Yirui Capsules Alleviate Atherosclerosis by Improving the Lipid Profile and Reducing Inflammation in Apolipoprotein E-Deficient Mice
Abstract
:1. Introduction
2. Materials and Methods
2.1. Animals
2.2. Blood Processing and Lipid Analysis
2.3. Atherosclerosis
2.4. Multiple Cytokine Measurements
2.5. Statistical Analyses
3. Results
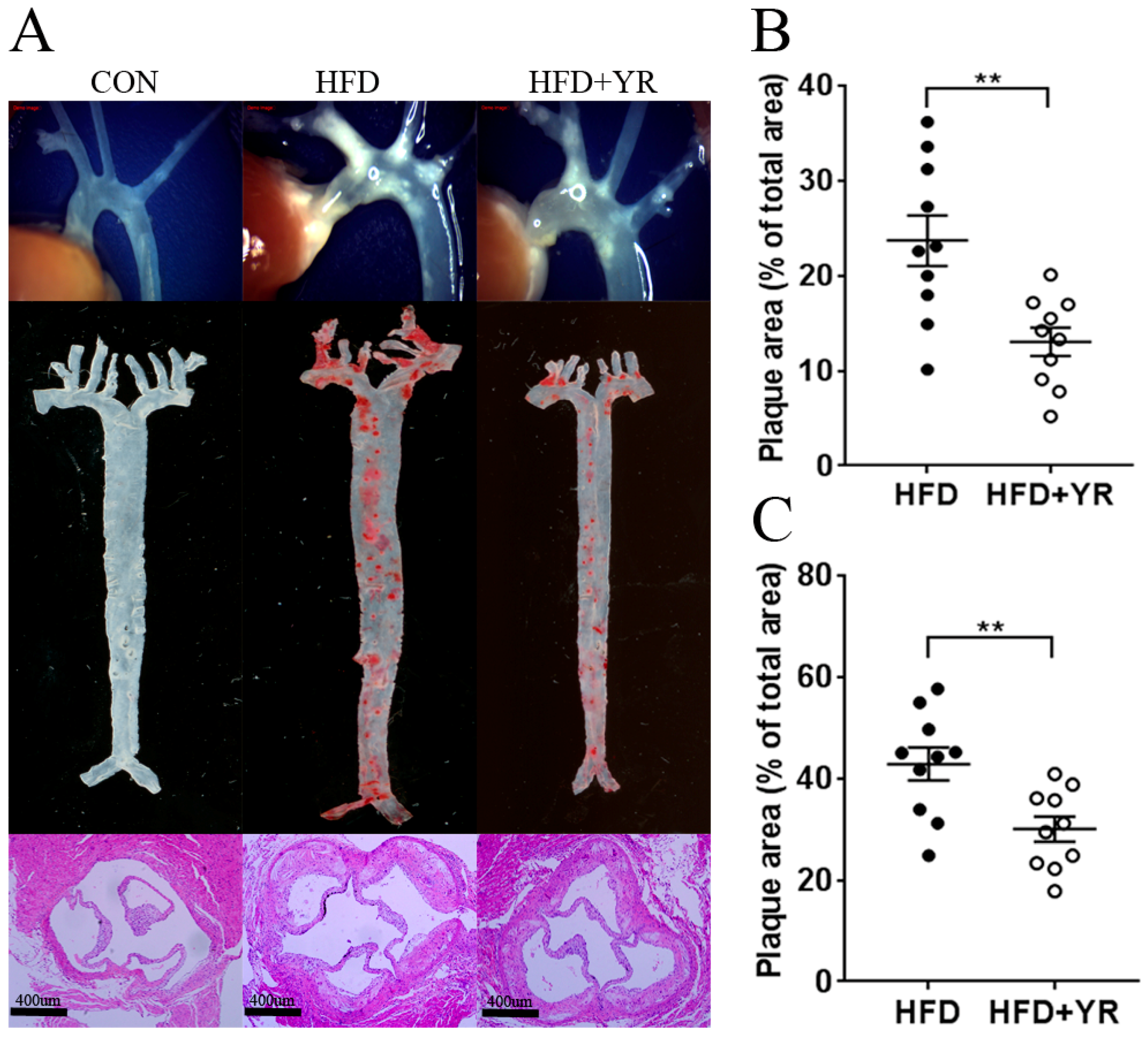
3.1. Effects of YR (Yirui) on Atherosclerotic Plaques
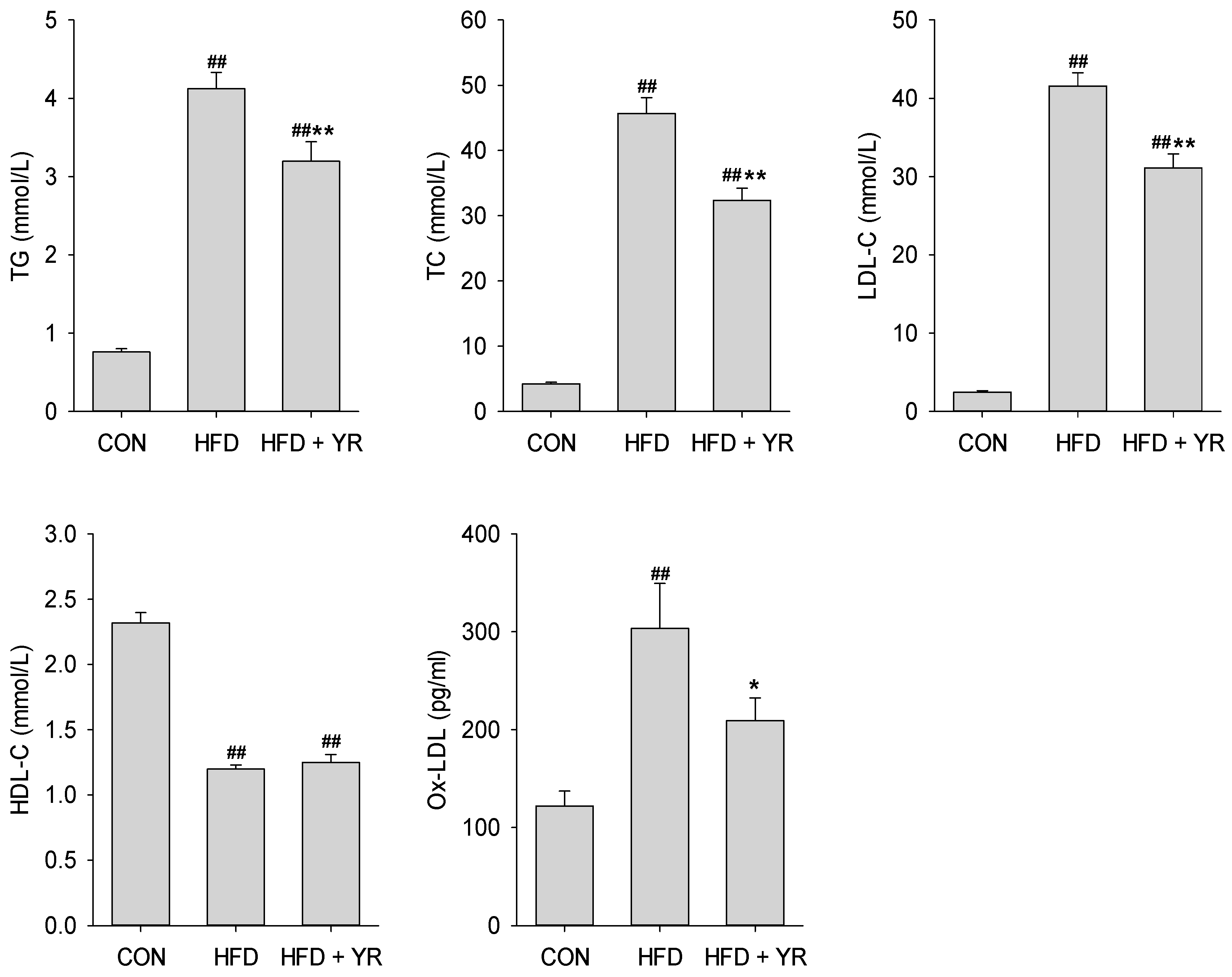
3.2. Effects of YR (Yirui) on the Serum Lipid Profile
3.3. Effects of YR (Yirui) on Cytokine and Chemokine Levels
4. Discussion
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Cardiovascular Diseases (CVDS). Available online: http://www.who.int/mediacentre/factsheets/fs317/en/ (accessed on 15 October 2017).
- Tonstad, S.; Despres, J.P. Treatment of lipid disorders in obesity. Expert Rev. Cardiovasc. Ther. 2011, 9, 1069–1080. [Google Scholar] [CrossRef] [PubMed]
- Tall, A.R.; Yvan-Charvet, L. Cholesterol, inflammation and innate immunity. Nat. Rev. Immunol. 2015, 15, 104–116. [Google Scholar] [CrossRef] [PubMed]
- Glass, C.K.; Witztum, J.L. Atherosclerosis. The road ahead. Cell 2001, 104, 503–516. [Google Scholar] [CrossRef]
- Gosmanova, E.O.; Le, N.A. Cardiovascular complications in CKD patients: Role of oxidative stress. Cardiol. Res. Pract. 2011, 2011, 156326. [Google Scholar] [CrossRef] [PubMed]
- Jones, N.L.; Reagan, J.W.; Willingham, M.C. The pathogenesis of foam cell formation: Modified LDL stimulates uptake of co-incubated LDL via macropinocytosis. Arterioscler. Thromb. Vasc. Biol. 2000, 20, 773–781. [Google Scholar] [CrossRef] [PubMed]
- Maiolino, G.; Rossitto, G.; Caielli, P.; Bisogni, V.; Rossi, G.P.; Calo, L.A. The role of oxidized low-density lipoproteins in atherosclerosis: The myths and the facts. Mediat. Inflamm. 2013, 2013, 714653. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Ren, Y.; Kang, L.; Zhang, L. Oxidized low-density lipoprotein increases the proliferation and migration of human coronary artery smooth muscle cells through the upregulation of osteopontin. Int. J. Mol. Med. 2014, 33, 1341–1347. [Google Scholar] [CrossRef] [PubMed]
- Steinberg, D.; Parthasarathy, S.; Carew, T.E.; Khoo, J.C.; Witztum, J.L. Beyond cholesterol. Modifications of low-density lipoprotein that increase its atherogenicity. N. Engl. J. Med. 1989, 320, 915–924. [Google Scholar] [PubMed]
- Trpkovic, A.; Resanovic, I.; Stanimirovic, J.; Radak, D.; Mousa, S.A.; Cenic-Milosevic, D.; Jevremovic, D.; Isenovic, E.R. Oxidized low-density lipoprotein as a biomarker of cardiovascular diseases. Crit. Rev. Clin. Lab. Sci. 2015, 52, 70–85. [Google Scholar] [CrossRef] [PubMed]
- Matsumoto, S.; Gotoh, N.; Hishinuma, S.; Abe, Y.; Shimizu, Y.; Katano, Y.; Ishihata, A. The role of hypertriglyceridemia in the development of atherosclerosis and endothelial dysfunction. Nutrients 2014, 6, 1236–1250. [Google Scholar] [CrossRef] [PubMed]
- Le, N.A.; Walter, M.F. The role of hypertriglyceridemia in atherosclerosis. Curr. Atheroscler. Rep. 2007, 9, 110–115. [Google Scholar] [CrossRef] [PubMed]
- Libby, P.; Ridker, P.M.; Maseri, A. Inflammation and atherosclerosis. Circulation 2002, 105, 1135–1143. [Google Scholar] [CrossRef] [PubMed]
- Gistera, A.; Hansson, G.K. The immunology of atherosclerosis. Nat. Rev. Nephrol. 2017, 13, 368–380. [Google Scholar] [CrossRef] [PubMed]
- Van Diepen, J.A.; Berbee, J.F.; Havekes, L.M.; Rensen, P.C. Interactions between inflammation and lipid metabolism: Relevance for efficacy of anti-inflammatory drugs in the treatment of atherosclerosis. Atherosclerosis 2013, 228, 306–315. [Google Scholar] [CrossRef] [PubMed]
- Cheng, L.; Pan, G.F.; Zhang, X.D.; Wang, J.L.; Wang, W.D.; Zhang, J.Y.; Wang, H.; Liang, R.X.; Sun, X.B. Yindanxinnaotong, a Chinese compound medicine, synergistically attenuates atherosclerosis progress. Sci. Rep. 2015, 5, 12333. [Google Scholar] [CrossRef] [PubMed]
- Zheng, J.; Liu, B.; Lun, Q.; Gu, X.; Pan, B.; Zhao, Y.; Xiao, W.; Li, J.; Tu, P. Longxuetongluo capsule inhibits atherosclerosis progression in high-fat diet-induced ApoE−/− mice by improving endothelial dysfunction. Atherosclerosis 2016, 255, 156–163. [Google Scholar] [CrossRef] [PubMed]
- China Food and Drug Administration. Available online: http://www.sda.gov.cn/WS01/CL1160/76528.html (accessed on 2 November 2017).
- Kuo, D.H.; Yeh, C.H.; Shieh, P.C.; Cheng, K.C.; Chen, F.A.; Cheng, J.T. Effect of Shanzha, a Chinese herbal product, on obesity and dyslipidemia in hamsters receiving high-fat diet. J. Ethnopharmacol. 2009, 124, 544–550. [Google Scholar] [CrossRef] [PubMed]
- Xia, W.; Sun, C.; Zhao, Y.; Wu, L. Hypolipidemic and antioxidant activities of Sanchi (radix notoginseng) in rats fed with a high fat diet. Phytomedicine 2011, 18, 516–520. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Jin, S.; Song, C.; Chen, C.; Zhang, Y.; Xiang, Y.; Xu, Y.; Feng, Y.; Wan, Q.; Jiang, H. The metabolic change of serum lysophosphatidylcholines involved in the lipid lowering effect of triterpenes from alismatis rhizoma on high-fat diet induced hyperlipidemia mice. J. Ethnopharmacol. 2016, 177, 10–18. [Google Scholar] [CrossRef] [PubMed]
- Lim, C.; Lim, S.; Lee, B.; Kim, B.; Cho, S. Effect of methanol extract of salviae miltiorrhizae radix in high-fat diet-induced hyperlipidemic mice. Chin. Med. 2017, 12, 29. [Google Scholar] [CrossRef] [PubMed]
- Wang, B.; Sun, J.; Shi, Y.; Le, G. Salvianolic acid B inhibits high-fat diet-induced inflammation by activating the Nrf2 pathway. J. Food Sci. 2017, 82, 1953–1960. [Google Scholar] [CrossRef] [PubMed]
- Tzang, B.S.; Yang, S.F.; Fu, S.G.; Yang, H.C.; Sun, H.L.; Chen, Y.C. Effects of dietary flaxseed oil on cholesterol metabolism of hamsters. Food Chem. 2009, 114, 1450–1455. [Google Scholar] [CrossRef]
- Liu, Z.; Xu, S.; Huang, X.; Wang, J.; Gao, S.; Li, H.; Zhou, C.; Ye, J.; Chen, S.; Jin, Z.G.; et al. Cryptotanshinone, an orally bioactive herbal compound from Danshen, attenuates atherosclerosis in apolipoprotein E-deficient mice: Role of lectin-like oxidized LDL receptor-1 (LOX-1). Br. J. Pharmacol. 2015, 172, 5661–5675. [Google Scholar] [CrossRef] [PubMed]
- Oh, J.U.; Jang, H.; Kim, S.J. Inhibition of inducible nitric oxide synthase and cyclooxygenase-2 by methanolic extract of crataegi fructus. Curr. Top. Nutraceutical Res. 2014, 12, 35–41. [Google Scholar]
- Han, C.W.; Kwun, M.J.; Kim, K.H.; Choi, J.Y.; Oh, S.R.; Ahn, K.S.; Lee, J.H.; Joo, M. Ethanol extract of Alismatis Rhizoma reduces acute lung inflammation by suppressing Nf-kappaB and activating Nrf2. J. Ethnopharmacol. 2013, 146, 402–410. [Google Scholar] [CrossRef] [PubMed]
- Zhao, G.; Etherton, T.D.; Martin, K.R.; Gillies, P.J.; West, S.G.; Kris-Etherton, P.M. Dietary alpha-linolenic acid inhibits proinflammatory cytokine production by peripheral blood mononuclear cells in hypercholesterolemic subjects. Am. J. Clin. Nutr. 2007, 85, 385–391. [Google Scholar] [PubMed]
- Haisong, Z.; Lang, Y.; Wenzhi, L.; Qichang, L.; Qing, W.; Yan, D.; Peixun, W. Effect of yirui capsules on serum inflammatory cytokines and hepatic alpha-7 nicotinic acetylcholine receptor expression in hyperlipidemia rats. J. Guangzhou Univ. Tradit. Chin. Med. 2015, 32, 1047–1051. [Google Scholar]
- Wang, Y.X.; Martin-McNulty, B.; Huw, L.Y.; da Cunha, V.; Post, J.; Hinchman, J.; Vergona, R.; Sullivan, M.E.; Dole, W.; Kauser, K. Anti-atherosclerotic effect of simvastatin depends on the presence of apolipoprotein e. Atherosclerosis 2002, 162, 23–31. [Google Scholar] [CrossRef]
- McKenney, J.M. Pharmacotherapy of dyslipidemia. Cardiovasc. Drugs Ther. 2001, 15, 413–422. [Google Scholar] [CrossRef] [PubMed]
- Castelli, W.P.; Garrison, R.J.; Wilson, P.W.; Abbott, R.D.; Kalousdian, S.; Kannel, W.B. Incidence of coronary heart disease and lipoprotein cholesterol levels. The framingham study. JAMA 1986, 256, 2835–2838. [Google Scholar] [CrossRef] [PubMed]
- Kannel, W.B.; Neaton, J.D.; Wentworth, D.; Thomas, H.E.; Stamler, J.; Hulley, S.B.; Kjelsberg, M.O. Overall and coronary heart disease mortality rates in relation to major risk factors in 325,348 men screened for the MRFIT. Multiple risk factor intervention trial. Am. Heart J. 1986, 112, 825–836. [Google Scholar] [CrossRef]
- Linton, M.F.; Fazio, S. Macrophages, inflammation, and atherosclerosis. Int. J. Obes. Relat. Metab. Disord. 2003, 27 (Suppl. 3), S35–S40. [Google Scholar] [CrossRef] [PubMed]
- Gordon, T.; Castelli, W.P.; Hjortland, M.C.; Kannel, W.B.; Dawber, T.R. High density lipoprotein as a protective factor against coronary heart disease. The framingham study. Am. J. Med. 1977, 62, 707–714. [Google Scholar] [CrossRef]
- Annema, W.; von Eckardstein, A. High-density lipoproteins. Multifunctional but vulnerable protections from atherosclerosis. Circ. J. 2013, 77, 2432–2448. [Google Scholar] [CrossRef] [PubMed]
- Hu, J.; Xi, D.; Zhao, J.; Luo, T.; Liu, J.; Lu, H.; Li, M.; Xiong, H.; Guo, Z. High-density lipoprotein and inflammation and its significance to atherosclerosis. Am. J. Med. Sci. 2016, 352, 408–415. [Google Scholar] [CrossRef] [PubMed]
- Liu, G.; Wang, B.; Zhang, J.; Jiang, H.; Liu, F. Total panax notoginsenosides prevent atherosclerosis in apolipoprotein E-knockout mice: Role of downregulation of CD40 and MMP-9 expression. J. Ethnopharmacol. 2009, 126, 350–354. [Google Scholar] [CrossRef] [PubMed]
- Lemieux, I.; Lamarche, B.; Couillard, C.; Pascot, A.; Cantin, B.; Bergeron, J.; Dagenais, G.R.; Despres, J.P. Total cholesterol/HDL cholesterol ratio vs LDL cholesterol/HDL cholesterol ratio as indices of ischemic heart disease risk in men: The Quebec Cardiovascular Study. Arch. Intern. Med. 2001, 161, 2685–2692. [Google Scholar] [CrossRef] [PubMed]
- Hansson, G.K. Inflammation, atherosclerosis, and coronary artery disease. N. Engl. J. Med. 2005, 352, 1685–1695. [Google Scholar] [CrossRef] [PubMed]
- Tousoulis, D.; Oikonomou, E.; Economou, E.K.; Crea, F.; Kaski, J.C. Inflammatory cytokines in atherosclerosis: Current therapeutic approaches. Eur. Heart J. 2016, 37, 1723–1732. [Google Scholar] [CrossRef] [PubMed]
- Kleemann, R.; Zadelaar, S.; Kooistra, T. Cytokines and atherosclerosis: A comprehensive review of studies in mice. Cardiovasc. Res. 2008, 79, 360–376. [Google Scholar] [CrossRef] [PubMed]
- Bertrand, M.J.; Tardif, J.C. Inflammation and beyond: New directions and emerging drugs for treating atherosclerosis. Expert Opin. Emerg. Drugs 2017, 22, 1–26. [Google Scholar] [CrossRef] [PubMed]
- Naura, A.S.; Hans, C.P.; Zerfaoui, M.; Errami, Y.; Ju, J.; Kim, H.; Matrougui, K.; Kim, J.G.; Boulares, A.H. High-fat diet induces lung remodeling in ApoE-deficient mice: An association with an increase in circulatory and lung inflammatory factors. Lab. Investig. 2009, 89, 1243–1251. [Google Scholar] [CrossRef] [PubMed]

© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xu, J.; Xia, Z.; Rong, S.; Gao, H.; Yang, W.; Li, J.; Ma, C.; Deng, Q.; Huang, Q.; Xiao, L.; et al. Yirui Capsules Alleviate Atherosclerosis by Improving the Lipid Profile and Reducing Inflammation in Apolipoprotein E-Deficient Mice. Nutrients 2018, 10, 142. https://doi.org/10.3390/nu10020142
Xu J, Xia Z, Rong S, Gao H, Yang W, Li J, Ma C, Deng Q, Huang Q, Xiao L, et al. Yirui Capsules Alleviate Atherosclerosis by Improving the Lipid Profile and Reducing Inflammation in Apolipoprotein E-Deficient Mice. Nutrients. 2018; 10(2):142. https://doi.org/10.3390/nu10020142
Chicago/Turabian StyleXu, Jiqu, Zumeng Xia, Shuang Rong, Hui Gao, Wei Yang, Jieliang Li, Congcong Ma, Qianchun Deng, Qingde Huang, Lingyun Xiao, and et al. 2018. "Yirui Capsules Alleviate Atherosclerosis by Improving the Lipid Profile and Reducing Inflammation in Apolipoprotein E-Deficient Mice" Nutrients 10, no. 2: 142. https://doi.org/10.3390/nu10020142




