The Reproducibility and Comparative Validity of a Non-Nutritive Sweetener Food Frequency Questionnaire
Abstract
:1. Introduction
2. Materials and Methods
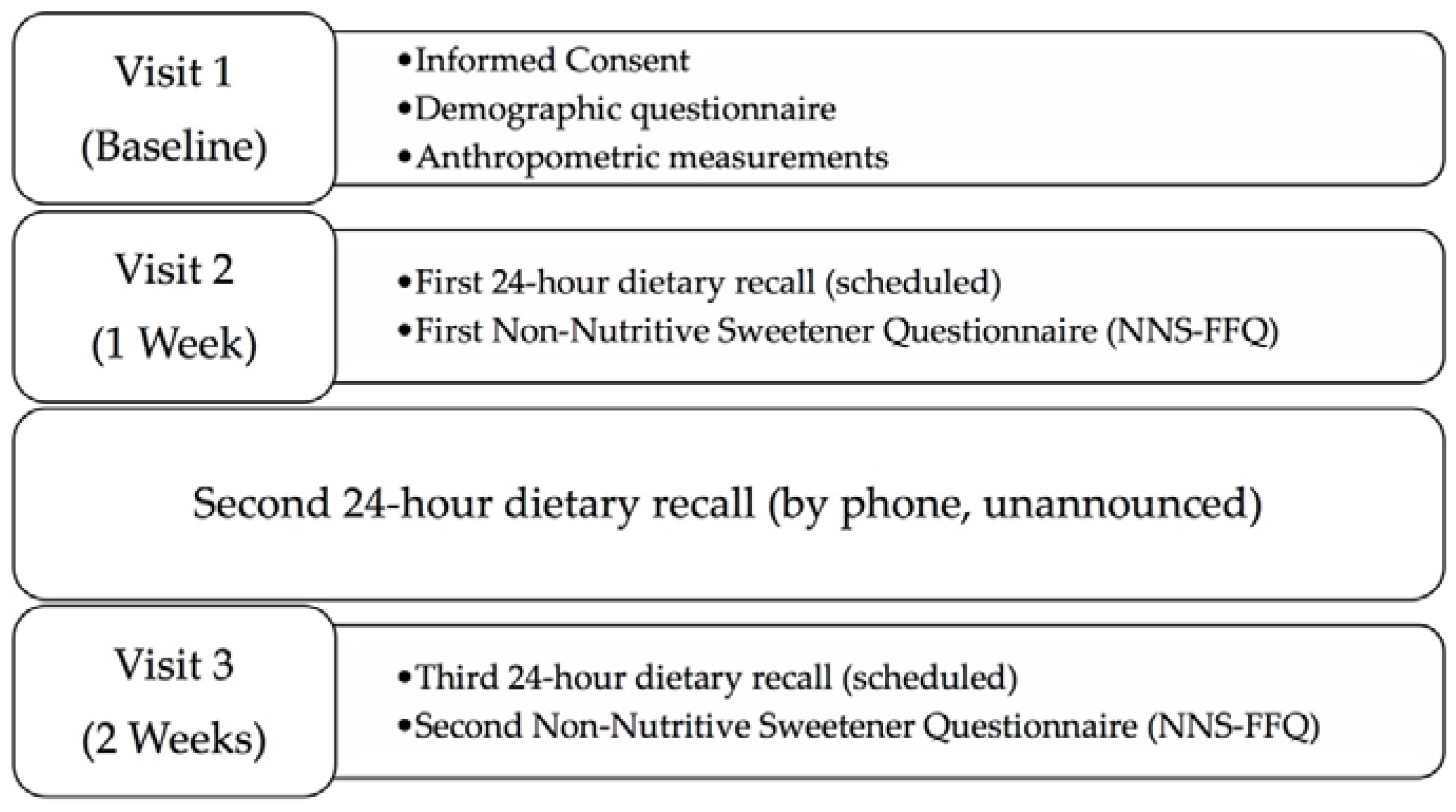
2.1. Subjects and Design
2.2. Development of the Non-Nutritive Sweetener Food Frequency Questionnaire (NNS-FFQ)
2.3. Dietary Recalls
2.4. Consumer versus Non-Consumer Analysis
2.5. Statistical Analysis
3. Results
3.1. Demographic Characteristics
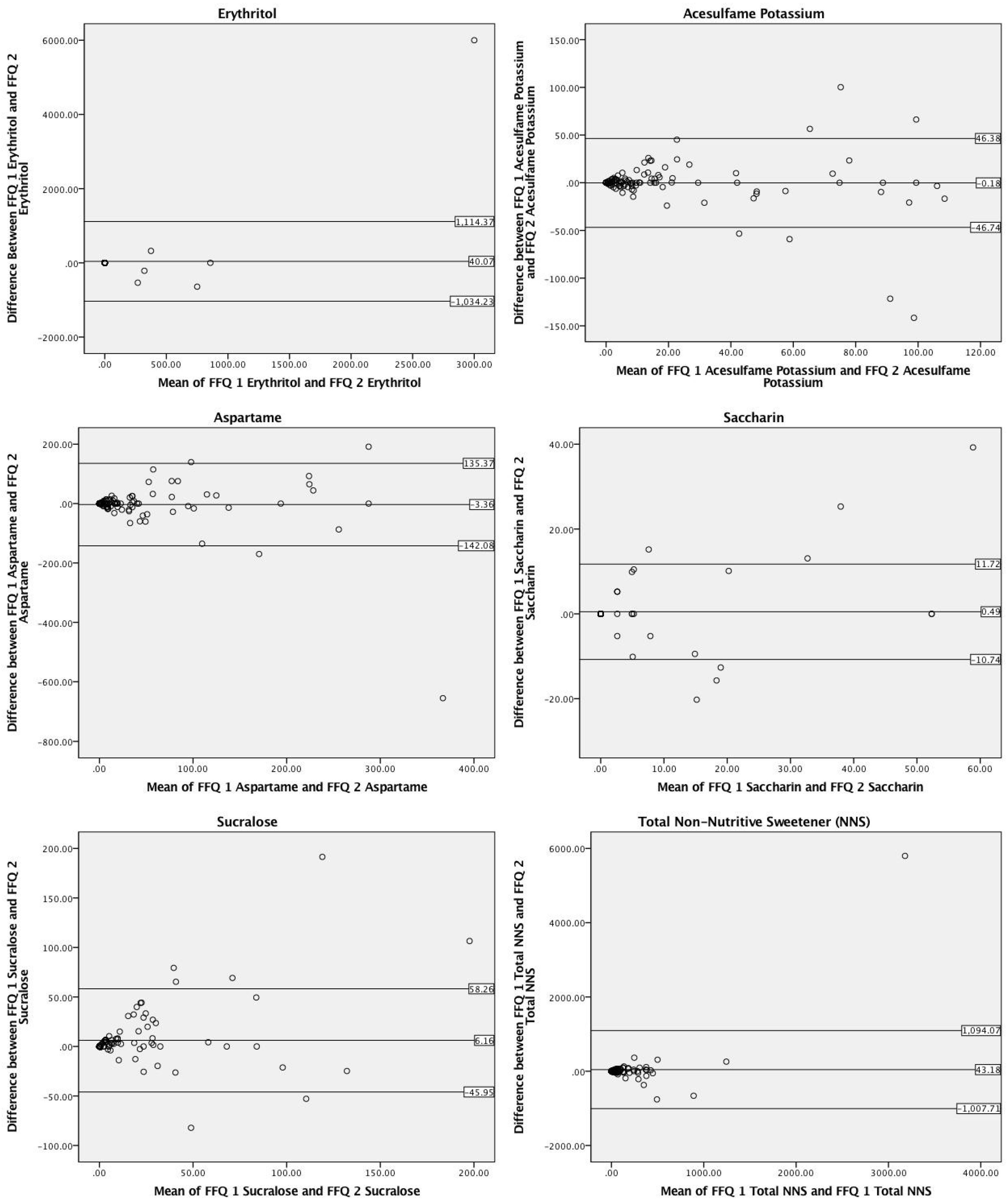
3.2. Test–Retest Reproducibility
3.3. Comparative Validity
4. Discussion
5. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Raben, A.; Richelsen, B. Artificial sweeteners: A place in the field of functional foods? Focus on obesity and related metabolic disorders. Curr. Opin. Clin. Nutr. 2012, 15, 597–604. [Google Scholar] [CrossRef] [PubMed]
- Roberts, M.W.; Wright, J.T. Nonnutritive, low caloric substitutes for food sugars: Clinical implications for addressing the incidence of dental caries and overweight/obesity. Int. J. Dent. 2012, 2012, 625701. [Google Scholar] [CrossRef] [PubMed]
- Gardner, C.; Wylie-Rosett, J.; Gidding, S.S.; Johnson, R.K.; Reader, D.; Lichtenstein, A.H. Nonnutritive sweeteners: Current use and health perspectives: A scientific statement from the American Heart Association and the American Diabetes Association. Circulation 2012, 126, 509–519. [Google Scholar] [CrossRef] [PubMed]
- Tate, D.F.; Turner-McGrievy, G.; Lyons, E.; Stevens, J.; Erickson, K.; Polzien, K.; Diamond, M.; Wang, X.; Popkin, B. Replacing caloric beverages with water or diet beverages for weight loss in adults: Main results of the Choose Healthy Options Consciously Everyday (CHOICE) randomized clinical trial. Am. J. Clin. Nutr. 2012, 95, 555–563. [Google Scholar] [CrossRef] [PubMed]
- Tandel, K.R. Sugar substitutes: Health controversy over perceived benefits. J. Pharmacol. Pharmacother. 2011, 2, 236–243. [Google Scholar] [CrossRef] [PubMed]
- Suez, J.; Korem, T.; Zilberman-Schapira, G.; Segal, E.; Elinav, E. Non-caloric artificial sweeteners and the microbiome: Findings and challenges. Gut Microbes 2015, 6, 149–155. [Google Scholar] [CrossRef] [PubMed]
- Swithers, S.E.; Davidson, T.L. A role for sweet taste: Calorie predictive relations in energy regulation by rats. Behav. Neurosci. 2008, 122, 161–173. [Google Scholar] [CrossRef] [PubMed]
- Swithers, S.E. Artificial sweeteners produce the counterintuitive effect of inducing metabolic derangements. Trends Endocrinol. Metab. 2013, 24, 431–441. [Google Scholar] [CrossRef] [PubMed]
- Mattes, R.D.; Popkin, B.M. Nonnutritive sweetener consumption in humans: Effects on appetite and food intake and their putative mechanisms. Am. J. Clin. Nutr. 2009, 89, 1–14. [Google Scholar] [CrossRef] [PubMed]
- U.S. Food and Drug Administration. High-Intensity Sweeteners. Available online: http://www.fda.gov/food/ingredientspackaginglabeling/foodadditivesingredients/ucm397716.htm (accessed on 8 November 2017).
- U.S. Food and Drug Administration. Generally Recognized As Safe (GRAS). Available online: http://www.fda.gov/Food/IngredientsPackagingLabeling/GRAS/default.htm (accessed on 8 November 2017).
- Mortensen, A. Sweeteners permitted in the European Union: Safety aspects. Scand. J. Food Nutr. 2006, 50, 104–116. [Google Scholar] [CrossRef] [Green Version]
- European Food Safety Authority. Sweeteners. Available online: https://www.efsa.europa.eu/en/topics/topic/sweeteners (accessed on 2 February 2018).
- Hedrick, V.E.; Passaro, E.M.; Davy, B.M.; You, W.; Zoellner, J.M. Characterization of non-nutritive sweetener intake in rural southwest Virginian adults living in a health-disparate region. Nutrients 2017, 9, 757. [Google Scholar] [CrossRef] [PubMed]
- Willett, W. Nutritional Epidemiology, 3rd ed.; Oxford University Press: New York, NY, USA, 2013. [Google Scholar]
- Thompson, F.E.; Subar, A.F. Dietary Assessment Methodology. In Nutrition in the Prevention and Treatment of Disease, 2nd ed.; Elsevier Inc.: Oxford, UK, 2008; pp. 3–22. [Google Scholar]
- Monsen, E.R.; Van Horn, L. Research: Successful Approaches; American Dietetic Association: Chicago, IL, USA, 1992. [Google Scholar]
- National Institute of Cancer Division of Cancer Control and Population Sciences. Register of Validated Short Dietary Assessment Instruments. Available online: https://epi.grants.cancer.gov/diet/shortreg/register.php (accessed on 8 November 2017).
- Federation of American Societies for Experimental Biology. The Evaluation of the Energy of Certain Sugar Alcohols Used as Food Ingredients; Life Sciences Research Office: Bethesda, MD, USA, 1994. [Google Scholar]
- Altman, D.G.; Bland, J.M. Measurement in medicine: The analysis of method comparison studies. J. R. Stat. Soc. Ser. D Stat. 1983, 32, 307–317. [Google Scholar] [CrossRef]
- Giavarina, D. Understanding Bland Altman analysis. Biochem. Med. 2015, 25, 141–151. [Google Scholar] [CrossRef] [PubMed]
- Myles, P.S.; Cui, J.I. Using the Bland-Altman method to measure agreement with repeated measures. Br. J. Anaesth. 2007, 99, 309–311. [Google Scholar] [CrossRef] [PubMed]
- Landis, J.R.; Koch, G.G. The measurement of observer agreement for categorical data. Biometrics 1977, 33, 159–174. [Google Scholar] [CrossRef] [PubMed]
- Block, G.; Hartman, A.M.; Dresser, C.M.; Carroll, M.D.; Gannon, J.; Gardner, L. A data-based approach to diet questionnaire design and testing. Am. J. Epidemiol. 1986, 124, 453–469. [Google Scholar] [CrossRef] [PubMed]
- Peters, J.C.; Beck, J.; Cardel, M.; Wyatt, H.R.; Foster, G.D.; Pan, Z.; Wojtanowski, A.C.; Vander Veur, S.S.; Herring, S.J.; Brill, C.; et al. The effects of water and non-nutritive sweetened beverages on weight loss and weight maintenance: A randomized clinical trial. Obesity 2016, 24, 297–304. [Google Scholar] [CrossRef] [PubMed]
- Peters, J.C.; Wyatt, H.R.; Foster, G.D.; Pan, Z.; Wojtanowski, A.C.; Vander Veur, S.S.; Herring, S.J.; Brill, C.; Hill, J.O. The effects of water and non-nutritive sweetened beverages on weight loss during a 12-week weight loss treatment program. Obesity 2014, 22, 1415–1421. [Google Scholar] [CrossRef] [PubMed]
- Chia, C.W.; Shardell, M.; Tanaka, T.; Liu, D.D.; Gravenstein, K.S.; Simonsick, E.M.; Egan, J.M.; Ferrucci, L. Chronic low-calorie sweetener use and risk of abdominal obesity among older adults: A cohort study. PLoS ONE 2016, 11, e0167241. [Google Scholar] [CrossRef] [PubMed]
- Fowler, S.P.; Williams, K.; Hazuda, H.P. Diet soda intake is associated with long-term increases in waist circumference in a biethnic cohort of older adults: The San Antonio Longitudinal Study of Aging. J. Am. Geriatr. Soc. 2015, 63, 708–715. [Google Scholar] [CrossRef] [PubMed]
- Fowler, S.P.; Williams, K.; Resendez, R.G.; Hunt, K.J.; Hazuda, H.P.; Stern, M.P. Fueling the obesity epidemic? Artificially sweetened beverage use and long-term weight gain. Obesity 2008, 16, 1894–1900. [Google Scholar] [CrossRef] [PubMed]
- Magnuson, B.A.; Burdock, G.A.; Doull, J.; Kroes, R.M.; Marsh, G.M.; Pariza, M.W.; Spencer, P.S.; Waddell, W.J.; Walker, R.; Williams, G.M. Aspartame: A safety evaluation based on current use levels, regulations, and toxicological and epidemiological studies. Crit. Rev. Toxicol. 2007, 37, 629–727. [Google Scholar] [CrossRef] [PubMed]
- Renwick, A. The metabolism of intense sweeteners. Xenobiotica 1986, 16, 1057–1071. [Google Scholar] [CrossRef] [PubMed]
- Grotz, V.L.; Munro, I.C. An overview of the safety of sucralose. Regul. Toxicol. Pharmacol. 2009, 55, 1–5. [Google Scholar] [CrossRef] [PubMed]
- Ma, Y.; Olendzki, B.C.; Pagoto, S.L.; Hurley, T.G.; Magner, R.P.; Ockene, I.S.; Schneider, K.L.; Merriam, P.A.; Hébert, J.R. Number of 24-hour diet recalls needed to estimate energy intake. Ann. Epidemiol. 2009, 19, 553–559. [Google Scholar] [CrossRef] [PubMed]
- Eck, L.H.; Klesges, L.M.; Klesges, R.C. Precision and estimated accuracy of two short-term food frequency questionnaires compared with recalls and records. J. Clin. Epidemiol. 1996, 49, 1195–1200. [Google Scholar] [CrossRef]
- Eck, L.H.; Klesges, R.C.; Hanson, C.L.; Slawson, D.; Portis, L.; Lavasque, M.E. Measuring short-term dietary intake: Development and testing of a 1-week food frequency questionnaire. J. Am. Diet. Assoc. 1991, 91, 940–945. [Google Scholar] [PubMed]
- Pérez Rodrigo, C.; Aranceta, J.; Salvador, G.; Varela-Moreiras, G. Food frequency questionnaires. Nutr. Hosp. 2015, 31 (Suppl. S3), 49–56. [Google Scholar] [PubMed]
- Block, G. Human Dietary Assessment: Methods and Issues. Prev. Med. 1989, 18, 653–660. [Google Scholar] [CrossRef]
- Walton, J. Dietary assessment methodology for nutritional assessment: A practical approach. Top. Clin. Nutr. 2015, 30, 33–46. [Google Scholar] [CrossRef]
- Gersovitz, M.; Madden, J.P.; Smiciklas-Wright, H. Validity of the 24-hr. dietary recall and seven-day record for group comparisons. J. Acad. Nutr. Diet. 1978, 73, 48–55. [Google Scholar]
- Thompson, F.E.; Byers, T. Dietary assessment resource manual. J. Nutr. 1994, 124 (Suppl. S11), 2245s–2317s. [Google Scholar] [PubMed]

| Characteristics | Total Sample (n = 123), n (%) |
|---|---|
| Sex | |
| Male | 54 (44) |
| Female | 69 (56) |
| Mean age ± SD (years) | 36.8 ± 16.6 |
| Race/Ethnicity | |
| Caucasian | 93 (75) |
| Asian/Pacific Islander | 17 (14) |
| African American | 7 (6) |
| Hispanic | 3 (2.5) |
| More than 1 race | 3 (2.5) |
| BMI (kg/m2) | |
| Mean BMI ± SD | 26.0 ± 5.7 |
| Underweight (≤18.4) | 1 (1) |
| Normal weight (18.5–24.9) | 68 (55) |
| Overweight (25–29.9) | 32 (26) |
| Obese (≥30) | 22 (18) |
| Education Level | |
| High-School Graduate | 6 (5) |
| Some College | 20 (16) |
| College Graduate | 45 (37) |
| Graduate School | 52 (42) |
| Household Income ($) | |
| ≤14,999 | 19 (15) |
| 15,000–29,999 | 29 (23.5) |
| 30,000–49,999 | 12 (10) |
| 50,000–99,999 | 29 (23.5) |
| ≥100,000 | 22 (18) |
| No response | 12 (10) |
| NNS Type | Number of Participants Reporting any Consumption via Dietary Recall n (%) | Number of Participants Reporting any Consumption via FFQ 1 n (%) | Number of Participants Reporting Any Consumption via FFQ 2 n (%) | Cohen’s κ |
|---|---|---|---|---|
| Acesulfame Potassium | 58 (47) | 93 (76) | 91 (74) | 0.681 *** |
| Aspartame | 68 (55) | 83 (68) | 84 (68) | 0.417 *** |
| Saccharin | 7 (6) | 21 (17) | 17 (14) | 0.601 *** |
| Sucralose | 27 (22) | 68 (55) | 53 (43) | 0.517 *** |
| Erythritol a | 2 (2) | 5 (4) | 5 (4) | n/a b |
| NNS Type | NNS-FFQ Time 1 Mean ± SD a (Median, Range) | NNS-FFQ Time 2 Mean ± SD a (Median, Range) | Spearman’s Correlation (rs) | Mean Difference (Mean ± SE) b |
|---|---|---|---|---|
| Acesulfame Potassium (mg) | 18.6 ± 28.9 (5.0, 0.0–132.5) | 18.8 ± 32.6 (5.0, 0.0–169.5) | 0.81 ** | 0.2 ± 2.1 |
| Aspartame (mg) | 35.3 ± 67.8 (7.0, 0.0–383.1) | 38.7 ± 85.1 (7.1, 0.0–694.5) | 0.81 ** | 3.4 ± 6.4 |
| Saccharin (mg) | 3.3 ± 11.5 (0.0, 0.0–78.5) | 2.9 ± 9.2 (0.0, 0.0–52.3) | 0.77 ** | 0.5 ± 0.5 |
| Sucralose (mg) | 18.2 ± 37.6 (2.7, 0.0–250.9) | 12.1 ± 28.0 (0.0, 0.0–144.6) | 0.81 ** | 6.2 ± 2.4 * |
| Erythritol (mg) c | 65.3 ± 548.7 (0.0, 0.0–6000.0) | 25.3 ± 137.9 (0.0, 0.0–1071.4) | 0.78 ** | 40.1 ± 49.4 |
| Total NNS (mg) | 140.8 ± 566.8 (24.0, 0.0–6079.1) | 97.6 ± 194.4 (24.6, 0.0–1221.0) | 0.92 ** | 43.8 ± 48.4 |
| Non-Nutritive Sweetener Type | NNS-FFQ 2 Mean ± SD a (Median, Range) | Dietary Recall Mean ± SD a (Median, Range) | Spearman’s Correlation (rs) | Mean Difference (Mean ± SE) b |
|---|---|---|---|---|
| Acesulfame Potassium (mg) | 18.8 ± 32.6 (6.0, 0.0–169.5) | 6.8 ± 16.4 (0.0, 0.0–99.3) | 0.51 ** | 12.0 ± 2.4 *** |
| Aspartame (mg) | 38.7 ± 85.1 (7.1, 0.0–694.5) | 36.5 ± 97.2 (1.2, 0.0–526.4) | 0.59 ** | 2.2 ± 6.7 |
| Saccharin (mg) | 2.9 ± 9.2 (0.0, 0.0–52.3) | 3.9 ± 19.6 (0.0, 0.0–170.9) | 0.55 ** | 1.0 ± 1.5 |
| Sucralose (mg) | 12.1 ± 28.0 (0.0, 0.0–144.6) | 8.0 ± 19.7 (0.0, 0.0–103.7) | 0.53 ** | 4.0 ± 2.1 |
| Erythritol (mg) c | 25.3 ± 137.9 (0.0, 0.0–1071.4) | 16.3 ± 134.0 (0.0, 0.0–1333.3) | −0.03 | 9.0 ± 17.5 |
| Total NNS (mg) | 97.6 ± 194.4 (25.9, 0.0–1221.0) | 72.4 ± 183.6 (8.0, 0.0–1335.7) | 0.55 ** | 25.3 ± 18.0 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Myers, E.A.; Passaro, E.M.; Hedrick, V.E. The Reproducibility and Comparative Validity of a Non-Nutritive Sweetener Food Frequency Questionnaire. Nutrients 2018, 10, 334. https://doi.org/10.3390/nu10030334
Myers EA, Passaro EM, Hedrick VE. The Reproducibility and Comparative Validity of a Non-Nutritive Sweetener Food Frequency Questionnaire. Nutrients. 2018; 10(3):334. https://doi.org/10.3390/nu10030334
Chicago/Turabian StyleMyers, Emily A., Erin M. Passaro, and Valisa E. Hedrick. 2018. "The Reproducibility and Comparative Validity of a Non-Nutritive Sweetener Food Frequency Questionnaire" Nutrients 10, no. 3: 334. https://doi.org/10.3390/nu10030334





