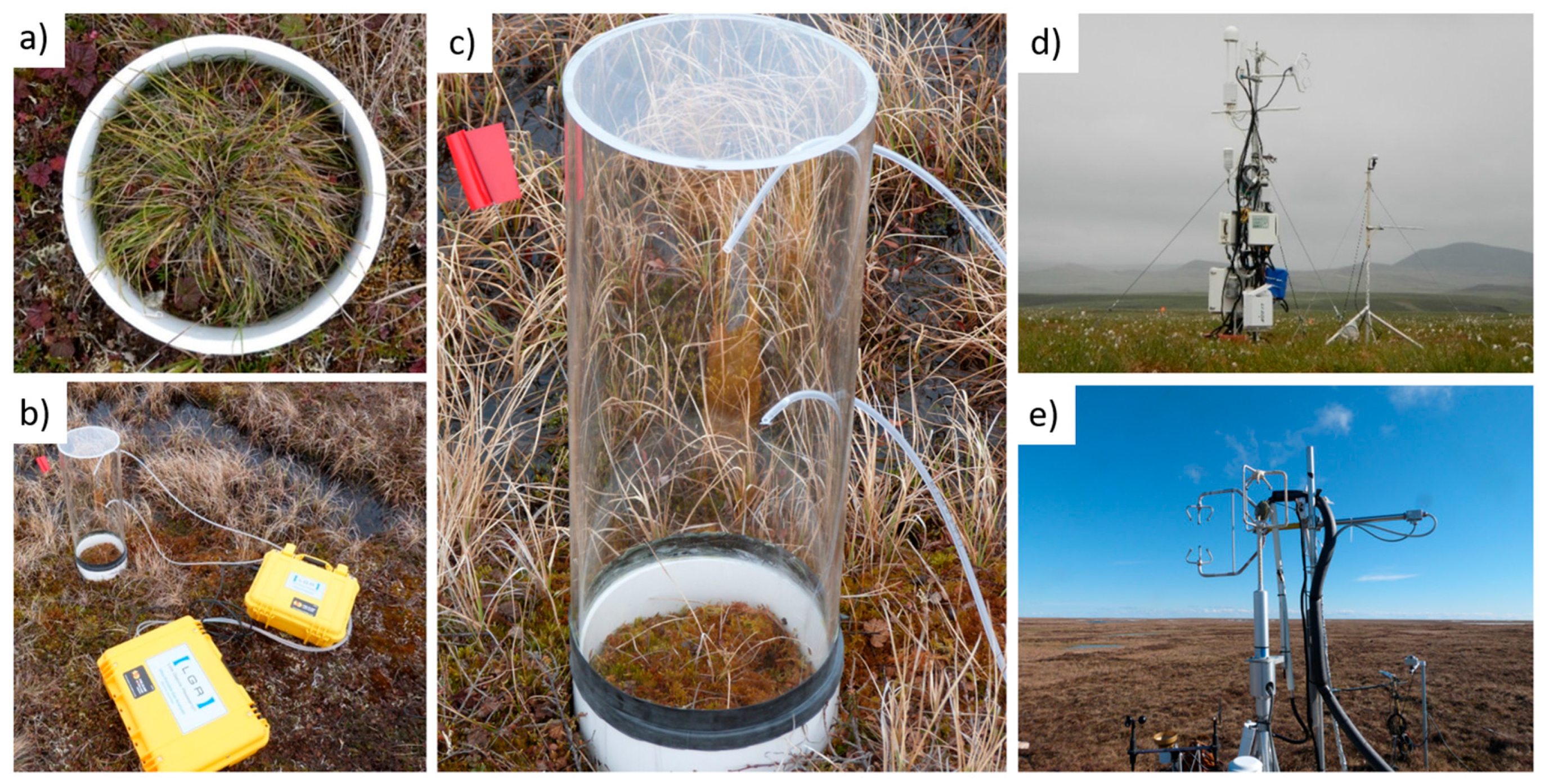
2.3. Vegetation Data
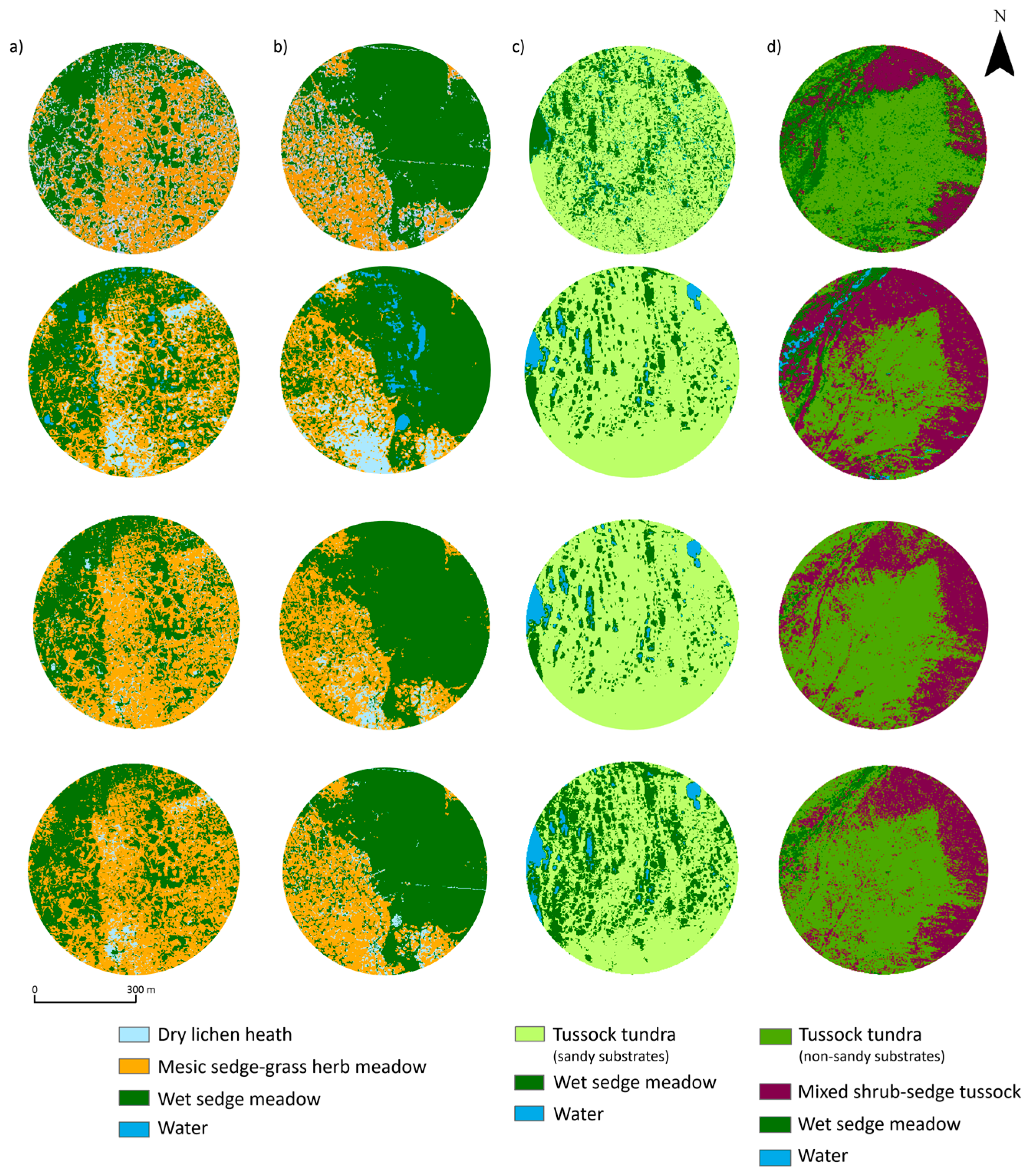
2.4. Vegetation Mapping
2.5. Measurements of CH4
2.6. Upscaling CH4 Emissions
3. Results
3.1. Mapping Tundra Vegetation
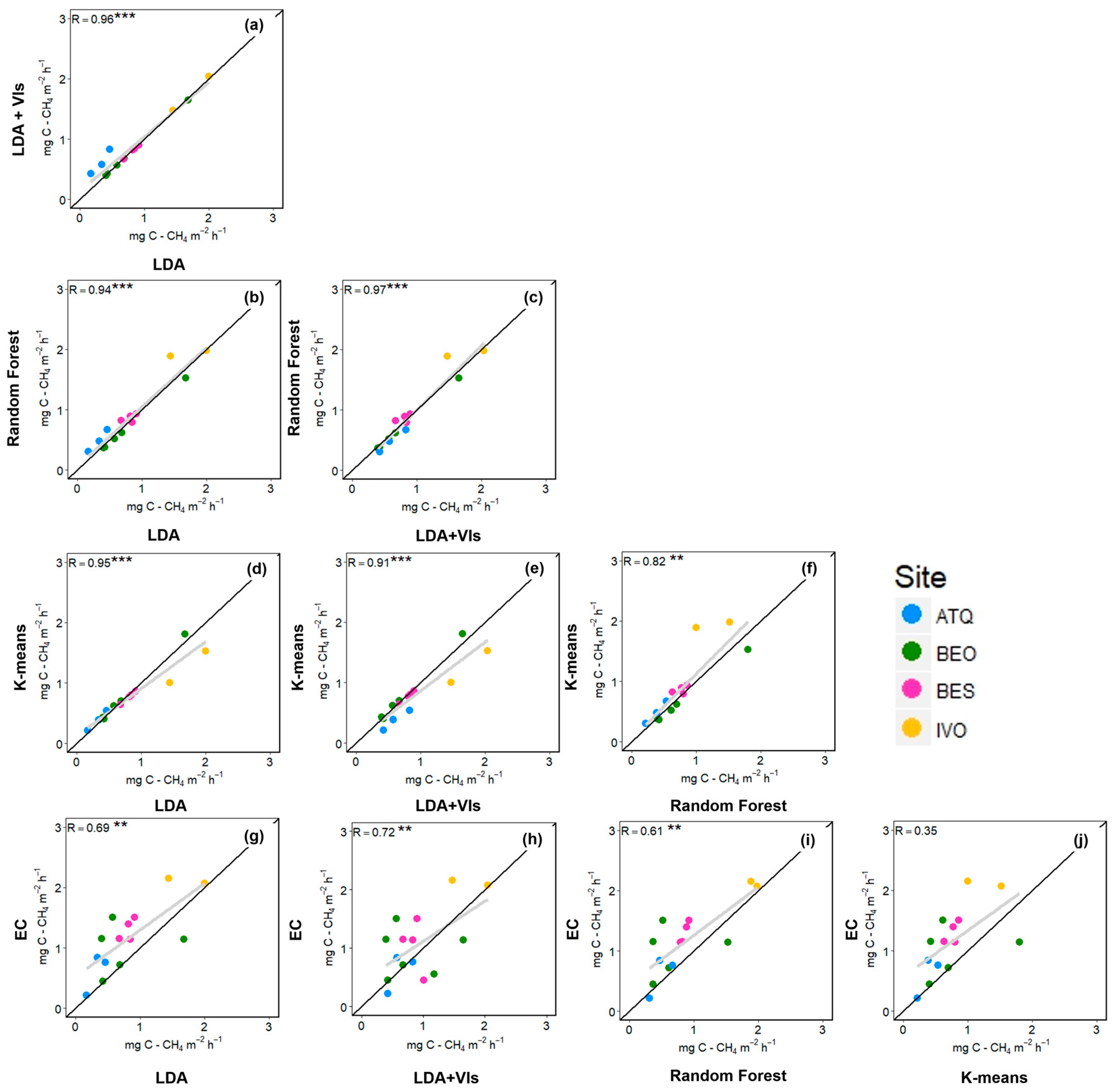
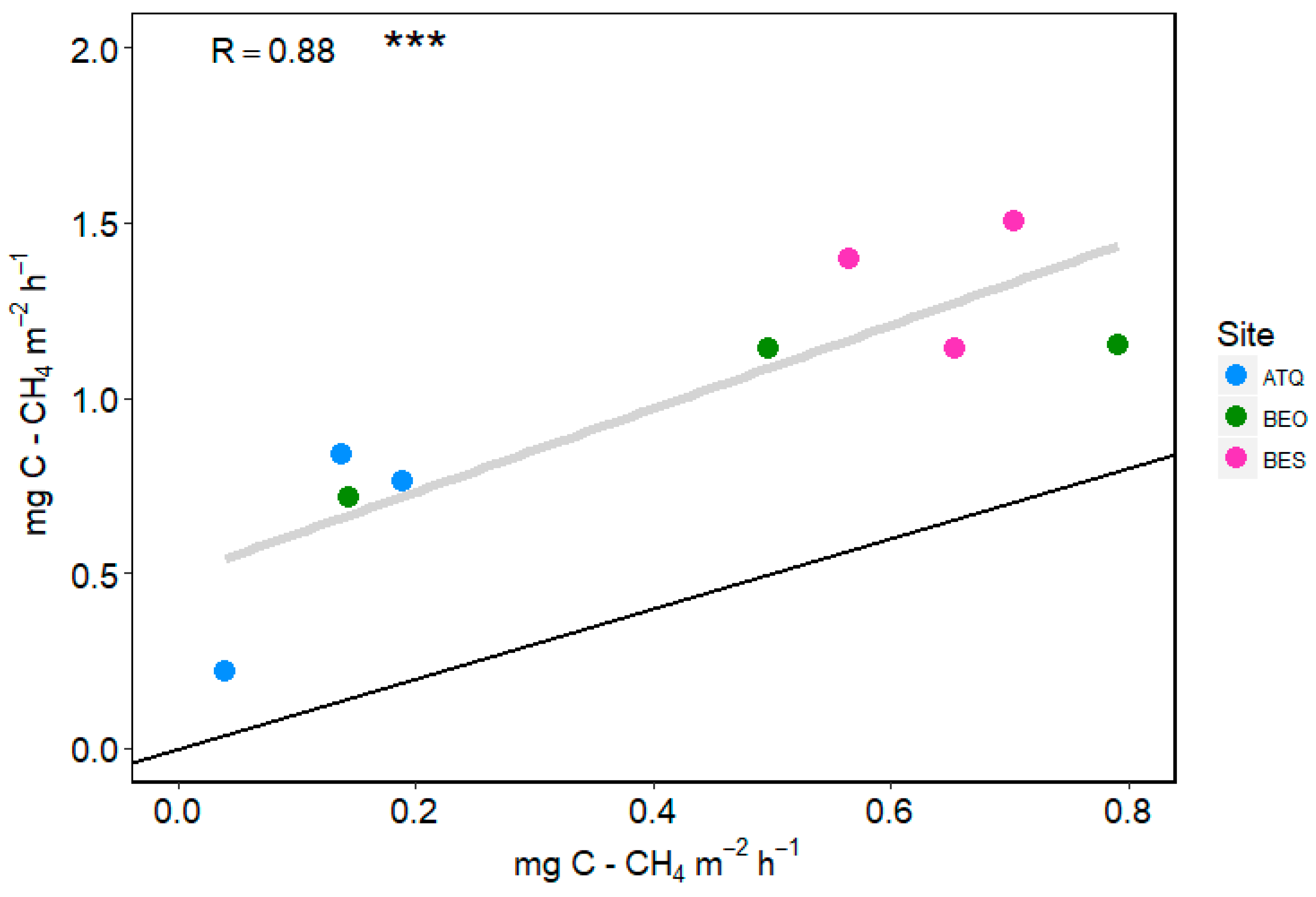
3.2. Upscaling CH4 Emissions
4. Discussion
4.1. Tundra Vegetation Mapping
4.2. Upscaled Flux Chamber Measurements
5. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Intergovernmental Panel on Climate Change (IPCC). Climate Change: The Physical Science Bases; Cambridge University Press: Cambridge, UK, 2013. [Google Scholar]
- Christensen, T.R. Methane emissions from Arctic tundra. Biogeochemistry 1993, 2, 117–139. [Google Scholar] [CrossRef]
- McGuire, A.D.; Christensen, T.R.; Hayes, D.; Heroult, A.; Euskirchen, E.; Kimball, J.S.; Koven, C.; Lafleur, P.; Miller, P.A.; Oechel, W.; et al. An assessment of the carbon balance of Arctic tundra comparisons among observations, process models and atmospheric inversions. Biogeosciences 2012, 9, 3185–3204. [Google Scholar] [CrossRef] [Green Version]
- Dlugokencky, E.J.; Nisbet, E.G.; Fisher, R.; Lowry, D. Global atmospheric methane: Budget, changes and dangers. Philos. Trans. R. Soc. Lond. A 2011, 369, 2058–2072. [Google Scholar] [CrossRef] [PubMed]
- Olefeldt, D.; Turetsky, M.R.; Crill, P.M.; McGuire, A.D. Environmental and physical controls on northern terrestrial methane emissions across permafrost zone. Glob. Chang. Biol. 2013, 19, 589–603. [Google Scholar] [CrossRef] [PubMed]
- Yvon-Durocher, G.; Allen, A.P.; Bastviken, D.; Conrad, R.; Gudasz, C.; St-Pierre, A.; Thanh-Duc, N.; del Giorgo, P.A. Methane fluxes show consistent temperature dependence across microbial to ecosystem scales. Nature 2014, 507, 488–495. [Google Scholar] [CrossRef] [PubMed]
- Oechel, W.C.; Vourlitis, G.L.; Hastings, S.J.; Zulueta, R.C.; Hinzman, L.; Kane, D. Acclimation of ecosystem CO2 exchange in the Alaskan Arctic in response to decadal climate warming. Nature 2000, 406, 978–981. [Google Scholar] [CrossRef] [PubMed]
- Chapin, F.S.; Sturm, M.; Serreze, M.C.; McFadden, J.P.; Key, J.R.; Lloyd, A.H.; McGuire, A.D.; Rupp, T.S.; Lynch, A.H.; Schimel, J.P.; et al. Role of land-surface changes in arctic summer warming. Science 2005, 310, 657–660. [Google Scholar] [CrossRef] [PubMed]
- Davidson, S.J.; Sloan, V.L.; Phoenix, G.K.; Wagner, R.; Fisher, J.P.; Oechel, W.C.; Zona, D. Vegetation type dominates the spatial variability in CH4 emissions across multiple Arctic tundra landscapes. Ecosystems 2016, 19, 1116–1132. [Google Scholar] [CrossRef]
- Zona, D.; Gioli, B.; Commane, R.; Lindaas, J.; Wofsy, S.C.; Miller, C.E.; Dinardo, S.J.; Dengel, S.; Sweeney, C.; Karion, A.; et al. Cold season emissions dominate the Arctic tundra methane budget. Proc. Natl. Acad. Sci. USA 2016, 113, 40–45. [Google Scholar] [CrossRef] [PubMed]
- Dinsmore, K.J.; Drewer, J.; Levy, P.E.; George, C.; Lohila, A.; Aurela, M.; Skiba, U.M. Growing season CH4 and N2O fluxes from a subarctic landscape in northern Finland from chamber to landscape scale. Biogeosciences 2017, 14, 799–815. [Google Scholar] [CrossRef]
- Heikkinen, J.E.P.; Virtanen, T.; Huttunen, J.T.; Elsakov, V.; Martikainen, P.J. Carbon balance in East European tundra. Glob. Biogeochem. Cycles 2004, 18, GB1023. [Google Scholar] [CrossRef]
- Petrescu, A.M.R.; van Beek, L.P.H.; van Huissteden, J.; Prigent, C.; Sachs, T.; Corradi, C.A.R.; Parmentrier, F.J.W.; Dolman, A.J. Modeling regional to global CH4 emissions of boreal and arctic wetlands. Glob. Biogeochem. Cycles 2010, 24, GB4009. [Google Scholar] [CrossRef]
- Melton, J.R.; Wania, R.; Hodson, E.L.; Poulter, B.; Ringeval, B.; Spahni, R.; Bohn, T.; Avis, C.A.; Beerling, D.J.; Chen, G.; et al. Present state of global wetland extent and wetland methane modelling: Conclusions from a model inter-comparison project (WETCHIMP). Biogeosciences 2013, 10, 753–788. [Google Scholar] [CrossRef]
- Sachs, T.; Giebels, M.; Boike, J.; Kutzbach, L. Environmental controls on CH4 emission from polygonal tundra on the microsite scale in the Lena river delta, Siberia. Glob. Chang. Biol. 2010, 16, 3096–3110. [Google Scholar] [CrossRef]
- King, J.Y.; Reeburgh, W.S.; Regli, S.K. Methane emission and transport by arctic sedges in Alaska: Results of a vegetation removal experiment. J. Geophys. Res. 1998, 103, 29083–29092. [Google Scholar] [CrossRef]
- Ström, L.; Ekberg, A.; Mastepanov, M.; Christensen, T.R. The effect of vascular plants on carbon turnover and methane emissions from a tundra wetland. Glob. Chang. Biol. 2003, 9, 1185–1192. [Google Scholar] [CrossRef]
- Zona, D.; Oechel, W.C.; Kochendorfer, J.; Paw, U.K.T.; Salyuk, A.N.; Olivas, P.C.; Oberbaeur, S.F.; Lipson, D.A. Methane fluxes during the initiation of a large-scale water table manipulation experiment in the Alaskan Arctic tundra. Glob. Biogeochem. Cycles 2009, 23, 1–11. [Google Scholar] [CrossRef]
- King, J.Y.; Reeburgh, W.S.; Thieler, K.K.; Kling, G.W.; Loya, W.M.; Johnson, L.C.; Nadelhoffer, K.J. Pulse-labelling studies of carbon cycling in Arctic tundra ecosystems: The contribution of photosynthates to methane emission. Glob. Biogeochem. Cycles 2002, 16, 1–8. [Google Scholar] [CrossRef]
- Christensen, T.R.; Ekberg, A.; Ström, L.; Mastepanov, M.; Panikov, N.; Öquist, M.; Svensson, B.H.; Nykänen, H.; Martikainen, P.J.; Oskarsson, H. Factors controllong large scale variations in methane emissions from wetlands. Geophys. Res. Lett. 2003, 30, 1414. [Google Scholar] [CrossRef]
- Bubier, J.L.; Moore, T.R.; Bellisario, L.; Comer, N.T.; Crill, P.M. Ecological controls on methane emissions from a northern peatland complex in the zone of discontinuous permafrost, Manitoba, Canada. Glob. Biogeochem. Cycles 1995, 9, 455–470. [Google Scholar] [CrossRef]
- Schimel, J.P. Plant transport and methane productions as a control on methane flux from arctic wet meadow tundra. Biogeochemistry 1995, 28, 183–200. [Google Scholar] [CrossRef]
- Fletcher, B.J.; Gornall, J.L.; Poyatos, R.; Press, M.C.; Stoy, P.C.; Huntley, B.; Baxter, B.; Phoenix, G.K. Photosynthesis and productivity in heterogeneous arctic tundra: Consequences for ecosystem function of mixing vegetation types at stand edges. J. Ecol. 2012, 100, 441–451. [Google Scholar] [CrossRef] [Green Version]
- Koelbener, A.; Ström, L.; Edwards, P.J.; Venterink, H.O. Plant species from mesotrophic wetlands cause relatively high methane emissions from peat soil. Plant Soil 2010, 326, 147–158. [Google Scholar] [CrossRef]
- Oechel, W.C.; Vourlitis, G.L.; Brooks, S.; Crawford, T.L.; Dumas, E. Intercomparison among chamber, tower, and aircraft net CO2 and energy fluxes measuring during the Arctic System Science Land-Atmosphere-Ice interactions (ARCSS-LAII) Flux Study. J. Geophys. Res. 1998, 103, 28993–29003. [Google Scholar] [CrossRef]
- Vourlitis, G.L.; Oechel, W.C.; Hope, A.; Stow, D.; Boynton, B.; Verfaillie, J., Jr.; Zulueta, R.; Hastings, S.J. Physiological models for scaling plol measurements of CO2 flux across an Arctic tundra landscape. Ecol. Appl. 2000, 10, 60–72. [Google Scholar]
- Schneider, J.; Grosse, G.; Wagner, D. Land cover classification of tundra environments in the Arctic Lena Delta based on Landsat 7 ETM+ data and its application for upscaling methane emissions. Remote Sens. Environ. 2009, 113, 380–391. [Google Scholar] [CrossRef] [Green Version]
- Forbrich, I.; Kutzbach, L.; Wille, C.; Becker, T.; Wu, J.; Wilmking, M. Cross-evaluation of measurements of peatland methane emissions on microform and ecosystem scales using high-resolution landcover classification and source weight modelling. Agric. For. Meteorol. 2011, 151, 864–874. [Google Scholar] [CrossRef]
- Ueyama, M.; Ichii, K.; Iwata, H.; Euskirchen, E.S.; Zona, D.; Rocha, A.V.; Harazono, Y.; Iwama, C.; Nakai, T.; Oechel, W.C. Upscaling terrestrial carbon dioxide fluxes in Alaska with satellite remote sensing and support vector regression. J. Geophys. Res. 2013, 118, 1266–1281. [Google Scholar] [CrossRef]
- Budischev, A.; Mi, Y.; van Huissteden, J.; Belelli-Marchesini, L.; Schaepman-Strub, G.; Parmentier, F.J.W.; Fratini, G.; Gallagher, A.; Maximov, T.C.; Dolman, A.J. Evaluation of a plot-scale methane emission model using eddy covariance observations and footprint modelling. Biogeosciences 2014, 11, 4651–4664. [Google Scholar] [CrossRef] [Green Version]
- Baldocchi, D.D. Assessing the eddy covariance technique for evaluating carbon dioxide exchange rates of ecosystems: Past, present and future. Glob. Chang. Biol. 2003, 9, 479–492. [Google Scholar] [CrossRef]
- Hope, A.S.; Fleming, J.B.; Vourlitis, G.; Stow, D.A.; Oechel, W.C.; Hack, T. Relating CO2 fluxes to spectral vegetation indices in tundra landscapes: Importance of footprint definition. Polar Rec. 1995, 177, 245–250. [Google Scholar] [CrossRef]
- Zhang, Y.; Sachs, T.; Li, C.; Boike, J. Upscaling methane fluxes from closed chambers to eddy covariance based on a permafrost integrated model. Glob. Chang. Biol. 2012, 18, 1428–1440. [Google Scholar] [CrossRef]
- Frey, K.E.; Smith, L.C. How well do we know northern land cover? Comparison of four global vegetation and wetland products with a new ground-truth database for West Siberia. Glob. Biogeochem. Cycles 2007, 21, GB1016. [Google Scholar] [CrossRef]
- Fox, A.M.; Huntley, B.; Lloyd, C.R.; Williams, M.; Baxter, R. Net ecosystem exchange over heterogeneous Arctic tundra: Scaling between chamber and eddy covariance measurements. Glob. Biogeochem. Cycles 2008, 22, 1–15. [Google Scholar] [CrossRef]
- Atkinson, D.M.; Treitz, P. Arctic ecological classifications derived from vegetation community and satellite spectral data. Remote Sens. 2012, 4, 3948–3971. [Google Scholar] [CrossRef]
- Bhatt, U.S.; Walker, D.A.; Raynolds, M.K.; Comiso, J.C.; Epstein, H.E.; Jia, G.; Gens, R.; Pinzon, J.E.; Tucker, C.J.; Tweedie, C.E.; et al. Circumpolar Arctic tundra vegetation change is linked to sea ice decline. Earth Interact. 2010, 4, 1–20. [Google Scholar] [CrossRef]
- Buchhorn, M.; Walker, D.A.; Heim, B.; Raynolds, M.K.; Epstein, H.E.; Schwieder, M. Ground-based hyperspectral characterization of Alaska tundra vegetation along environmental gradients. Remote Sens. 2013, 5, 3971–4005. [Google Scholar] [CrossRef] [Green Version]
- Muster, S.; Langer, M.; Heim, B.; Westermann, S.; Boike, J. Subpixel heterogeneity of ice-wedge polygonal tundra: A multi-scale analysis of land cover and evapotranspiration in the Lena River Delta, Siberia. Tellus B 2012, 64, 17301. [Google Scholar] [CrossRef]
- Davidson, S.J.; Santos, M.J.; Sloan, V.L.; Watts, J.D.; Phoenix, G.K.; Oechel, W.C.; Zona, D. Mapping Arctic tundra vegetation communities using field spectroscopy and multispectral satellite data in north Alaska. Remote Sens. 2016, 8, 978. [Google Scholar] [CrossRef]
- Langford, Z.; Kumar, J.; Hoffman, F.M.; Norby, R.J.; Wullschleger, S.D.; Sloan, V.L.; Iversen, C.M. Mapping Arctic plant functional type distributions in the Barrow Environmental Observatory using WorldView-2 and LiDAR Datasets. Remote Sens. 2016, 8, 733. [Google Scholar] [CrossRef]
- Stow, D.A.; Hope, A.; McGuire, D.; Verbyla, D.; Gamon, J.; Huemmrich, F.; Houston, S.; Racine, C.; Sturm, M.; Tape, K.; et al. Remote sensing of vegetation and land-cover change in Arctic tundra ecosystems. Remote Sens. Environ. 2004, 89, 281–308. [Google Scholar] [CrossRef]
- Bartsch, A.; Höfler, A.; Kroisleitner, C.; Trofaier, A. Land cover mapping in northern high latitude permafrost regions with satellite data: Achievements and remaining challenges. Remote Sens. 2016, 8, 979. [Google Scholar] [CrossRef]
- Komárková, V.; Webber, P.J. Two low Arctic vegetation maps near Atkasook, Alaska. Arct. Alp. Res. 1980, 12, 447–472. [Google Scholar] [CrossRef]
- Oechel, W.C.; Laskowski, C.A.; Burba, G.; Gioli, B.; Kalhori, A.A.M. Annual patterns and budget of CO2 flux in an Arctic tussock tundra ecosystem. J. Geophys. Res. Biogeosci. 2014, 119, 323–339. [Google Scholar] [CrossRef]
- Kormann, R.; Meixner, F.X. An analytical footprint model for non-neutral stratification. Bound. Layer Meteorol. 2001, 99, 207–224. [Google Scholar] [CrossRef]
- Burba, G.; Anderson, D. A Brief Practical Guide to Eddy Covariance Flux Measurements: Principles and Workflow Examples for Scientific and Industrial Applications; Li-COR Biosciences: Lincoln, NE, USA, 2010. [Google Scholar]
- Wegmann, M.; Leutner, B.; Dech, S. Remote Sensing and GIS for Ecologists: Using Open Source Software; Pelagic Publishing: Exeter, UK, 2016. [Google Scholar]
- Chavez, P.S., Jr. Image-based atmospheric corrections—revisited and improved. Photogramm. Eng. Remote Sens. 1996, 62, 1025–1036. [Google Scholar]
- Vanonckelen, S.; Lhermitte, S.; Van Rompaey, A. The effect of atmospheric and topographic correction methods on land cover classification accuracy. Int. J. Appl. Earth Obs. Geoinf. 2013, 24, 9–21. [Google Scholar] [CrossRef]
- Stehman, S.V. Sampling designs for accuracy assessment of land cover. Int. J. Remote Sens. 2009, 30, 5243–5272. [Google Scholar] [CrossRef]
- Moody, D.I.; Brumby, S.P.; Rowland, J.C.; Altmann, G.L. Land cover classification in multispectral imagery using clustering of sparse approximations over learned feature dictionaries. J. Appl. Remote Sens. 2014, 8, 084793. [Google Scholar] [CrossRef]
- Bratsch, S.N.; Epstein, E.; Bucchorn, M.; Walker, D.A. Differentiating among four Arctic tundra plant communities at Ivotuk, Alaska using field spectroscopy. Remote Sens. 2016, 8, 51. [Google Scholar] [CrossRef]
- Gong, P.; Pu, R.; Yu, B. Conifer species recognition: An exploratory analysis of in situ hyperspectral data. Remote Sens. Environ. 1997, 62, 189–200. [Google Scholar] [CrossRef]
- Clark, M.L.; Roberts, D.A.; Clark, D.B. Hyperspectral discrimination of tropical tree species at leaf to crown scales. Remote Sens. Environ. 2005, 96, 375–398. [Google Scholar] [CrossRef]
- Chapman, D.S.; Bonn, A.; Kunin, W.E.; Cornell, S.J. Random Forest characterization of upland vegetation management burning from aerial imagery. J. Biogeogr. 2010, 37, 37–46. [Google Scholar] [CrossRef]
- Bradter, U.; Thom, T.J.; Atringham, J.D.; Kunin, W.E.; Benton, T.G. Prediction of national vegetation classification communities in the British uplands using environmental data at multiple spatial scales, aerial images and the classifier random forest. J. Appl. Ecol. 2011, 48, 1057–1065. [Google Scholar] [CrossRef]
- Han, K.-S.; Champeaux, J.-L.; Roujean, J.-L. A land cover classification product over France at 1 km resolution using SPOT4/VEGETATION data. Remote Sens. Environ. 2004, 92, 52–66. [Google Scholar] [CrossRef]
- Hartigan, J.A. Clustering Algorithms; John Wiley & Sons, Inc.: New York, NY, USA, 1975; p. 351. [Google Scholar]
- MacKay, D. Information Theory, Inference, and Learning Algorithms; Cambridge University Press: Cambridge, UK, 2003. [Google Scholar]
- Lin, D.H.; Johnson, D.R.; Andresen, C.; Tweedie, C.E. High spatial resolution decade-time scale land cover changes at multiple locations in the Bergingian Arctic (1948–2000s). Environ. Res. Lett. 2012, 9, 1–14. [Google Scholar] [CrossRef]
- Schwaller, M.R. A Geobotanical investigation based on linear discriminant and profile analyses of airborne thematic mapper simulator data. Remote Sens. Environ. 1987, 23, 23–34. [Google Scholar] [CrossRef]
- Bandos, T.V.; Bruzzone, L.; Camps-Valls, G. Classification of hyperspectral images with regularized linear discriminant analysis. IEEE Trans. Geosci. Remote Sens. 2009, 47, 862–873. [Google Scholar] [CrossRef]
- Reynolds, J.; Wesson, K.; Desbiez, A.L.-J.; Ochoa-Quintero, J.M.; Leimgruber, P. Using remote sensing and Random Forest to assess the conservation status of critical cerrado habitats in Mato Gross do Sul, Brazil. Land 2016, 5, 12. [Google Scholar] [CrossRef]
- Van Beijma, S.; Comber, A.; Lamb, A. Random forest classification of salt marsh vegetation habitats using quad polarmetric airborne SAR, elevation and optical RS data. Remote Sens. Environ. 2014, 149, 118–129. [Google Scholar] [CrossRef]
- Breiman, L. Random Forests. Mach. Learn. 2001, 45, 5–32. [Google Scholar] [CrossRef]
- Breiman, L.; Friedman, J.; Stone, C.J.; Olshen, R.A. Classification and Regression Trees; Brooks: Belmont, CA, USA; Wadsworth, OH, USA, 1984. [Google Scholar]
- Gislason, P.O.; Benediktsson, J.A.; Sveinsson, J.R. Random Forests for land cover classification. Pattern Recognit. Lett. 2006, 27, 294–300. [Google Scholar] [CrossRef]
- Rodriguez-Galiano, V.F.; Ghimire, B.; Rogan, J.; Chica-Olmo, M.; Rigol-Sanchez, J.P. An assessment of the effectiveness of a random forest classifier for land-cover classification. ISPRS J. Photogramm. Remote Sens. 2012, 67, 93–104. [Google Scholar] [CrossRef]
- Pal, M. Random Forest classifier for remote sensing classification. Int. J. Remote Sens. 2005, 26, 217–222. [Google Scholar] [CrossRef]
- Hayes, M.N.; Miller, S.N.; Murphy, M.A. High-resolution landcover classification using Random Forest. Remote Sens. Lett. 2014, 5, 112–121. [Google Scholar] [CrossRef]
- Huemmrich, K.F.; Gamon, J.A.; Tweedie, C.E.; Entcheva Campbell, P.K.; Landis, D.R.; Middleton, E.M. Arctic tundra vegetation functional types based on photosynthetic physiology and optical properties. IEEE J. Sel. Top. Appl. Earth Obs. Remote Sens. 2013, 6, 265–275. [Google Scholar] [CrossRef]
- Rouse, J.W.; Haas, R.H.; Schell, J.A.; Deering, D.W. Monitoring vegetation systems in the Great Plains with ERTS. In Proceedings of the Third Earth Resources Technology Satellite-1 Symposium, Washington, DC, USA, 10–14 December 1974. [Google Scholar]
- Gao, B.-C. NDWI—A normalized difference water index for remote sensing of vegetation liquid water from space. Remote Sens. Environ. 1996, 58, 257–266. [Google Scholar] [CrossRef]
- Huete, A.; Didan, K.; Miura, T.; Rodriguez, E.P.; Gao, X.; Ferreira, L.G. Overview of the radiometric and biophysical performance of the MODIS vegetation indices. Remote Sens. Environ. 2002, 83, 195–213. [Google Scholar] [CrossRef]
- Congalton, R.G. A Review of assessing the accuracy of classifications of remotely sensed data. Remote Sens. Environ. 1991, 37, 35–46. [Google Scholar] [CrossRef]
- Cohen, J. A coefficient of agreement for nominal scales. Educ. Psychol. Meas. 1960, 20, 37–46. [Google Scholar] [CrossRef]
- Brennan, R.L.; Prediger, D.J. Coefficient Kappa: Some uses, misuses and alternatives. Educ. Physiol. Meas. 1981, 41, 687–699. [Google Scholar] [CrossRef]
- Vickers, D.; Mahrt, L. Quality control and flux sampling problems for tower and aircraft data. J. Atmos. Ocean. Technol. 1997, 14, 512–526. [Google Scholar] [CrossRef]
- Goodrich, J.P.; Oechel, W.C.; Gioli, B.; Moreaux, V.; Murphy, P.C.; Burba, G.; Zona, D. Impact of different eddy covariance sensors, site set-up and maintenance on the annual balance of CO2 and CH4 in the harsh Arctic environment. Agric. Forest Meteorol. 2016, 228, 239–251. [Google Scholar] [CrossRef]
- Van der Molen, M.K.; van Huissteden, J.; Parmentier, F.J.W.; Petrescu, A.M.R.; Dolman, A.J.; Maximov, T.C.; Kononov, A.V.; Karsanaev, S.V.; Suzdalov, D.A. The growing season greenhouse gas balance of a continental tundra site in the Indigirka lowlands, NE Siberia. Biogeosciences 2007, 4, 985–1003. [Google Scholar] [CrossRef] [Green Version]
- Parmentier, F.J.W.; van Huissteden, J.; van der Molen, M.K.; Schaepman-Strub, G.; Karsanaev, S.A.; Maximov, T.C.; Dolman, A.J. Spatial and temporal dynamics in eddy covariance observations of methane fluxes at a tundra site in northeastern Siberia. J. Geophys. Res. 2011, 116, G03016. [Google Scholar] [CrossRef]
- Cook, R. Detection of influential observations in linear regression. Technometrics 1977, 19, 15–18. [Google Scholar]
- Walker, D.A.; Raynolds, M.K.; Daniëls, F.J.A.; Eythor, E.; Elvebakk, A.; Gould, W.A.; Katenin, A.E.; Kholod, S.; Markon, C.J.; Melnikov, E.; et al. The circumpolar Arctic vegetation map. J. Veg. Sci. 2005, 16, 267–282. [Google Scholar] [CrossRef]
- Kupková, L.; Červená, L.; Suchá, R.; Jakešová, L.; Zagajewski, B.; Březina, S.; Albrechtová, J. Classification of tundra vegetation in the Krokonše Mts. National Park using APEX, AISA Dual and Sentinel-2A data. Eur. J. Remote Sens. 2017, 50, 29–46. [Google Scholar] [CrossRef]
- Greaves, H.E.; Vierling, L.A.; Eitel, J.U.H.; Boelman, N.T.; Magney, T.S.; Prager, C.M.; Griffin, K.L. High-resolution mapping of aboveground shrub biomass in Arctic tundra using airborne LiDAR and imagery. Remote Sens. Environ. 2016, 184, 361–373. [Google Scholar] [CrossRef]
- Fraser, R.H.; Olthof, I.; Lantz, T.C.; Schmitt, C. UAV photogrammetry for mapping vegetation in the low-Arctic. Arct. Sci. 2016, 2, 79–102. [Google Scholar] [CrossRef]
- Laidler, G.J.; Treitz, P. Biophysical remote sensing of arctic environments. Prog. Phys. Geogr. 2003, 27, 44–68. [Google Scholar] [CrossRef]
- Kutzbach, L.; Wagner, D.; Pfeiffer, E.-M. Effect of microrelief and vegetation on methane emission from wet polygonal tundra, Lena Delta, Northern Siberia. Biogeochemistry 2004, 69, 341–362. [Google Scholar] [CrossRef] [Green Version]
- Marushchak, M.E.; Friborg, T.; Biasi, C.; Johansson, T.; Kiepe, I.; Lümatainen, M.; Lind, S.E.; Martikainen, P.J.; Virtanen, T.; Soegaard, H.; et al. Methane dynamics in the subarctic tundra: Combining stable isotope analyses, plot- and ecosystem-scale flux measurements. Biogeosciences 2016, 13, 597–608. [Google Scholar] [CrossRef]
- Riutta, R.; Laine, J.; Aurela, M.; Rinne, J.; Vesala, T.; Laurila, T.; Haapanala, S.; Philatie, M.; Tuittila, E.-S. Spatial variation in plant community functions regulates carbon gas dynamics in a boreal fen ecosystem. Tellus B 2007, 59, 838–852. [Google Scholar] [CrossRef]
- Xu, X.; Riley, W.J.; Koven, C.D.; Billesbach, D.P.; Chang, R.Y.-W.; Commane, R.; Euskirchen, E.S.; Hartery, S.; Harazono, Y.; Iwata, H.; et al. A multi-scale comparison of modeled and observed seasonal methane emissions in northern wetlands. Biogeosciences 2016, 13, 5043–5056. [Google Scholar] [CrossRef]
- Pirk, N.; Sievers, J.; Mastepanov, M.; Parmentier, F.J.W.; Crill, P.; Christensen, T.R. Calculations of automatic chamber flux measurements of methane and carbon dioxide using short time series of concentrations. Biogeosciences 2016, 13, 903–912. [Google Scholar] [CrossRef]
- Zona, D.; Lipson, D.A.; Paw, U.K.T.; Oberbauer, S.F.; Olivas, P.; Gioli, B.; Oechel, W.C. Increased CO2 loss from vegetated drained lake tundra ecosystems due to flooding. Glob. Biogeochem. Cycles 2012, 26, GB2004. [Google Scholar] [CrossRef]
- Sabrekov, A.F.; Runkle, B.R.K.; Glagolev, M.V.; Kleptsova, I.E.; Maksyutov, S.S. Seasonal variability as a source of uncertainty in the West Siberian regional CH4 flux upscaling. Envrion. Res. Lett. 2014, 9, 045008. [Google Scholar] [CrossRef]
- Sturtevant, C.S.; Oechel, W.C.; Zona, D.; Kim, V.; Emerson, C.E. Soil moisture control over autumn methane flux, Arctic Coastal Plain of Alaska. Biogeosciences 2012, 9, 1423–1440. [Google Scholar] [CrossRef]
- Bubier, J.; Moore, T.; Savage, K.; Crill, P. A comparison of methane flux in a boreal landscape between a dry and wet year. Glob. Biogeochem. Cycles 2005, 19, 1–11. [Google Scholar] [CrossRef]
- Matthes, J.C.; Sturtevant, C.; Verfaille, J.; Knox, S.; Baldocchi, D. Parsing the variability in CH4 flux at a spatially heterogeneous wetland: Integrating multiple eddy covariance towers with high-resolution flux footprint analysis. J. Geophys. Res. Biogeosci. 2014, 119, 1322–1339. [Google Scholar] [CrossRef]
| Site | Microtopographic Position | Chamber Scale Vegetation Type | EC Tower Scale Vegetation Type |
|---|---|---|---|
| Barrow-BEO/BES | High centre | Moss-lichen | Dry lichen heath |
| Flat centre | Dry graminoid | Mesic sedge-grass-herb meadow | |
| Rim | Dry graminoid | ||
| Low centre | Wet sedge | Wet sedge meadow | |
| Trough | Wet sedge | ||
| Drained lake basin | Wet sedge | ||
| Atqasuk | Ridge-tussock | Tussock sedge | Tussock tundra (sandy substrates) |
| Ridge-inter-tussock | Moss-shrub | ||
| Pool | Wet sedge | Wet sedge meadow | |
| Ivotuk | Plateau-tussock | Tussock sedge | Tussock tundra (non-sandy substrates) |
| Plateau-inter-tussock | Moss-shrub | ||
| Plateau-hollow | Moss only | ||
| Wet meadow | Wet sedge | Wet sedge meadow |
| Site | LDA | LDA + VIs | KM | RF |
|---|---|---|---|---|
| Barrow-BEO/BES | ||||
| Classification accuracy (%) | 65 | 64 | 60 | 65 |
| Kappa | 0.43 | 0.43 | 0.35 | 0.44 |
| Atqasuk | ||||
| Classification accuracy (%) | 88 | 80 | 86 | 80 |
| Kappa | 0.71 | 0.56 | 0.67 | 0.55 |
| Ivotuk | ||||
| Classification accuracy (%) | 67 | 73 | 82 | 74 |
| Kappa | 0.39 | 0.52 | 0.73 | 0.53 |
| Site Vegetation Community (%) | LDA | LDA + VIs | KM | RF |
|---|---|---|---|---|
| Barrow-BEO | ||||
| Mesic sedge-grass-herb meadow | 52.5 | 51.8 | 38.8 | 41.1 |
| Dry lichen heath | 3.8 | 5.5 | 11.8 | 19.2 |
| Wet sedge meadow | 43.6 | 42.7 | 47.1 | 39.8 |
| Water | N/A | N/A | 2.2 | N/A |
| Barrow-BES | ||||
| Mesic sedge-grass-herb meadow | 28.8 | 29.3 | 22 | 22.6 |
| Dry lichen heath | 5.1 | 5.5 | 11.9 | 12.2 |
| Wet sedge meadow | 66.1 | 65.2 | 62.7 | 65.5 |
| Water | N/A | N/A | 3.4 | N/A |
| Atqasuk | ||||
| Tussock tundra (sandy substrates) | 83.9 | 63.5 | 80.1 | 74.4 |
| Wet sedge meadow | 12.6 | 32.5 | 16.4 | 25.6 |
| Water | 3.5 | 4 | 3.5 | 2.0 |
| Ivotuk | ||||
| Tussock tundra (non-sandy substrates) | 59.9 | 59.4 | 44.4 | 57.6 |
| Mixed shrub-sedge tussock | 38.9 | 34.9 | 46.5 | 18.3 |
| Wet sedge meadow | 1.2 | 5.7 | 7.6 | 24.0 |
| Water | N/A | N/A | 1.5 | N/A |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Davidson, S.J.; Santos, M.J.; Sloan, V.L.; Reuss-Schmidt, K.; Phoenix, G.K.; Oechel, W.C.; Zona, D. Upscaling CH4 Fluxes Using High-Resolution Imagery in Arctic Tundra Ecosystems. Remote Sens. 2017, 9, 1227. https://doi.org/10.3390/rs9121227
Davidson SJ, Santos MJ, Sloan VL, Reuss-Schmidt K, Phoenix GK, Oechel WC, Zona D. Upscaling CH4 Fluxes Using High-Resolution Imagery in Arctic Tundra Ecosystems. Remote Sensing. 2017; 9(12):1227. https://doi.org/10.3390/rs9121227
Chicago/Turabian StyleDavidson, Scott J., Maria J. Santos, Victoria L. Sloan, Kassandra Reuss-Schmidt, Gareth K. Phoenix, Walter C. Oechel, and Donatella Zona. 2017. "Upscaling CH4 Fluxes Using High-Resolution Imagery in Arctic Tundra Ecosystems" Remote Sensing 9, no. 12: 1227. https://doi.org/10.3390/rs9121227