Potential of RapidEye and WorldView-2 Satellite Data for Improving Rice Nitrogen Status Monitoring at Different Growth Stages
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Area
2.2. Experimental Design
2.3. Determining N Status Indicators with Plant Sampling and Analysis
2.4. Field Spectral Measurements and Re-Sampling
2.5. Data Analysis
3. Results
3.1. Variability of the N Status Indicators
3.2. Correlation between N Indicators and Vegetation Indices
3.3. Stepwise Multiple Linear Regression Analysis
3.4. Partial Least Squares Regression Modeling
3.5. Validation of the Estimation Models
4. Discussion
4.1. Impacts of Growth Stages on N Status Monitoring
4.2. Importance of the Red Edge and Other Bands for N Status Estimation
4.3. Limitations of This Study
5. Conclusions and Future Outlooks
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Yao, Y.; Miao, Y.; Huang, S.; Gao, L.; Ma, X.; Zhao, G.; Jiang, R.; Chen, X.; Zhang, F.; Yu, K.; et al. Active canopy sensor-based precision N management strategy for rice. Agron. Sustain. Dev. 2012, 32, 925–933. [Google Scholar] [CrossRef]
- Zhao, G.; Miao, Y.; Wang, H.; Su, M.; Fan, M.; Zhang, F.; Jiang, R.; Zhangh, Z.; Liu, C.; Liu, P.; et al. A preliminary precision rice management system for increasing both grain yield and nitrogen use efficiency. Field Crop. Res. 2013, 154, 23–30. [Google Scholar] [CrossRef]
- Huang, S.; Miao, Y.; Zhao, G.; Yuan, F.; Ma, X.; Tan, C.; Yu, W.; Gnyp, M.; Lenz-Wiedemann, V.I.S.; Rascher, U.; et al. Satellite remote sensing-based in-season diagnosis of rice nitrogen status in Northeast China. Remote Sens. 2015, 7, 10646–10667. [Google Scholar] [CrossRef]
- Yao, Y.; Miao, Y.; Cao, Q.; Wang, H.; Gnyp, M.L.; Bareth, G.; Khosla, R.; Yang, W.; Liu, F.; Liu, C. In-season estimation of rice nitrogen status with an active crop canopy sensor. IEEE J. Sel. Top. Appl. Earth Obs. Remote Sens. 2014, 7, 4403–4413. [Google Scholar] [CrossRef]
- Li, F.; Miao, Y.; Zhang, F.; Cui, Z.; Li, R.; Chen, X.; Zhang, H.; Schroder, J.; Raun, W.R.; Jia, L. In-season optical sensing improves nitrogen-use efficiency for winter wheat. Soil Sci. Soc. Am. J. 2009, 73, 1566–1574. [Google Scholar] [CrossRef]
- Cao, Q.; Miao, Y.; Feng, G.; Gao, X.; Li, F.; Liu, B.; Yue, S.; Cheng, S.; Ustin, S.; Khosla, R. Active canopy sensing of winter wheat nitrogen status: An evaluation of two sensor systems. Comput. Electron. Agric. 2015, 112, 54–67. [Google Scholar] [CrossRef]
- Cao, Q.; Miao, Y.; Shen, J.; Yu, W.; Yuan, F.; Cheng, S.; Huang, S.; Wang, H.; Yang, W.; Liu, F. Improving in-season estimation of rice yield potential and responsiveness to topdressing nitrogen application with Crop Circle active canopy sensor. Precis. Agric. 2016, 17, 136–154. [Google Scholar] [CrossRef]
- Xia, T.; Miao, Y.; Wu, D.; Shao, H.; Khosla, R.; Mi, G. Active optical sensing of spring maize for in-season diagnosis of nitrogen status based on nitrogen nutrition index. Remote Sens. 2016, 8, 605. [Google Scholar] [CrossRef]
- Schmidt, J.P.; Dellinger, A.E.; Beegle, D.B. Nitrogen recommendations for corn: An on-the-go sensor compared with current recommendation methods. Agron. J. 2009, 101, 916–924. [Google Scholar] [CrossRef]
- Diacono, M.; Rubino, P.; Montemurro, F. Precision nitrogen management of wheat: A reivew. Agron. Sustain. Dev. 2013, 33, 219–241. [Google Scholar] [CrossRef]
- Holland, K.H.; Schepers, J.S. Use of a virtual-reference concept to interpret active crop canopy sensor data. Precis. Agric. 2013, 14, 71–85. [Google Scholar] [CrossRef]
- Mulla, D.J. Twenty five years of remote sensing in precision agriculture: Key advances and remaining knowledge gaps. Biosyst. Eng. 2013, 114, 358–371. [Google Scholar] [CrossRef]
- Mulla, D.J.; Miao, Y. Precision Farming. In Land Resources Monitoring, Modeling, and Mapping with Remote Sensing; Thenkabail, P.S., Ed.; CRC Press: Boca Raton, FL, USA, 2016. [Google Scholar]
- Zhao, Q.; Lenz-Wiedemann, V.I.S.; Yuan, F.; Jiang, R.; Miao, Y.; Zhang, F.; Bareth, G. Investigating within-field variability of rice from high resolution satellite imagery in Qixing Farm County, Northeast China. ISPRS Int. J. Geo-Inf. 2015, 4, 236–261. [Google Scholar] [CrossRef]
- Beeri, O.; Phillips, R.; Carson, P.; Leibig, M. Alternate satellite models for estimation of sugar beet residue nitrogen credit. Agric. Ecosyst. Environ. 2005, 107, 21–35. [Google Scholar] [CrossRef]
- Claverie, M.; Demarez, V.; Duchemin, B.; Hagolle, O.; Ducrot, D.; Marais-Sicre, C.; Dejoux, J.F.; Huc, M.; Keravec, P.; Béziat, P.; et al. Maize and sunflower biomass estimation in southwest France using high spatial and temporal resolution remote sensing data. Remote Sens. Environ. 2012, 124, 844–857. [Google Scholar] [CrossRef] [Green Version]
- Tang, Y.L.; Huang, J.F.; Wang, R.C.; Wang, F.M. Comparsion of yield estimation simulated models of rice by remote sensing. Trans. Chin. Soc. Agric. Eng. 2004, 20, 166–171. [Google Scholar]
- Magney, T.S.; Eitel, J.U.; Vierling, L.A. Mapping wheat nitrogen uptake from RapidEye vegetation indices. Precis. Agric. 2016. [Google Scholar] [CrossRef]
- Eitel, J.U.H.; Long, D.S.; Gessler, P.E.; Smith, A.M.S. Using in-situ measurements to evaluate the new RapidEye™ satellite series for prediction of wheat nitrogen status. Int. J. Remote Sens. 2007, 28, 4183–4190. [Google Scholar] [CrossRef]
- Eitel, J.U.H.; Vierling, L.A.; Litvak, M.E.; Long, D.S.; Schulth, U.; Ager, A.A.; Krofcheck, D.J.; Stoscheck, L. Broadband, red edge information from satellites improves early stress detection in a New Mexico conifer woodland. Remote Sens. Environ. 2011, 115, 3640–3646. [Google Scholar] [CrossRef]
- Asam, S.; Fabritius, H.; Klein, D.; Conrad, C.; Dech, S. Derivation of leaf area index for grassland within alpine upland using multi-temporal RapidEye data. Int. J. Remote Sens. 2013, 34, 8628–8652. [Google Scholar] [CrossRef]
- Kim, H.O.; Yeom, J.M. Multi-temporal spectral analysis of rice fields in South Korea using MODIS and RapidEye satellite imagery. J. Astron. Space Sci. 2012, 29, 407–411. [Google Scholar] [CrossRef]
- Ramoelo, A.; Skidmore, A.K.; Cho, M.A.; Schlerf, M.; Mathieu, R.; Heitkönig, I.M.A. Regional estimation of savanna grass nitrogen using the red-edge band of the spaceborne RapidEye sensor. Int. J. Appl. Earth Obs. Geoinf. 2012, 19, 151–162. [Google Scholar] [CrossRef]
- Mutanga, O.; Adam, E.; Cho, M.A. High density biomass estimation for wetland vegetation using WorldView-2 imagery and random forest regression algorithm. Int. J. Appl. Earth Obs. Geoinf. 2012, 18, 399–406. [Google Scholar] [CrossRef]
- Yang, C.M.; Liu, C.C.; Wang, Y.W. Using FORMOSAT-2 satellite data to estimate leaf area index of rice crop. J. Photogram. Remote Sens. 2008, 13, 253–260. [Google Scholar]
- Bsaibes, A.; Courault, D.; Baret, F.; Weiss, M.; Olioso, A.; Jacob, F.; Hagolle, O.; Marloie, O.; Bertrand, N.; Desfond, V.; et al. Albedo and LAI estimates from FORMOSAT-2 data for crop monitoring. Remote Sens. Environ. 2009, 113, 716–729. [Google Scholar] [CrossRef]
- Bausch, W.C.; Khosla, R. QuickBird satellite versus ground-based multi-spectral data for estimating nitrogen status of irrigated maize. Precis. Agric. 2010, 11, 274–290. [Google Scholar] [CrossRef]
- Thenkabail, P.S.; Smith, R.B.; Pauw, E.D. Hyperspectral vegetation indices and their relationships with agricultural crop characteristics. Remote Sens. Environ. 2000, 71, 158–182. [Google Scholar] [CrossRef]
- Mutanga, O.; Skidmore, A.K. Narrow band vegetation indices overcome the saturation problem in biomass estimation. Int. J. Remote Sens. 2004, 25, 3999–4014. [Google Scholar] [CrossRef]
- Gnyp, M.L.; Miao, Y.; Yuan, F.; Ustin, S.L.; Yu, K.; Yao, Y.; Huang, S.; Bareth, G. Hyperspectral canopy sensing of paddy rice aboveground biomass at different growth stages. Field Crop. Res. 2014, 155, 42–55. [Google Scholar] [CrossRef]
- Van Niel, T.G.; McVicar, T.R. Current and potential uses of optical remotesensing in rice-based irrigation systems: A review. Aust. J. Agric. Res. 2004, 55, 155–185. [Google Scholar]
- Nguy-Robertson, A.; Gitelson, A.; Peng, Y.; Viñaet, A.; Arkebauer, T.; Rundquist, D. Green leaf area index estimation in maize and soybean: Combining vegetation indices to achieve maximal sensitivity. Agron. J. 2012, 104, 1336–1347. [Google Scholar] [CrossRef]
- Cao, Q.; Miao, Y.; Wang, H.; Huang, S.; Chen, S.; Khosla, R.; Jiang, R. Non-destructive estimation of rice plant nitrogen status with Crop Circle multispectral active canopy sensor. Field Crop. Res. 2013, 154, 133–144. [Google Scholar] [CrossRef]
- Kanke, Y.; Tubaña, B.; Dalen, M.; Harrell, D. Evaluation of red and red-edge reflectance-based vegetation indices for rice biomass and grain yield prediction models in paddy fields. Precis. Agric. 2016, 17, 507–530. [Google Scholar] [CrossRef]
- Stroppiana, D.; Fava, F.; Boschetti, M.; Brivio, P.A. Estimation of nitrogen content in crops and pastures using hyperspectral vegetation indices. In Hyperspectral Remote Sensing of Vegetation; Thenkabail, P.S., Lyon, J.G., Huete, A., Eds.; CRC Press: Boca Raton, FL, USA, 2012; pp. 245–262. [Google Scholar]
- Wold, S.; Trygg, J.; Berglund, A.; Antti, H. Some recent developments in PLS modeling. Chemometr. Intell. Lab. 2001, 58, 131–150. [Google Scholar] [CrossRef]
- Hansen, P.M.; Schjoerring, J.K. Reflectance measurement of canopy biomass and nitrogen status in wheat crops using normalized difference vegetation indices and partial least squares regression. Remote Sens. Environ. 2003, 86, 542–553. [Google Scholar] [CrossRef]
- Nguyen, H.T.; Lee, B.W. Assessment of rice leaf growth and nitrogen status by hyperspectral canopy reflectance and partial least square regression. Eur. J. Agron. 2006, 24, 349–356. [Google Scholar] [CrossRef]
- Wang, Y.; Yang, Y. Effects of agriculture reclamation on hydrologic characteristics in the Sanjiang Plain. China Geogr. Sci. 2001, 11, 163–167. [Google Scholar] [CrossRef]
- Yan, M.; Deng, W.; Chen, P. Climate change in the Sanjiang Plain disturbed by large-scale reclamation. J. Geogr. Sci. 2002, 12, 405–412. [Google Scholar]
- Xing, B.; Dudas, M.J.; Zhang, Z.; Qi, X. Pedogenetic characteristics of albic soils in the three river plain, Heilongjiang Province. Acta Pedol. Sin. 1994, 31, 95–104. [Google Scholar]
- Greenwood, D.J.; Neeteson, J.J.; Draycott, A. Quantitative relationships for the dependence of growth rate of arable crops on their nitrogen content, dry weight and aerial environment. Plant Soil 1986, 91, 281–301. [Google Scholar] [CrossRef]
- Lemaire, G.; Jeuffroy, M.H.; Gastal, F. Diagnosis tool for plant and crop N status in vegetative stage: Theory and practices for crop N management. Eur. J. Agron. 2008, 28, 614–624. [Google Scholar] [CrossRef]
- Justes, E.; Mary, B.; Meynard, J.M.; Machet, J.M.; Thelier-Huche, L. Determination of a critical nitrogen dilution curve for winter wheat crops. Ann. Bot. 1994, 74, 397–407. [Google Scholar] [CrossRef]
- Gastal, F.; Farruggia, A.; Hacquet, J. The nitrogen nutrition index of grass can be evaluated through determination of N concentration of upper leaves. In Proceedings of the 2001 11th Nitrogen Workshop, Reims, France, 9–12 September 2001; pp. 449–450.
- Farruggia, A.; Gastal, F.; Scholefield, D. Assessment of the nitrogen status of grassland. Grass Forage Sci. 2004, 59, 113–120. [Google Scholar] [CrossRef]
- Chen, P.; Haboudane, D.; Tremblay, N.; Wang, J.; Vigneault, P.; Li, B. New spectral indicator assessing the efficiency of crop nitrogen treatment in corn and wheat. Remote Sens. Environ. 2010, 114, 1987–1997. [Google Scholar] [CrossRef]
- Cilia, C.; Panigada, C.; Rossini, M.; Meroni, M.; Busetto, L.; Amaducci, S.; Boschetti, M.; Picchi, V.; Colombo, R. Nitrogen status assessment for variable rate fertilization in maize through hyperspectral imagery. Remote Sens. 2014, 6, 6549–6565. [Google Scholar] [CrossRef] [Green Version]
- Jordan, C.F. Derivation of leaf-area index from quality of light on the forest floor. Ecology 1969, 50, 663–666. [Google Scholar] [CrossRef]
- Gitelson, A.A.; Viña, A.; Ciganda, V.; Rundquist, D.C.; Arkebauer, T.J. Remote estimation of canopy chlorophyll content in crops. Geophys. Res. Lett. 2005, 32, L08403. [Google Scholar] [CrossRef]
- Rouse, J.W.; Has, R.H.; Schell, J.A.; Deering, D.W. Monitoring vegetation systems in the great plains with ERTS. In Proceedings of the Third ERTS Symposium (NASA), Washington, DC, USA, 10–14 December 1973.
- Gitelson, A.A.; Kaufman, Y.J.; Merzlyak, M.N. Use of a green channel in remote sensing of global vegetation from EOS-MODIS. Remote Sens. Environ. 1996, 58, 289–298. [Google Scholar] [CrossRef]
- Rondeaux, G.; Steven, M.; Baret, F. Optimization of soil-adjusted vegetation indices. Remote Sens. Environ. 1996, 55, 95–107. [Google Scholar] [CrossRef]
- Daughtry, C.S.T.; Walthall, C.L.; Kim, M.S.; Colstoun, E.B.; McMurtrey, J.E., III. Estimating cron leaf chlorophyll concentration from leaf and canopy reflectance. Remote Sens. Environ. 2000, 74, 229–239. [Google Scholar] [CrossRef]
- Broge, N.H.; Leblanc, E. Comparing prediction power and stability of broadband and hyperspectral vegetation indices for estimation of green leaf area index and canopy chlorophyll density. Remote Sens. Environ. 2000, 76, 156–172. [Google Scholar] [CrossRef]
- Haboudane, D.; Miller, J.R.; Tremblay, N.; Zarco-Tejada, P.J.; Dextraze, L. Integrated narrow-band vegetation indices for prediction of crop chlorophyll content for application to precision agriculture. Remote Sens. Environ. 2002, 81, 416–426. [Google Scholar] [CrossRef]
- Fitzgerald, G.; Rodriguez, D.; O’Leary, G. Measuring and predicting canopy nitrogen nutrition in wheat using a spectral index-The canopy chlorophyll content index (CCCI). Field Crop. Res. 2010, 116, 318–324. [Google Scholar] [CrossRef]
- Dash, J.; Curran, P.J. The MERIS terrestrial chlorophyll index. Int. J. Remote Sens. 2004, 31, 5513–5532. [Google Scholar] [CrossRef]
- Clarke, T.R.; Moran, M.S.; Barnes, E.M.; Pinter, P.J.; Qi, J. Planar domain indices: A method for measuring a quality of a single component in two-component pixels. In Proceedings of the IGARSS 2001: IEEE International Geoscience and Remote Sensing Symposium, Sydney, Australia, 9–13 July 2001.
- Wu, C.; Niu, Z.; Tang, Q.; Huang, W. Estimating chlorophyll content from hyperspectral vegetation indices: Modeling and validation. Agric. For. Meteorol. 2008, 148, 1230–1241. [Google Scholar] [CrossRef]
- Chong, I.; Jun, C. Performance of some variable selection methods when multicollinearity is present. Chemom. Intell. Lab. 2005, 78, 103–112. [Google Scholar] [CrossRef]
- Li, X.; Zhang, Y.; Bao, Y.; Luo, J.; Jin, X.; Xu, X.; Song, X.; Yang, G. Exploring the best hyperspectral features for LAI estimation using partial least squares regression. Remote Sens. 2014, 6, 6221–6241. [Google Scholar] [CrossRef]
- Plénet, D.; Lemaire, G. Relationships between dynamics of nitrogen uptake and dry matter accumulation in maize crops. Determination of critical N concentration. Plant Soil 1999, 216, 65–82. [Google Scholar] [CrossRef]
- Ata-Ul-Karim, S.T.; Yao, X.; Liu, X.; Cao, W.; Zhu, Y. Development of critical nitrogen dilution curve of Japonica rice in Yangtze River Reaches. Field Crop. Res. 2013, 149, 49–158. [Google Scholar] [CrossRef]
- Yu, K.; Li, F.; Gnyp, M.L.; Miao, Y.; Bareth, G.; Chen, X. Remotely detecting canopy nitrogen concentration and uptake of paddy rice in the Northeast China Plain. ISPRS J. Photogramm. Remote Sens. 2013, 78, 102–115. [Google Scholar] [CrossRef]
- Li, F.; Miao, Y.; Feng, G.; Yuan, F.; Yue, S.; Gao, X.; Liu, Y.; Liu, B.; Ustin, S.L.; Chen, X. Improving estimation of summer maize nitrogen status with red edge-based spectral vegetation indices. Field Crop. Res. 2014, 157, 111–123. [Google Scholar] [CrossRef]
- Mistele, B.; Schmidhalter, U. Estimating the nitrogen nutrition index using spectral canopy reflectance measurements. Eur. J. Agron. 2008, 29, 184–190. [Google Scholar] [CrossRef]
- Shiratsuchi, L.; Ferguson, R.; Shanahan, J.; Adamchuk, V.; Rundquist, D.; Marx, D.; Slater, G. Water and nitrogen effects on active canopy sensor vegetationindices. Agron. J. 2011, 103, 1815–1826. [Google Scholar] [CrossRef]
- Cho, M.A.; Skidmore, A.K. A new technique for extracting the red edge position from hyperspectral data: The linear extrapolation method. Remote Sens. Environ. 2006, 101, 181–193. [Google Scholar] [CrossRef]
- Clevers, J.G.P.W.; De Jong, S.M.; Epema, G.F.; Van Der Meer, F.D.; Bakker, W.H.; Skidmore, A.K.; Scholte, K.H. Derivation of the red edge index using the MERIS standard band setting. Int. J. Remote Sens. 2002, 23, 3169–3184. [Google Scholar] [CrossRef]
- Haboudane, D.; Tremblay, N.; Miller, J.R.; Vigneault, P. Remote estimation of crop chlorophyll content using spectral indices derived from hyperspectral data. IEEE Trans. Geosci. Remote Sens. 2008, 46, 423–437. [Google Scholar] [CrossRef]
- Zarco-Tejada, P.J.; Miller, J.R.; Morales, A.; Berjon, A.; Aguera, J. Hyperspectral indices and model simulation for chlorophyll estimation in open-canopy treecrops. Remote Sens. Environ. 2004, 90, 463–476. [Google Scholar] [CrossRef]
- Carter, G.A. Responses of leaf spectral reflectance to plant stress. Am. J. Bot. 1993, 80, 239–243. [Google Scholar] [CrossRef]
- Carter, G.A.; Knapp, A.K. Leaf optical properties in higher plants: Linking spectral characteristics to stress and chlorophyll concentration. Am. J. Bot. 2001, 88, 677–684. [Google Scholar] [CrossRef] [PubMed]
- Herrmann, I.; Pimstein, A.; Karnieli, A.; Cohen, Y.; Alchanatis, V.; Bonfil, D.J. LAI assessment of wheat and potato crops by VENμS and Sentinel-2 bands. Remote Sens. Environ. 2011, 115, 2141–2151. [Google Scholar] [CrossRef]
- Koppe, W.; Gnyp, M.L.; Hütt, C.; Yao, Y.; Miao, Y.; Chen, X.; Bareth, G. Rice monitoring with multi-temporal and dual-polarimetric TerraSAR-X data. Int. J. Appl. Earth Obs. Geoinf. 2013, 21, 568–576. [Google Scholar] [CrossRef]



| Experiment | Site | Year | Cultivar | N Rates (kg·ha−1) | Transplanting/Harvesting Date | Sampling Stage |
|---|---|---|---|---|---|---|
| 1 | 1 | 2008 | Kongyu 131 | 0, 35, 70, 105, 140 | 29 May/21 September | PI, SE, HE |
| 2 | 2 | 2008 | Kongyu 131 | 0, 35, 70, 105, 140 | 13 May/22 September | PI, SE, HE |
| 3 | 1 | 2009 | Kongyu 131 | 0, 35, 70, 105, 140 | 24 May/27 September | SE, HE |
| 4 | 2 | 2009 | Kongyu 131 | 0, 35, 70, 105, 140 | 20 May/27 September | PI,SE, HE |
| 5 | 1 | 2011 | Kongyu 131 | 0, 70, 100, 130,160 | 17 May/21 September | PI |
| 6 | 1 | 2011 | Longjing 21 | 0, 70, 100, 130, 160 | 19 May/21 September | PI |
| 7 | 1 | 2008 | Kongyu 131 | 0, 23, 45, 68, 91 | 29 May/21 September | HE |
| 8 | 2 | 2008 | Kongyu 131 | 0, 23, 45, 68, 91 | 13 May/22 September | HE |
| 9 | 1 | 2009 | Kongyu 131 | 0, 23, 45, 68, 91 | 24 May/27 September | SE, HE |
| 10 | 2 | 2009 | Kongyu 131 | 0, 23, 45, 68, 91 | 20 May/27 September | SE, HE |
| Properties | FORMOSAT-2 (F2) | RapidEye (RY) | WorldView-2 (WV2) |
|---|---|---|---|
| Type | Sun-synchronous | Sun-synchronous | Sun-synchronous |
| Launch time | 4 May 2004 | 8 August 2008 | 9 October 2009 |
| Orbit altitude (km) | 891 | 620 | 770 |
| Spatial Resolution for Multispectral bands (m) | 8 | 6.5 | 2 |
| Spatial Resolution for Panchromatic bands (m) | 2 | - | 0.5 |
| Revisit time (Day) | 1 | <1 | 1.1 |
| Swath width (km) | 24 | 80 | 16.4 |
| Band settings | 450–520 nm (Blue: FB) 520–600 nm (Green: FG) 630–690 nm (Red: FR) 760–900 nm (NIR1: FNIR1) | 440–510 nm (Blue: RB) 520–590 nm (Green: RG) 630–685 nm (Red: RR) 690–730 nm (Red edge: RRE) 760–900 nm (NIR1: RNIR1) | 400–450 nm (Coastal: WVC) 450–510 nm (Blue: WVB) 510–581 nm (Green: WVG) 585–625 nm (Yellow: WVY) 630–690 nm (Red: WVR) 705–745 nm (Red Edge: WVRE) 770–895 nm (NIR1: WVNIR1) 860–1040 nm (NIR2: WVNIR2) |
| Vegetation Index | Formula | Satellite Sensors | Reference |
|---|---|---|---|
| Ration Vegetation Index (RVI) | NIR/R | F2, RY, WV2 | [49] |
| Chlorophyll Index (CI) | (NIR/G) − 1 | F2, RY, WV2 | [50] |
| Normalized Difference Vegetation Index (NDVI) | (NIR − R)/(NIR + R) | F2, RY, WV2 | [51] |
| Green NDVI (GNDVI) | (NIR − G)/(NIR + G) | F2, RY, WV2 | [52] |
| Optimized Soil Adjusted Vegetation Index (OSAVI) | (1 + 0.16) × ((NIR − R)/(NIR + R + 0.16)) | F2, RY, WV2 | [53] |
| Modified Chlorophyll Absorption in Reflectance Index (MCARI) | ((NIR − R)− 0.2(R − G)) × (NIR/R) | F2, RY, WV2 | [54] |
| Triangular Vegetation Index (TVI) | 0.5 × (120(NIR − G) − 200(R − G)) | F2, RY, WV2 | [55] |
| Modified Transformed Chlorophyll Absorption in Reflectance Index (TCARI) | 3 × ((NIR − R) − 0.2(NIR − G)(NIR/R)) | F2, RY, WV2 | [56] |
| MCARI/OSAVI | MCARI/OSAVI | F2, RY, WV2 | [56] |
| TCARI/OSAVI | TCARI/OSAVI | F2, RY, WV2 | [56] |
| Red Edge Chlorophyll Index (RECI) | (NIR/RE) − 1 | RY, WV2 | [50] |
| Normalized difference Red Edge Index (NDRE) | (NIR − RE)/(NIR + RE) | RY, WV2 | [57] |
| MERIS Terrestrial Chlorophyll Index (MTCI) | (NIR − RE)/(RE − R) | RY, WV2 | [58] |
| Canopy Chlorophyll Content Index (CCCI) | (NDRE − NDREmin)/(NDREmax − NDREmin) | RY, WV2 | [57] |
| Nitrogen Planar Domain Index (NDPI) | (RECI − RECImin)/(RECImax − RECImin) | RY, WV2 | [59] |
| Red Edge OSAVI (REOSAVI) | (1 + 0.16) × ((NIR − RE)/(NIR + RE + 0.16)) | RY, WV2 | [60] |
| Red Edge MCARI (REMCARI) | ((NIR − RE) − 0.2(RE − G)) × (NIR/RE) | RY, WV2 | [60] |
| Red Edge Triangular Vegetation Index (RETVI) | 0.5 × (120(NIR − G) − 200(RE − G)) | RY, WV2 | [55] |
| Red Edge TCARI (RETCARI) | 3 × ((NIR − RE) − 0.2(NIR − G)(NIR/RE)) | RY, WV2 | [60] |
| REMCARI/REOSAVI | REMCARI/REOSAVI | RY, WV2 | [60] |
| RETCARI/REOSAVI | RETCARI/REOSAVI | RY, WV2 | [60] |
| Stage | Calibration Dataset | Validation Dataset | |||||||
|---|---|---|---|---|---|---|---|---|---|
| AGB (t·ha−1) | PNC (%) | PNU (kg·ha−1) | NNI | AGB (t·ha−1) | PNC (%) | PNU (kg·ha−1) | NNI | ||
| PI | N | 57 | 57 | 57 | 57 | 28 | 28 | 28 | 28 |
| Mean | 1.11 | 2.47 | 27.53 | 0.96 | 1.05 | 2.46 | 26.09 | 0.94 | |
| SD | 0.50 | 0.17 | 12.71 | 0.11 | 0.48 | 0.21 | 11.84 | 0.10 | |
| CV | 45.02 | 6.97 | 46.17 | 11.40 | 45.79 | 8.45 | 45.40 | 10.63 | |
| SE | N | 92 | 92 | 92 | 92 | 45 | 45 | 45 | 45 |
| Mean | 1.78 | 2.36 | 40.13 | 1.01 | 1.83 | 2.39 | 41.32 | 1.02 | |
| SD | 0.88 | 0.36 | 16.96 | 0.14 | 0.99 | 0.35 | 18.25 | 0.12 | |
| CV | 49.36 | 15.11 | 42.26 | 13.74 | 54.17 | 14.64 | 44.17 | 12.04 | |
| HE | N | 98 | 98 | 98 | 98 | 49 | 49 | 49 | 49 |
| Mean | 6.28 | 1.62 | 103.34 | 1.09 | 5.93 | 1.60 | 95.41 | 1.05 | |
| SD | 1.49 | 0.28 | 36.20 | 0.24 | 1.45 | 0.29 | 31.09 | 0.22 | |
| CV | 23.75 | 17.06 | 35.03 | 21.97 | 24.46 | 18.11 | 32.59 | 21.18 | |
| All | N | 247 | 247 | 247 | 247 | 122 | 122 | 122 | 122 |
| Min | 0.20 | 0.83 | 4.39 | 0.53 | 0.14 | 0.96 | 3.17 | 0.65 | |
| Max | 9.92 | 3.15 | 205.64 | 1.63 | 9.21 | 3.35 | 195.37 | 1.63 | |
| Mean | 3.41 | 2.09 | 62.30 | 1.03 | 3.30 | 2.09 | 59.55 | 1.02 | |
| SD | 2.59 | 0.48 | 42.36 | 0.19 | 2.45 | 0.50 | 37.94 | 0.17 | |
| CV | 75.95 | 22.97 | 67.99 | 18.45 | 74.24 | 23.92 | 63.71 | 16.67 | |
| PI Stage | SE Stage | HE Stage | All | ||||
|---|---|---|---|---|---|---|---|
| Index | AGB | Index | AGB | Index | AGB | Index | AGB |
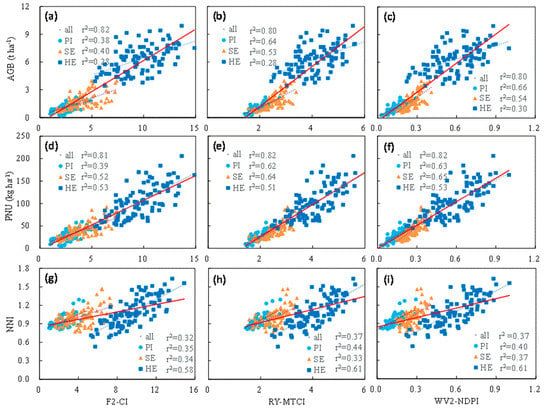
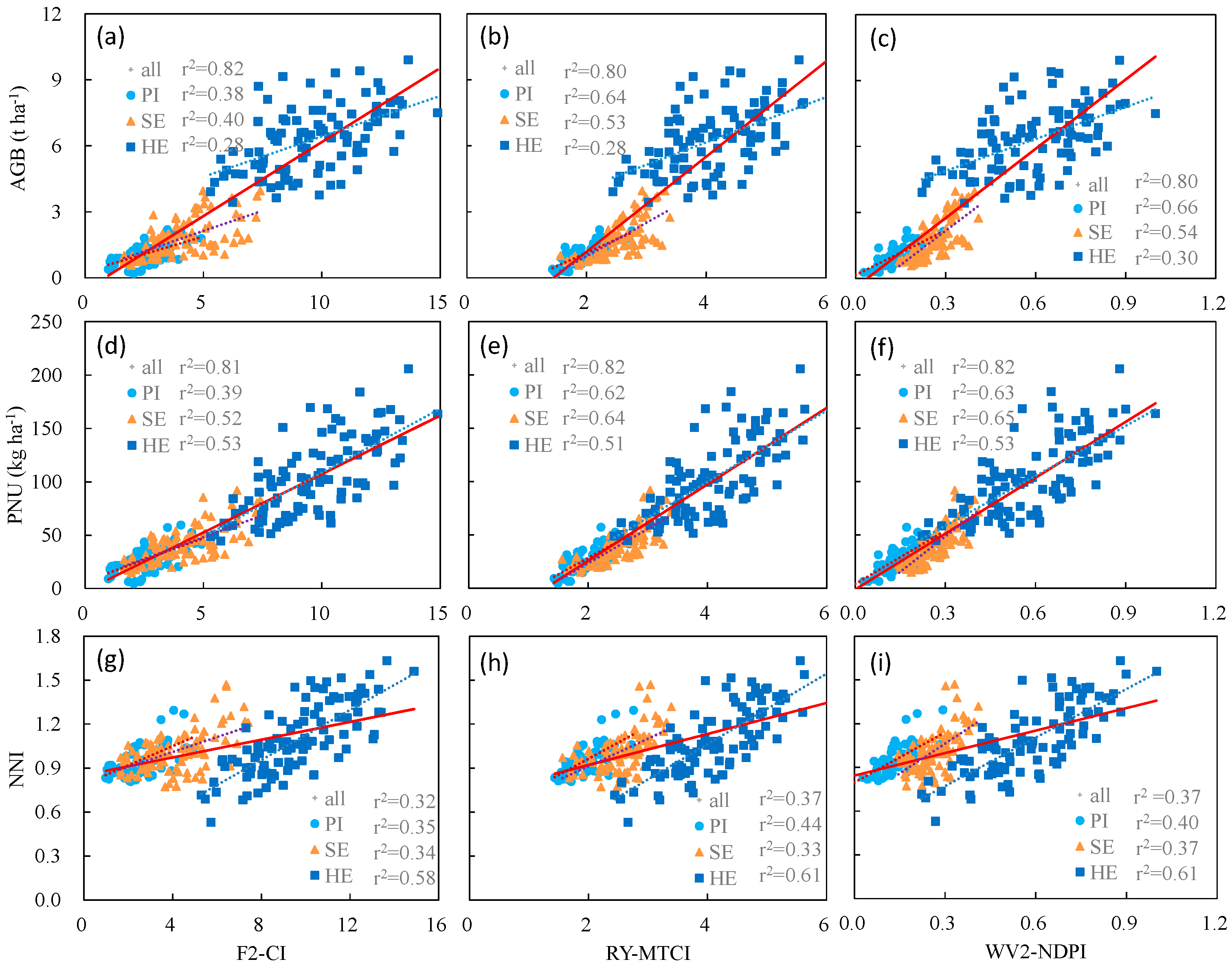
| F2-CI | 0.39 ** | F2-GNDVI | 0.41 ** | F2-CI | 0.28 ** | F2-CI | 0.82 ** |
| F2-GNDVI | 0.35 ** | F2-OSAVI | 0.41 ** | F2-GNDVI | 0.27 ** | F2-RVI | 0.80 ** |
| F2-MCARI/OSAVI | 0.33 ** | F2-NDVI | 0.41 ** | F2-RVI | 0.21 ** | F2-MCARI/OSAVI | 0.77 ** |
| F2-TCARI/OSAVI | 0.34 ** | F2-CI | 0.40 ** | F2-NDVI | 0.20 ** | F2-TCARI/OSAVI | 0.77 ** |
| F2-RVI | 0.33 ** | F2-TVI | 0.39 ** | F2-TCARI/OSAVI | 0.18 ** | F2-MCARI | 0.75 ** |
| RY-MTCI | 0.64 ** | RY-MTCI | 0.53 ** | RY-MTCI | 0.28 ** | RY-CI | 0.82 ** |
| RY-CCCI | 0.61 ** | RY-CCCI | 0.51 ** | RY-CCCI | 0.28 ** | RY-RECI | 0.81 ** |
| RY-NDPI | 0.59 ** | RY-NDPI | 0.50 ** | RY-NDPI | 0.28 ** | RY-NDPI | 0.81 ** |
| RY-RECI | 0.46 ** | RY-RECI | 0.47 ** | RY-RECI | 0.28 ** | RY-RVI | 0.80 ** |
| RY-NDRE | 0.43 ** | RY-NDRE | 0.46 ** | RY-NDRE | 0.28 ** | RY-MTCI | 0.80 ** |
| WV2-NDPI | 0.65 ** | WV2-MTCI | 0.57 ** | WV2-NDPI | 0.30 ** | WV2-CI | 0.82 ** |
| WV2-MTCI | 0.62 ** | WV2-NDPI | 0.54 ** | WV2-MTCI | 0.30 ** | WV2-RECI | 0.82 ** |
| WV2-RETVI | 0.57 ** | WV2-RECI | 0.51 ** | WV2-RECI | 0.30 ** | WV2-MTCI | 0.81 ** |
| WV2-RECI | 0.54 ** | WV2-NDRE | 0.50 ** | WV2-NDRE | 0.30 ** | WV2-RETVI | 0.81 ** |
| WV2-NDRE | 0.53 ** | WV2-RETVI | 0.47 ** | WV2-CCCI | 0.30 ** | WV2-NDPI | 0.80 ** |
| Index | PNC | Index | PNC | Index | PNC | Index | PNC |
| F2-CI | F2-NDVI | 0.06 * | F2-CI | 0.53 ** | F2-OSAVI | 0.42 ** | |
| F2-GNDVI | F2-GNDVI | F2-GNDVI | 0.52 ** | F2-TVI | 0.41 ** | ||
| F2-RVI | F2-OSAVI | F2-NDVI | 0.46 ** | F2-NDVI | 0.39 ** | ||
| F2-TCARI/OSAVI | F2-CI | F2-RVI | 0.44 ** | F2-RVI | 0.39 ** | ||
| F2-TCARI | F2-RVI | F2-TCARI/OSAVI | 0.42 ** | F2-GNDVI | 0.39 ** | ||
| RY-RETCARI/REOSAVI | RY-RETCARI | 0.09 ** | RY-RECI | 0.57 ** | RY-OSAVI | 0.42 ** | |
| RY-GNDVI | RY-NDVI | 0.06 * | RY-MTCI | 0.56 ** | RY-REOSAVI | 0.42 ** | |
| RY-RECI | RY-NDRE | 0.05 * | RY-NDPI | 0.56 ** | RY-TVI | 0.41 ** | |
| RY-NDPI | RY-MTCI | RY-NDRE | 0.55 ** | RY-GNDVI | 0.40 ** | ||
| RY-MTCI | RY-GNDVI | RY-RETCARI/REOSAVI | 0.55 ** | RY-RETVI | 0.40 ** | ||
| WV2-GNDVI | WV2-MTCI | 0.07 * | WV2-REOSAVI | 0.57 ** | WV2-RETCARI | 0.44 ** | |
| WV2-RECI | WV2-NDVI | 0.06 * | WV2-RECI | 0.56 ** | WV2-OSAVI | 0.42 ** | |
| WV2-NDPI | WV2-NDRE | 0.05 * | WV2-MTCI | 0.56 ** | WV2-REOSAVI | 0.41 ** | |
| WV2-NDRE | WV2-GNDVI | WV2-NDRE | 0.56 ** | WV2-TVI | 0.41 ** | ||
| WV2-CI | WV2-RECI | WV2-NDPI | 0.55 ** | WV2-GNDVI | 0.39 ** | ||
| PI Stage | SE Stage | HE Stage | All | ||||
|---|---|---|---|---|---|---|---|
| Index | PNU | Index | PNU | Index | PNU | Index | PNU |
| F2-CI | 0.39 ** | F2-CI | 0.52 ** | F2-CI | 0.50 ** | F2-CI | 0.81 ** |
| F2-GNDVI | 0.35 ** | F2-TVI | 0.52 ** | F2-GNDVI | 0.48 ** | F2-RVI | 0.77 ** |
| F2-TCARI/OSAVI | 0.34 ** | F2-GNDVI | 0.50 ** | F2-RVI | 0.40 ** | F2-MCARI/OSAVI | 0.76 ** |
| F2-RVI | 0.33 ** | F2-OSAVI | 0.50 ** | F2-NDVI | 0.39 ** | F2-TCARI/OSAVI | 0.76 ** |
| F2-MCARI/OSAVI | 0.33 ** | F2-MCARI/OSAVI | 0.49 ** | F2-TCARI/OSAVI | 0.36 ** | F2-MCARI | 0.75 ** |
| RY-MTCI | 0.62 ** | RY-MTCI | 0.64 ** | RY-NDPI | 0.52 ** | RY-NDPI | 0.83 ** |
| RY-CCCI | 0.59 ** | RY-CCCI | 0.62 ** | RY-RECI | 0.52 ** | RY-MTCI | 0.82 ** |
| RY-NDPI | 0.58 ** | RY-NDPI | 0.61 ** | RY-MTCI | 0.51 ** | RY-CI | 0.81 ** |
| RY-RECI | 0.46 ** | RY-RECI | 0.57 ** | RY-RETCARI | 0.51 ** | RY-RECI | 0.81 ** |
| RY-NDRE | 0.43 ** | RY-RETVI | 0.56 ** | RY-RETCARI/REOSAVI | 0.51 ** | RY-REMCARI | 0.79 ** |
| WV2-NDPI | 0.63 ** | WV2-NDPI | 0.65 ** | WV2-RECI | 0.62 ** | WV2-NDPI | 0.82 ** |
| WV2-MTCI | 0.60 ** | WV2-MTCI | 0.64 ** | WV2-NDPI | 0.61 ** | WV2-MTCI | 0.82 ** |
| WV2-RETVI | 0.54 ** | WV2-RETVI | 0.61 ** | WV2-MTCI | 0.61 ** | WV2-RECI | 0.82 ** |
| WV2-RECI | 0.53 ** | WV2-RECI | 0.60 ** | WV2-NDRE | 0.61 ** | WV2-CI | 0.81 ** |
| WV2-NDRE | 0.52 ** | WV2-NDRE | 0.59 ** | WV2-REOSAVI | 0.61 ** | WV2-REMCARI | 0.81 ** |
| Index | NNI | Index | NNI | Index | NNI | Index | NNI |
| F2-CI | 0.35 ** | F2-TCARI | 0.34 ** | F2-CI | 0.58 ** | F2-CI | 0.32 ** |
| F2-TCARI/OSAVI | 0.32 ** | F2-TCARI/OSAVI | 0.33 ** | F2-GNDVI | 0.57 ** | F2-TCARI | 0.30 ** |
| F2-RVI | 0.31 ** | F2-MCARI | 0.33 ** | F2-NDVI | 0.48 ** | F2-MCARI | 0.29 ** |
| F2-GNDVI | 0.31 ** | F2-MCARI/OSAVI | 0.32 ** | F2-RVI | 0.47 ** | F2-TCARI/OSAVI | 0.29 ** |
| F2-MCARI/OSAVI | 0.29 ** | F2-CI | 0.30 ** | F2-TCARI/OSAVI | 0.44 ** | F2-RVI | 0.28 ** |
| RY-MTCI | 0.44 ** | RY-REMCARI | 0.35 ** | RY-NDPI | 0.61 ** | RY-RETCARI/REOSAVI | 0.37 ** |
| RY-NDPI | 0.44 ** | RY-CCCI | 0.34 ** | RY-RECI | 0.61 ** | RY-MTCI | 0.37 ** |
| RY-RECI | 0.38 ** | RY-TCARI | 0.34 ** | RY-MTCI | 0.61 ** | RY-NDPI | 0.35 ** |
| RY-CCCI | 0.36 ** | RY-MTCI | 0.33 ** | RY-NDRE | 0.60 ** | RY-CCCI | 0.35 ** |
| RY-NDRE | 0.36 ** | RY-REMCARI/REOSAVI | 0.33 ** | RY-RETCARI/REOSAVI | 0.60 ** | RY-RETCARI | 0.34 ** |
| WV2-MTCI | 0.41 ** | WV2-NDPI | 0.37 ** | WV2-RECI | 0.62 ** | WV2-NDPI | 0.37 ** |
| WV2-RECI | 0.41 ** | WV2-REMCARI | 0.36 ** | WV2-NDPI | 0.61 ** | WV2-MTCI | 0.35 ** |
| WV2-NDRE | 0.41 ** | WV2-RETVI | 0.36 ** | WV2-MTCI | 0.61 ** | WV2-CCCI | 0.35 ** |
| WV2-NDPI | 0.40 ** | WV2-TCARI | 0.34 ** | WV2-NDRE | 0.61 ** | WV2-RECI | 0.34 ** |
| WV2-RETVI | 0.38 ** | WV2-TCARI/OSAVI | 0.33 ** | WV2-REOSAVI | 0.61 ** | WV2-REMCARI | 0.33 ** |
| AGB | PNU | NNI | PNC | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| PI | SE | HE | All | PI | SE | HE | All | PI | SE | HE | All | PI | SE | HE | All | |
| Based on F2 bands | ||||||||||||||||
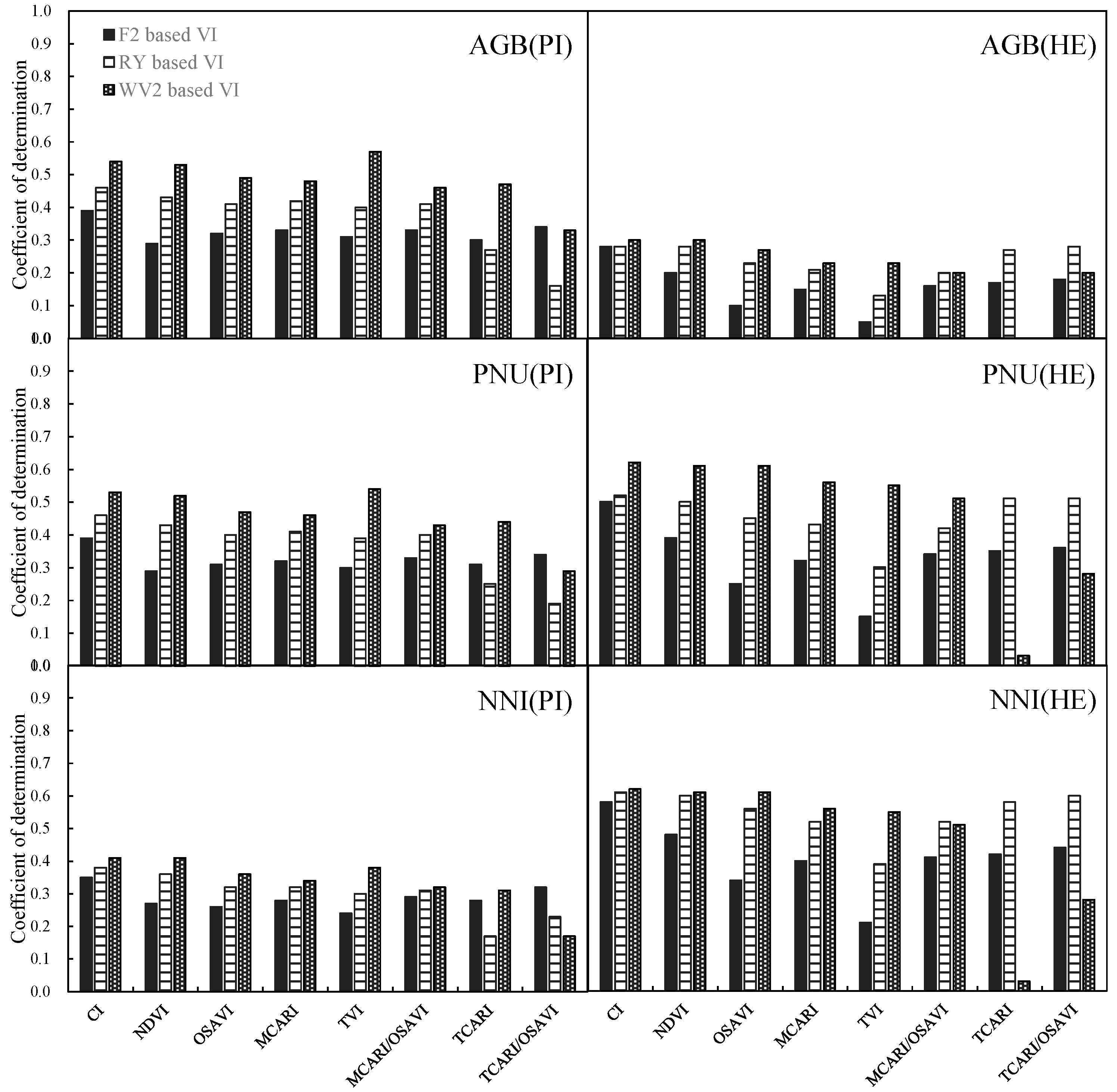
| R2 | 0.61 ** | 0.51 ** | 0.29 ** | 0.82 ** | 0.60 ** | 0.66 ** | 0.50 ** | 0.81 ** | 0.45 ** | 0.30 ** | 0.57 ** | 0.36 ** | 0.08 * | 0.22 ** | 0.51 ** | 0.43 ** |
| Band | NIR1 | R | G | NIR1 | NIR1 | NIR1 | R | NIR1 | NIR1 | NIR1 | R | NIR1 | G | R | R | NIR1 |
| G | B | NIR1 | G | G | G | NIR1 | G | G | NIR1 | G | B | NIR1 | R | |||
| B | B | B | B | G | B | B | G | B | NIR1 | G | ||||||
| R | R | R | G | |||||||||||||
| Based on RY bands | ||||||||||||||||
| R2 | 0.68 ** | 0.55 ** | 0.29 ** | 0.82 ** | 0.66 ** | 0.68 ** | 0.50 ** | 0.82 ** | 0.46 ** | 0.50 ** | 0.59 ** | 0.38 ** | 0.07 * | 0.20 ** | 0.57 ** | 0.43 ** |
| Band | NIR1 | NIR1 | G | NIR1 | NIR1 | NIR1 | R | NIR1 | R | NIR1 | R | NIR1 | G | R | NIR1 | NIR1 |
| RE | RE | NIR1 | RE | RE | RE | NIR1 | RE | NIR1 | RE | NIR1 | RE | B | RE | R | ||
| R | G | R | B | RE | R | RE | R | RE | R | NIR1 | G | |||||
| B | B | G | ||||||||||||||
| Based on WV2 bands | ||||||||||||||||
| R2 | 0.76 ** | 0.63 ** | 0.31 ** | 0.82 ** | 0.71 ** | 0.69 ** | 0.52 ** | 0.82 ** | 0.52 ** | 0.49 ** | 0.61 ** | 0.38 ** | 0.09 ** | 0.10 ** | 0.56 ** | 0.43 ** |
| Band | NIR1 | NIR1 | Y | NIR1 | NIR1 | NIR1 | NIR1 | NIR1 | NIR1 | NIR1 | NIR1 | NIR1 | Y | R | R | NIR2 |
| RE | RE | NIR1 | RE | RE | RE | RE | RE | RE | RE | RE | RE | B | NIR2 | R | ||
| NIR2 | G | G | R | R | G | NIR2 | R | G | RE | |||||||
| C | R | NIR2 | Y | |||||||||||||
| Y | Y | C | ||||||||||||||
| AGB | PNC | |||||||
|---|---|---|---|---|---|---|---|---|
| PI | SE | HE | All | PI | SE | HE | All | |
| Based on F2 bands | ||||||||
| R2 | 0.64 | 0.56 | 0.31 | 0.82 | 0.09 | 0.22 | 0.54 | 0.43 |
| RMSEC | 0.30 | 0.58 | 1.23 | 1.11 | 0.16 | 0.31 | 0.19 | 0.36 |
| Based on RY bands | ||||||||
| R2 | 0.71 | 0.57 | 0.30 | 0.82 | 0.11 | 0.23 | 0.56 | 0.44 |
| RMSEC | 0.26 | 0.57 | 1.24 | 1.11 | 0.16 | 0.31 | 0.18 | 0.36 |
| Based on WV2 bands | ||||||||
| R2 | 0.78 | 0.67 | 0.38 | 0.84 | 0.24 | 0.31 | 0.60 | 0.43 |
| RMSEC | 0.23 | 0.50 | 1.17 | 1.02 | 0.15 | 0.29 | 0.17 | 0.36 |
| PNU | NNI | |||||||
| PI | SE | HE | All | PI | SE | HE | All | |
| Based on F2 bands | ||||||||
| R2 | 0.62 | 0.68 | 0.50 | 0.81 | 0.46 | 0.50 | 0.58 | 0.36 |
| RMSEC | 7.76 | 9.61 | 25.50 | 18.32 | 0.08 | 0.10 | 0.15 | 0.15 |
| Based on RY bands | ||||||||
| R2 | 0.69 | 0.69 | 0.50 | 0.82 | 0.49 | 0.52 | 0.59 | 0.36 |
| RMSEC | 7.02 | 9.44 | 25.44 | 18.05 | 0.08 | 0.10 | 0.15 | 0.15 |
| Based on WV2 bands | ||||||||
| R2 | 0.75 | 0.78 | 0.55 | 0.83 | 0.55 | 0.56 | 0.62 | 0.43 |
| RMSEC | 6.24 | 7.87 | 24.22 | 17.56 | 0.07 | 0.09 | 0.15 | 0.14 |
| AGB | PNU | |||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| PI | SE | HE | PI | SE | HE | |||||||||||||
| F2 | RY | WV2 | F2 | RY | WV2 | F2 | RY | WV2 | F2 | RY | WV2 | F2 | RY | WV2 | F2 | RY | WV2 | |
| Best performed VI-based models | ||||||||||||||||||
| R2 | 0.36 | 0.76 | 0.64 | 0.57 | 0.73 | 0.75 | 0.26 | 0.28 | 0.32 | 0.37 | 0.73 | 0.62 | 0.66 | 0.78 | 0.72 | 0.47 | 0.46 | 0.45 |
| RMSEP | 0.39 | 0.24 | 0.29 | 0.67 | 0.53 | 0.70 | 1.27 | 1.25 | 1.23 | 9.33 | 6.16 | 7.27 | 10.92 | 8.71 | 9.73 | 23.77 | 24.06 | 24.20 |
| REr (%) | 36.56 | 22.73 | 27.40 | 36.90 | 29.08 | 38.35 | 21.46 | 21.13 | 20.66 | 35.75 | 23.61 | 27.88 | 26.43 | 21.08 | 23.54 | 24.91 | 25.22 | 25.36 |
| SMLR-based models | ||||||||||||||||||
| R2 | 0.69 | 0.77 | 0.85 | 0.65 | 0.77 | 0.82 | 0.39 | 0.39 | 0.39 | 0.73 | 0.78 | 0.84 | 0.76 | 0.78 | 0.76 | 0.49 | 0.50 | 0.49 |
| RMSEP | 0.27 | 0.23 | 0.19 | 0.62 | 0.53 | 0.45 | 1.19 | 1.19 | 1.18 | 6.27 | 5.56 | 4.74 | 9.83 | 9.28 | 9.36 | 23.04 | 22.98 | 23.14 |
| REr (%) | 25.56 | 21.95 | 17.81 | 33.90 | 28.87 | 24.78 | 19.98 | 19.99 | 19.83 | 24.03 | 21.30 | 18.16 | 23.79 | 22.45 | 22.66 | 24.14 | 24.08 | 24.25 |
| PLSR-based models | ||||||||||||||||||
| R2 | 0.65 | 0.77 | 0.84 | 0.76 | 0.79 | 0.78 | 0.38 | 0.39 | 0.33 | 0.70 | 0.77 | 0.81 | 0.76 | 0.77 | 0.72 | 0.50 | 0.49 | 0.47 |
| RMSEP | 0.28 | 0.23 | 0.19 | 0.55 | 0.52 | 0.48 | 1.18 | 1.18 | 1.23 | 6.49 | 5.59 | 5.12 | 9.76 | 9.34 | 9.92 | 23.07 | 23.14 | 23.54 |
| REr (%) | 26.79 | 21.62 | 18.10 | 30.27 | 28.45 | 26.17 | 19.91 | 19.93 | 20.72 | 24.88 | 21.43 | 19.64 | 23.63 | 22.59 | 24.01 | 24.18 | 24.26 | 24.68 |
| NNI | PNC | |||||||||||||||||
| PI | SE | HE | PI | SE | HE | |||||||||||||
| F2 | RY | WV2 | F2 | RY | WV2 | F2 | RY | WV2 | F2 | RY | WV2 | F2 | RY | WV2 | F2 | RY | WV2 | |
| Best performed VI-based models | ||||||||||||||||||
| R2 | 0.37 | 0.45 | 0.41 | 0.28 | 0.32 | 0.27 | 0.43 | 0.41 | 0.38 | - | - | - | 0.13 | 0.02 | 0.24 | 0.26 | 0.24 | 0.20 |
| RMSEP | 0.08 | 0.07 | 0.08 | 0.11 | 0.10 | 0.11 | 0.17 | 0.18 | 0.18 | - | - | - | 0.33 | 0.34 | 0.31 | 0.25 | 0.26 | 0.27 |
| REr (%) | 8.41 | 7.79 | 8.14 | 10.31 | 10.06 | 10.94 | 16.28 | 16.81 | 17.29 | - | - | - | 13.77 | 14.44 | 13.1 | 15.67 | 16.17 | 16.67 |
| SMLR-based models | ||||||||||||||||||
| R2 | 0.55 | 0.52 | 0.44 | 0.28 | 0.25 | 0.30 | 0.46 | 0.48 | 0.46 | 0.12 | 0.11 | 0.09 | 0.25 | 0.21 | 0.37 | 0.30 | 0.36 | 0.30 |
| RMSEP | 0.07 | 0.07 | 0.07 | 0.11 | 0.11 | 0.11 | 0.17 | 0.16 | 0.17 | 0.19 | 0.20 | 0.20 | 0.30 | 0.31 | 0.29 | 0.24 | 0.23 | 0.24 |
| REr (%) | 7.18 | 7.28 | 7.86 | 10.34 | 11.05 | 10.44 | 15.81 | 15.48 | 15.89 | 7.92 | 7.94 | 7.97 | 12.58 | 12.98 | 12.36 | 15.19 | 14.48 | 15.17 |
| PLS-based models | ||||||||||||||||||
| R2 | 0.62 | 0.56 | 0.54 | 0.28 | 0.27 | 0.24 | 0.48 | 0.47 | 0.44 | 0.14 | 0.21 | 0.34 | 0.25 | 0.30 | 0.48 | 0.36 | 0.35 | 0.30 |
| RMSEP | 0.06 | 0.07 | 0.07 | 0.11 | 0.11 | 0.11 | 0.16 | 0.16 | 0.17 | 0.19 | 0.19 | 0.17 | 0.30 | 0.29 | 0.25 | 0.23 | 0.23 | 0.25 |
| REr (%) | 6.68 | 7.00 | 7.14 | 10.53 | 10.70 | 10.81 | 15.44 | 15.64 | 16.12 | 7.85 | 7.62 | 6.96 | 12.64 | 12.27 | 10.56 | 14.43 | 14.65 | 15.41 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Huang, S.; Miao, Y.; Yuan, F.; Gnyp, M.L.; Yao, Y.; Cao, Q.; Wang, H.; Lenz-Wiedemann, V.I.S.; Bareth, G. Potential of RapidEye and WorldView-2 Satellite Data for Improving Rice Nitrogen Status Monitoring at Different Growth Stages. Remote Sens. 2017, 9, 227. https://doi.org/10.3390/rs9030227
Huang S, Miao Y, Yuan F, Gnyp ML, Yao Y, Cao Q, Wang H, Lenz-Wiedemann VIS, Bareth G. Potential of RapidEye and WorldView-2 Satellite Data for Improving Rice Nitrogen Status Monitoring at Different Growth Stages. Remote Sensing. 2017; 9(3):227. https://doi.org/10.3390/rs9030227
Chicago/Turabian StyleHuang, Shanyu, Yuxin Miao, Fei Yuan, Martin L. Gnyp, Yinkun Yao, Qiang Cao, Hongye Wang, Victoria I. S. Lenz-Wiedemann, and Georg Bareth. 2017. "Potential of RapidEye and WorldView-2 Satellite Data for Improving Rice Nitrogen Status Monitoring at Different Growth Stages" Remote Sensing 9, no. 3: 227. https://doi.org/10.3390/rs9030227
APA StyleHuang, S., Miao, Y., Yuan, F., Gnyp, M. L., Yao, Y., Cao, Q., Wang, H., Lenz-Wiedemann, V. I. S., & Bareth, G. (2017). Potential of RapidEye and WorldView-2 Satellite Data for Improving Rice Nitrogen Status Monitoring at Different Growth Stages. Remote Sensing, 9(3), 227. https://doi.org/10.3390/rs9030227