Absorption of Vitamin A and Carotenoids by the Enterocyte: Focus on Transport Proteins
Abstract
:1. Introduction
2. Overview of Vitamin A and Carotenoid Fate during the Digestion Process
| Transport proteins in the enterocyte | ||
|---|---|---|
 | Liver: 10,800–23,500
Fatty fish: 800−1000 Butter: 700 | CRBPII, possibly ABCA1 |
 | ||
 | Raw carrot: 8840
Canned carrot: 5780 Cooked spinach: 5240 | SR-BI, CD36 |
 | Cooked carrot: 468 | |
 | Orange juice: 880
Mandarin juice: 920 | |
 | Cooked spinach: 7040
Lettuce: 2640 | SR-BI,
NPC1L1, possibly ABCG5 |
 | Tomato sauce: 15,920
Tomatoes: 3030 Watermelon: 4870 | SR-BI |
3. Absorption of Vitamin A and Carotenoids by the Enterocyte (Figure 1)
3.1. Apical Uptake and Efflux
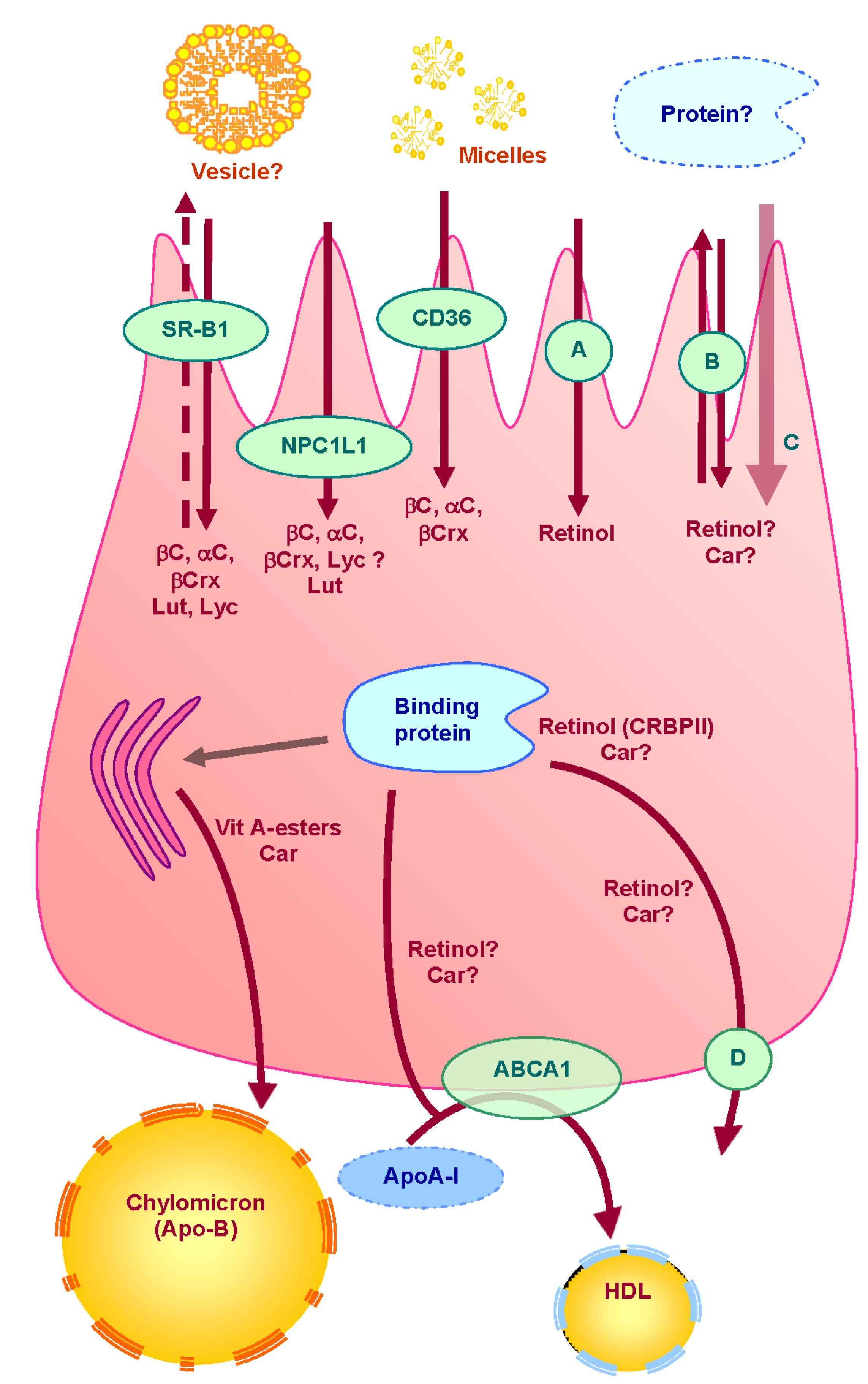
3.2. Intracellular Metabolism
3.3. Cytosolic Transport
3.4. Basolateral Secretion
4. Consequences of the Involvement of Vitamin A and Carotenoid Intestinal Transporters
5. Conclusions
List of abbreviations
| ARAT | Acyl-CoA Acyl Transferase |
| ABCA1 | ATP Binding Cassette A1 |
| ABCG5 | ATP Binding Cassette G5 |
| ABCG8 | ATP Binding Cassette G8 |
| BBM | Brush Border Membrane |
| BCMO1 | β-carotene-15,15′-monooxygenase |
| BCDO2 | β-carotene-9′,10′-dioxygenase |
| CBP | Carotenoid-Binding protein |
| CD36 | Cluster Determinant 36 |
| CRBPII | Cellular Retinol Binding Protein II |
| HR-LBP | Human Retinal Lutein-Binding Protein |
| ISX | Intestine-Specific Homebox |
| L-FABP | Liver Fatty-Acid-Binding Protein |
| LRA | Lecithin Retinol Acyl Transferase |
| NPC1L1 | Niemann-Pick C1-Like 1 |
| RBP | Retinol Binding Protein |
| RBPR2 | RBP-receptor 2 |
| SR-BI | Scavenger Receptor class B type 1 |
| STRA6 | STimulated by Retinoic Acid 6 |
Conflicts of Interest
References
- Gerster, H. Vitamin A—Functions, dietary requirements and safety in humans. Int. J. Vitam. Nutr. Res. 1997, 67, 71–90. [Google Scholar]
- Hollander, D.; Muralidhara, K.S. Vitamin A1 intestinal absorption in vivo: Influence of luminal factors on transport. Am. J. Physiol. Gastrointest. Liver Physiol. 1977, 232, E471–E477. [Google Scholar]
- Hollander, D.; Ruble, P.E. Beta-carotene intestinal absorption: Bile, fatty acid, pH, and flow rate effects on transport. Am. J. Physiol. 1978, 235, E686–E691. [Google Scholar]
- Hollander, D. Intestinal absorption of vitamin A, E, D, and K. J. Lab. Clin. Med. 1981, 97, 449–462. [Google Scholar]
- Borel, P. Factors affecting intestinal absorption of highly lipophilic food microconstituents (fat-soluble vitamins, carotenoids and phytosterols). Clin. Chem. Lab. Med. 2003, 41, 979–994. [Google Scholar] [CrossRef]
- Martin, A. Apports Nutritionnels Conseillés Pour La Population Française, 3rd ed.; Tec & Doc Lavoisier: Paris, France, 2001; p. 605. [Google Scholar]
- Reboul, E.; Richelle, M.; Perrot, E.; Desmoulins-Malezet, C.; Pirisi, V.; Borel, P. Bioaccessibility of carotenoids and vitamin E from their main dietary sources. J. Agric. Food Chem. 2006, 54, 8749–8755. [Google Scholar] [CrossRef]
- Dhuique-Mayer, C.; Borel, P.; Reboul, E.; Caporiccio, B.; Besancon, P.; Amiot, M.J. Beta-cryptoxanthin from citrus juices: Assessment of bioaccessibility using an in vitro digestion/Caco-2 cell culture model. Br. J. Nutr. 2007, 97, 883–890. [Google Scholar] [CrossRef]
- Hedren, E.; Diaz, V.; Svanberg, U. Estimation of carotenoid accessibility from carrots determined by an in vitro digestion method. Eur. J. Clin. Nutr. 2002, 56, 425–430. [Google Scholar] [CrossRef]
- Borel, P.; Grolier, P.; Armand, M.; Partier, A.; Lafont, H.; Lairon, D.; Azais-Braesco, V. Carotenoids in biological emulsions: Solubility, surface-to-core distribution, and release from lipid droplets. J. Lipid Res. 1996, 37, 250–261. [Google Scholar]
- Tyssandier, V.; Reboul, E.; Dumas, J.F.; Bouteloup-Demange, C.; Armand, M.; Marcand, J.; Sallas, M.; Borel, P. Processing of vegetable-borne carotenoids in the human stomach and duodenum. Am. J. Physiol. Gastrointest. Liver Physiol. 2003, 284, G913–G923. [Google Scholar]
- Borel, P.; Pasquier, B.; Armand, M.; Tyssandier, V.; Grolier, P.; Alexandre-Gouabau, M.C.; Andre, M.; Senft, M.; Peyrot, J.; Jaussan, V.; et al. Processing of vitamin A and E in the human gastrointestinal tract. Am. J. Physiol. Gastrointest. Liver Physiol. 2001, 280, G95–G103. [Google Scholar]
- Carrière, F.; Barrowman, J.A.; Verger, R.; Laugier, R. Secretion and contribution to lipolysis of gastric and pancreatic lipases during a test meal in humans. Gastroenterology 1993, 105, 876–888. [Google Scholar]
- Lombardo, D.; Guy, O. Studies on the substrate specificity of a carboxyl ester hydrolase from human pancreatic juice. II. Action on cholesterol esters and lipid-soluble vitamin esters. Biochim. Biophys. Acta 1980, 611, 147–155. [Google Scholar] [CrossRef]
- Zahalka, H.A.; Shee; Cheng, C.; Burton, G.W.; Ingold, K.U. Hydrolysis of stereoisomeric alpha-tocopheryl acetates catalyzed by bovine cholesterol esterase. Biochim. Biophys. Acta 1987, 921, 481–485. [Google Scholar] [CrossRef]
- Lauridsen, C.; Hedemann, M.S.; Jensen, S.K. Hydrolysis of tocopheryl and retinyl esters by porcine carboxyl ester hydrolase is affected by their carboxylate moiety and bile acids. J. Nutr. Biochem. 2001, 12, 219–224. [Google Scholar] [CrossRef]
- Breithaupt, D.E.; Bamedi, A.; Wirt, U. Carotenol fatty acid esters: Easy substrates for digestive enzymes? Comp. Biochem. Physiol. B Biochem. Mol. Biol. 2002, 132, 721–728. [Google Scholar] [CrossRef]
- Van Bennekum, A.M.; Li, L.; Piantedosi, R.; Shamir, R.; Vogel, S.; Fisher, E.A.; Blaner, W.S.; Harrison, E.H. Carboxyl ester lipase overexpression in rat hepatoma cells and CEL deficiency in mice have no impact on hepatic uptake or metabolism of chylomicron-retinyl ester. Biochemistry 1999, 38, 4150–4156. [Google Scholar] [CrossRef]
- Weng, W.; Li, L.; Van Bennekum, A.M.; Potter, S.H.; Harrison, E.H.; Blaner, W.S.; Breslow, J.L.; Fisher, E.A. Intestinal absorption of dietary cholesteryl ester is decreased but retinyl ester absorption is normal in carboxyl ester lipase knockout mice. Biochemistry 1999, 38, 4143–4149. [Google Scholar] [CrossRef]
- Erlanson, C.; Borgstrom, B. The identity of vitamin A esterase activity of rat pancreatic juice. Biochim. Biophys. Acta 1968, 167, 629–631. [Google Scholar] [CrossRef]
- Lindstrom, M.B.; Sternby, B.; Borgstrom, B. Concerted action of human carboxyl ester lipase and pancreatic lipase during lipid digestion in vitro: Importance of the physicochemical state of the substrate. Biochim. Biophys. Acta 1988, 959, 178–184. [Google Scholar] [CrossRef]
- Reboul, E.; Berton, A.; Moussa, M.; Kreuzer, C.; Crenon, I.; Borel, P. Pancreatic lipase and pancreatic lipase-related protein 2, but not pancreatic lipase-related protein 1, hydrolyze retinyl palmitate in physiological conditions. Biochim. Biophys. Acta 2006, 1761, 4–10. [Google Scholar] [CrossRef]
- Rigtrup, K.M.; Kakkad, B.; Ong, D.E. Purification and partial characterization of a retinyl ester hydrolase from the brush border of rat small intestine mucosa: Probable identity with brush border phospholipase B. Biochemistry 1994, 33, 2661–2666. [Google Scholar] [CrossRef]
- Rigtrup, K.M.; Mcewen, L.R.; Said, H.M.; Ong, D.E. Retinyl ester hydrolytic activity associated with human intestinal brush border membranes. Am. J. Clin. Nutr. 1994, 60, 111–116. [Google Scholar]
- Rigtrup, K.M.; Ong, D.E. A retinyl ester hydrolase activity intrinsic to the brush border membrane of rat small intestine. Biochemistry 1992, 31, 2920–2926. [Google Scholar] [CrossRef]
- Levin, G.; Mokady, S. Incorporation of all-trans- or 9-cis-beta-carotene into mixed micelles in vitro. Lipids 1995, 30, 177–179. [Google Scholar] [CrossRef]
- Tyssandier, V.; Lyan, B.; Borel, P. Main factors governing the transfer of carotenoids from emulsion lipid droplets to micelles. Biochim. Biophys. Acta 2001, 1533, 285–292. [Google Scholar] [CrossRef]
- Borel, P.; Armand, M.; Pasquier, B.; Senft, M.; Dutot, G.; Melin, C.; Lafont, H.; Lairon, D. Digestion and absorption of tube-feeding emulsions with different droplet sizes and compositions in the rat. J. Parenter. Enter. Nutr. 1994, 18, 534–543. [Google Scholar]
- Bengtsson, A.; Larsson Alminger, M.; Svanberg, U. In vitro bioaccessibility of beta-carotene from heat-processed orange-fleshed sweet potato. J. Agric. Food Chem. 2009, 57, 9693–9698. [Google Scholar] [CrossRef]
- Kirilenko, V.N.; Gregoriadis, G. Fat soluble vitamins in liposomes: Studies on incorporation efficiency and bile salt induced vesicle disintegration. J. Drug Target. 1993, 1, 361–368. [Google Scholar] [CrossRef]
- Noy, N.; Kelleher, D.J.; Scotto, A.W. Interactions of retinol with lipid bilayers: Studies with vesicles of different radii. J. Lipid Res. 1995, 36, 375–382. [Google Scholar]
- Perez, M.D.; Calvo, M. Interaction of b-lactoglobulin with retinol and fatty acids and its role as a possible biological function for this protein: A review. J. Dairy Sci. 1995, 78, 978–988. [Google Scholar] [CrossRef]
- Said, H.M.; Ong, D.E.; Shingleton, J.L. Intestinal uptake of retinol: Enhancement by bovine milk beta-lactoglobuline. Am. J. Clin. Nutr. 1989, 49, 690–694. [Google Scholar]
- Godovac-Zimmermann, J. The Structural motif of B-Lactoglobulin and Retinol-Binding Protein: A basic framework for binding and transport of small Hydrophobic molecules. Trends Biochem. Sci. 1988, 13, 64–66. [Google Scholar] [CrossRef]
- Dufour, E.; Haertle, T. Binding of retinoids and beta-carotene to beta-lactoglobulin. Influence of protein modifications. Biochim. Biophys. Acta 1991, 1079, 316–320. [Google Scholar] [CrossRef]
- Sivakumar, B.; Reddy, V. Absorption of labelled vitamin A in children during infection. Br. J. Nutr. 1972, 27, 299–304. [Google Scholar] [CrossRef]
- O’neill, M.E.; Thurnham, D.I. Intestinal absorption of β-carotene, lycopene and lutein in men and women following a standard meal: Response curves in the triacylglycerol-rich lipoprotein fraction. Br. J. Nutr. 1998, 79, 149–159. [Google Scholar] [CrossRef]
- Novotny, J.A.; Dueker, S.R.; Zech, L.A.; Clifford, A.J. Compartmental analysis of the dynamics of beta-carotene metabolism in an adult volunteer. J. Lipid Res. 1995, 36, 1825–1838. [Google Scholar]
- Van Vliet, T.; Schreurs, W.H.; van Den Berg, H. Intestinal beta-carotene absorption and cleavage in men: Response of beta-carotene and retinyl esters in the triglyceride-rich lipoprotein fraction after a single oral dose of beta-carotene. Am. J. Clin. Nutr. 1995, 62, 110–116. [Google Scholar]
- Faulks, R.M.; Hart, D.J.; Wilson, P.D.; Scott, K.J.; Southon, S. Absorption of all-trans and 9-cis beta-carotene in human ileostomy volunteers. Clin. Sci. 1997, 93, 585–591. [Google Scholar]
- Van Lieshout, M.; West, C.E.; van Breemen, R.B. Isotopic tracer techniques for studying the bioavailability and bioefficacy of dietary carotenoids, particularly beta-carotene, in humans: A review. Am. J. Clin. Nutr. 2003, 77, 12–28. [Google Scholar]
- Van Loo-Bouwman, C.A.; Naber, T.H.; van Breemen, R.B.; Zhu, D.; Dicke, H.; Siebelink, E.; Hulshof, P.J.; Russel, F.G.; Schaafsma, G.; West, C.E. Vitamin A equivalency and apparent absorption of beta-carotene in ileostomy subjects using a dual-isotope dilution technique. Br. J. Nutr. 2010, 103, 1836–1843. [Google Scholar] [CrossRef]
- Drevon, C.A. Absorption, transport and metabolism of vitamin E. Free Radic. Res. Commun. 1991, 14, 229–246. [Google Scholar] [CrossRef]
- Traber, M.G.; Sies, H. Vitamin E in humans: Demand and delivery. Annu. Rev. Nutr. 1996, 16, 321–347. [Google Scholar] [CrossRef]
- Cohn, W. Bioavailability of vitamin E. Eur. J. Clin. Nutr. 1997, 51 (Suppl. 1), S80–S85. [Google Scholar]
- Borel, P.; Lietz, G.; Goncalves, A.; Szabo De Edelenyi, F.; Lecompte, S.; Curtis, P.; Goumidi, L.; Caslake, M.J.; Miles, E.A.; Packard, C.; et al. CD36 and SR-BI are involved in cellular uptake of provitamin A carotenoids by Caco-2 and HEK cells, and some of their genetic variants are associated with plasma concentrations of these micronutrients in humans. J. Nutr. 2013, 143, 448–456. [Google Scholar] [CrossRef]
- During, A.; Harrison, E.H. Mechanisms of provitamin A (carotenoid) and vitamin A (retinol) transport into and out of intestinal Caco-2 cells. J. Lipid Res. 2007, 48, 2283–2294. [Google Scholar] [CrossRef]
- Quick, T.C.; Ong, D.E. Vitamin A metabolism in the human intestinal caco-2 cell line. Biochemistry 1990, 29, 1116–1123. [Google Scholar]
- Kawaguchi, R.; Yu, J.; Honda, J.; Hu, J.; Whitelegge, J.; Ping, P.; Wiita, P.; Bok, D.; Sun, H. A membrane receptor for retinol binding protein mediates cellular uptake of vitamin A. Science 2007, 315, 820–825. [Google Scholar] [CrossRef]
- Bouillet, P.; Sapin, V.; Chazaud, C.; Messaddeq, N.; Decimo, D.; Dolle, P.; Chambon, P. Developmental expression pattern of Stra6, a retinoic acid-responsive gene encoding a new type of membrane protein. Mech. Dev. 1997, 63, 173–186. [Google Scholar] [CrossRef]
- Isken, A.; Golczak, M.; Oberhauser, V.; Hunzelmann, S.; Driever, W.; Imanishi, Y.; Palczewski, K.; von Lintig, J. RBP4 disrupts vitamin A uptake homeostasis in a STRA6-deficient animal model for Matthew-Wood syndrome. Cell Metab. 2008, 7, 258–268. [Google Scholar] [CrossRef]
- Alapatt, P.; Guo, F.; Komanetsky, S.M.; Wang, S.; Cai, J.; Sargsyan, A.; Rodríguez Díaz, E.; Bacon, B.T.; Aryal, P.; Graham, T.E. Liver retinol transporter and receptor for serum retinol-binding protein (RBP4). J. Biol. Chem. 2013, 288, 1250–1265. [Google Scholar] [CrossRef]
- Borel, P.; Grolier, P.; Mekki, N.; Boirie, Y.; Rochette, Y.; Le Roy, B.; Alexandre-Gouabau, M.C.; Lairon, D.; Azais-Braesco, V. Low and high responders to pharmacological doses of beta-carotene: Proportion in the population, mechanisms involved and consequences on beta-carotene metabolism. J. Lipid Res. 1998, 39, 2250–2260. [Google Scholar]
- Jeanes, Y.M.; Hall, W.L.; Lodge, J.K. Comparative (2)H-labelled alpha-tocopherol biokinetics in plasma, lipoproteins, erythrocytes, platelets and lymphocytes in normolipidaemic males. Br. J. Nutr. 2005, 94, 92–99. [Google Scholar] [CrossRef]
- During, A.; Hussain, M.M.; Morel, D.W.; Harrison, E.H. Carotenoid uptake and secretion by CaCo-2 cells: Beta-carotene isomer selectivity and carotenoid interactions. J. Lipid Res. 2002, 43, 1086–1095. [Google Scholar] [CrossRef]
- Reboul, E.; Thap, S.; Perrot, E.; Amiot, M.J.; Lairon, D.; Borel, P. Effect of the main dietary antioxidants (carotenoids, gamma-tocopherol, polyphenols, and vitamin C) on alpha-tocopherol absorption. Eur. J. Clin. Nutr. 2007, 61, 1167–1173. [Google Scholar] [CrossRef]
- Reboul, E.; Thap, S.; Tourniaire, F.; Andre, M.; Juhel, C.; Morange, S.; Amiot, M.J.; Lairon, D.; Borel, P. Differential effect of dietary antioxidant classes (carotenoids, polyphenols, vitamin C and vitamin E) on lutein absorption. Br. J. Nutr. 2007, 97, 440–446. [Google Scholar] [CrossRef]
- Hageman, S.H.; She, L.; Furr, H.C.; Clark, R.M. Excess vitamin E decreases canthaxanthin absorption in the rat. Lipids 1999, 34, 627–631. [Google Scholar] [CrossRef]
- Kiefer, C.; Sumser, E.; Wernet, M.F.; von Lintig, J. A class B scavenger receptor mediates the cellular uptake of carotenoids in Drosophila. Proc. Natl. Acad. Sci. USA 2002, 99, 10581–10586. [Google Scholar] [CrossRef]
- Lobo, M.V.; Huerta, L.; Ruiz-Velasco, N.; Teixeiro, E.; de La Cueva, P.; Celdran, A.; Martín-Hidalgo, A.; Vega, M.A.; Bragado, R. Localization of the lipid receptors CD36 and CLA-1/SR-BI in the human gastrointestinal tract: Towards the identification of receptors mediating the intestinal absorption of dietary lipids. J. Histochem. Cytochem. 2001, 49, 1253–1260. [Google Scholar] [CrossRef]
- Terpstra, V.; van Amersfoort, E.S.; van Velzen, A.G.; Kuiper, J.; van Berkel, T.J. Hepatic and extrahepatic scavenger receptors: Function in relation to disease. Arterioscler. Thromb. Vasc. Biol. 2000, 20, 1860–1872. [Google Scholar] [CrossRef]
- Hauser, H.; Dyer, J.H.; Nandy, A.; Vega, M.A.; Werder, M.; Bieliauskaite, E.; Weber, F.E.; Compassi, S.; Gemperli, A.; Boffelli, D.; et al. Identification of a receptor mediating absorption of dietary cholesterol in the intestine. Biochemistry 1998, 37, 17843–17850. [Google Scholar] [CrossRef]
- Bietrix, F.; Yan, D.; Nauze, M.; Rolland, C.; Bertrand-Michel, J.; Comera, C.; Schaak, S.; Barbaras, R.; Groen, A.K.; Perret, B.; et al. Accelerated lipid absorption in mice overexpressing intestinal SR-BI. J. Biol. Chem. 2006, 281, 7214–7219. [Google Scholar] [CrossRef]
- Reboul, E.; Borel, P. Proteins involved in uptake, intracellular transport and basolateral secretion of fat-soluble vitamins and carotenoids by mammalian enterocytes. Prog. Lipid Res. 2011, 50, 388–402. [Google Scholar] [CrossRef]
- Reboul, E.; Abou, L.; Mikail, C.; Ghiringhelli, O.; Andre, M.; Portugal, H.; Jourdheuil-Rahmani, D.; Amiot, MJ.; Lairon, D.; Borel, P. Lutein transport by Caco-2 TC-7 cells occurs partly by a facilitated process involving the scavenger receptor class B type I (SR-BI). Biochem. J. 2005, 387, 455–461. [Google Scholar] [CrossRef]
- Van Bennekum, A.; Werder, M.; Thuahnai, S.T.; Han, C.H.; Duong, P.; Williams, D.L.; Wettstein, P.; Schulthess, G.; Phillips, M.C. Class B scavenger receptor-mediated intestinal absorption of dietary beta-carotene and cholesterol. Biochemistry 2005, 44, 4517–4525. [Google Scholar] [CrossRef]
- During, A.; Doraiswamy, S.; Harrison, E.H. Xanthophylls are preferentially taken up compared with beta-carotene by retinal cells via a SRBI-dependent mechanism. J. Lipid Res. 2008, 49, 1715–1724. [Google Scholar] [CrossRef]
- Moussa, M.; Landrier, J.F.; Reboul, E.; Ghiringhelli, O.; Comera, C.; Collet, X.; Fröhlich, K.; Böhm, V.; Borel, P. Lycopene absorption in human intestinal cells and in mice involves scavenger receptor class B type I but not Niemann-Pick C1-like 1. J. Nutr. 2008, 138, 1432–1436. [Google Scholar]
- Tandon, N.N.; Kralisz, U.; Jamieson, G.A. Identification of glycoprotein IV (CD36) as a primary receptor for platelet-collagen adhesion. J. Biol. Chem. 1989, 264, 7576–7583. [Google Scholar]
- Goudriaan, J.R.; Dahlmans, V.E.; Febbraio, M.; Teusink, B.; Romijn, J.A.; Havekes, L.M.; Voshol, P.J. Intestinal lipid absorption is not affected in CD36 deficient mice. Mol. Cell. Biochem. 2002, 239, 199–202. [Google Scholar] [CrossRef]
- Drover, V.A.; Ajmal, M.; Nassir, F.; Davidson, N.O.; Nauli, A.M.; Sahoo, D.; Tso, P.; Abumrad, N.A. CD36 deficiency impairs intestinal lipid secretion and clearance of chylomicrons from the blood. J. Clin. Investig. 2005, 115, 1290–1297. [Google Scholar]
- Sakudoh, T.; Iizuka, T.; Narukawa, J.; Sezutsu, H.; Kobayashi, I.; Kuwazaki, S.; Banno, Y.; Kitamura, A.; Sugiyama, H.; Takada, N.; et al. A CD36-related transmembrane protein is coordinated with an intracellular lipid-binding protein in selective carotenoid transport for cocoon coloration. J. Biol. Chem. 2010, 285, 7739–7751. [Google Scholar] [CrossRef]
- Moussa, M.; Gouranton, E.; Gleize, B.; Yazidi, C.E.; Niot, I.; Besnard, P.; Borel, P.; Landrier, J.F. CD36 is involved in lycopene and lutein uptake by adipocytes and adipose tissue cultures. Mol. Nutr. Food Res. 2011, 55, 578–584. [Google Scholar] [CrossRef]
- Ring, A.; Le Lay, S.; Pohl, J.; Verkade, P.; Stremmel, W. Caveolin-1 is required for fatty acid translocase (FAT/CD36) localization and function at the plasma membrane of mouse embryonic fibroblasts. Biochim. Biophys. Acta 2006, 1761, 416–423. [Google Scholar] [CrossRef] [Green Version]
- Davies, J.P.; Levy, B.; Ioannou, Y.A. Evidence for a Niemann-pick C (NPC) gene family: Identification and characterization of NPC1L1. Genomics 2000, 65, 137–145. [Google Scholar] [CrossRef]
- Altmann, S.W.; Davis, H.R., Jr.; Zhu, L.J.; Yao, X.; Hoos, L.M.; Tetzloff, G.; Iyer, S.P.; Maguire, M.; Golovko, A.; Zeng, M.; et al. Niemann-Pick C1 Like 1 protein is critical for intestinal cholesterol absorption. Science 2004, 303, 1201–1204. [Google Scholar] [CrossRef]
- Davies, J.P.; Scott, C.; Oishi, K.; Liapis, A.; Ioannou, Y.A. Inactivation of NPC1L1 causes multiple lipid transport defects and protects against diet-induced hypercholesterolemia. J. Biol. Chem. 2005, 280, 12710–12720. [Google Scholar]
- Garcia-Calvo, M.; Lisnock, J.; Bull, H.G.; Hawes, B.E.; Burnett, D.A.; Braun, M.P.; Crona, J.H.; Davis, H.R., Jr.; Dean, D.C.; Detmers, P.A.; et al. The target of ezetimibe is Niemann-Pick C1-Like 1 (NPC1L1). Proc. Natl. Acad. Sci. USA 2005, 102, 8132–8137. [Google Scholar] [CrossRef]
- Duval, C.; Touche, V.; Tailleux, A.; Fruchart, J.C.; Fievet, C.; Clavey, V.; Staels, B.; Lestavel, S. Niemann-Pick C1 like 1 gene expression is down-regulated by LXR activators in the intestine. Biochem. Biophys. Res. Commun. 2006, 340, 1259–1263. [Google Scholar] [CrossRef]
- Davis, H.R., Jr.; Zhu, L.J.; Hoos, L.M.; Tetzloff, G.; Maguire, M.; Liu, J.; Yao, X.; Iyer, S.P.; Lam, M.H.; Lund, E.G.; et al. Niemann-Pick C1 Like 1 (NPC1L1) is the intestinal phytosterol and cholesterol transporter and a key modulator of whole-body cholesterol homeostasis. J. Biol. Chem. 2004, 279, 33586–33592. [Google Scholar] [CrossRef]
- During, A.; Dawson, H.D.; Harrison, E.H. Carotenoid transport is decreased and expression of the lipid transporters SR-BI, NPC1L1, and ABCA1 is downregulated in Caco-2 cells treated with ezetimibe. J. Nutr. 2005, 135, 2305–2312. [Google Scholar]
- Sato, Y.; Suzuki, R.; Kobayashi, M.; Itagaki, S.; Hirano, T.; Noda, T.; Mizuno, S.; Sugawara, M.; Iseki, K. Involvement of cholesterol membrane transporter Niemann-Pick C1-like 1 in the intestinal absorption of lutein. J. Pharm. Pharm. Sci. 2012, 15, 256–264. [Google Scholar]
- Cai, L.; Eckhardt, E.R.; Shi, W.; Zhao, Z.; Nasser, M.; de Villiers, W.J.; van der Westhuyzen, D.R. Scavenger receptor class B type I reduces cholesterol absorption in cultured enterocyte CaCo-2 cells. J. Lipid Res. 2004, 45, 253–262. [Google Scholar]
- Reboul, E.; Goncalves, A.; Comera, C.; Bott, R.; Nowicki, M.; Landrier, J.F.; Jourdheuil-Rahmani, D.; Dufour, C.; Collet, X.; Borel, P. Vitamin D intestinal absorption is not a simple passive diffusion: Evidences for involvement of cholesterol transporters. Mol. Nutr. Food Res. 2011, 55, 691–702. [Google Scholar] [CrossRef]
- Reboul, E.; Klein, A.; Bietrix, F.; Gleize, B.; Malezet-Desmoulins, C.; Schneider, M.; Margotat, A.; Lagrost, L.; Collet, X.; Borel, P. Scavenger receptor class B type I (SR-BI) is involved in vitamin E transport across the enterocyte. J. Biol. Chem. 2006, 281, 4739–4745. [Google Scholar]
- Herron, K.L.; Mcgrane, M.M.; Waters, D.; Lofgren, I.E.; Clark, R.M.; Ordovas, J.M.; Fernandez, M.L. The ABCG5 polymorphism contributes to individual responses to dietary cholesterol and carotenoids in eggs. J. Nutr. 2006, 136, 1161–1165. [Google Scholar]
- Harrison, E.H. Mechanisms of digestion and absorption of dietary vitamin A. Annu. Rev. Nutr. 2005, 25, 87–103. [Google Scholar] [CrossRef]
- Batten, M.L.; Imanishi, Y.; Maeda, T.; Tu, D.C.; Moise, A.R.; Bronson, D.; Possin, D.; Van Gelder, R.N.; Baehr, W.; Palczewski, K. Lecithin-retinol acyltransferase is essential for accumulation of all-trans-retinyl esters in the eye and in the liver. J. Biol. Chem. 2004, 279, 10422–10432. [Google Scholar]
- Reboul, E.; Trompier, D.; Moussa, M.; Klein, A.; Landrier, J.F.; Chimini, G.; Borel, P. ATP-binding cassette transporter A1 is significantly involved in the intestinal absorption of alpha- and gamma-tocopherol but not in that of retinyl palmitate in mice. Am. J. Clin. Nutr. 2009, 89, 177–184. [Google Scholar]
- Sauvant, P.; Mekki, N.; Charbonnier, M.; Portugal, H.; Lairon, D.; Borel, P. Amounts and types of fatty acids in meals affect the pattern of retinoids secreted in human chylomicrons after a high-dose preformed vitamin A intake. Metabolism 2003, 52, 514–519. [Google Scholar] [CrossRef]
- Amengual, J.; Golczak, M.; Palczewski, K.; von Lintig, J. Lecithin: Retinol acyltransferase is critical for cellular uptake of vitamin A from serum retinol-binding protein. J. Biol. Chem. 2012, 287, 24216–24227. [Google Scholar] [CrossRef]
- Kawaguchi, R.; Zhong, M.; Kassai, M.; Ter-Stepanian, M.; Sun, H. STRA6-catalyzed vitamin A influx, efflux, and exchange. J. Membr. Biol. 2012, 245, 731–745. [Google Scholar] [CrossRef]
- Castenmiller, J.J.M.; West, C.E. Bioavailability and bioconversion of carotenoids. Annu. Rev. Nutr. 1998, 18, 19–38. [Google Scholar] [CrossRef]
- Lobo, G.P.; Amengual, J.; Palczewski, G.; Babino, D.; von Lintig, J. Carotenoid-oxygenases: Key players for carotenoid function and homeostasis in mammalian biology. Biochim. Biophys. Acta 2011, 1821, 78–81. [Google Scholar]
- Bauernfeind, J.C. Carotenoid vitamin A precursors and analogs in foods and feeds. J. Agric. Food Chem. 1972, 20, 456–473. [Google Scholar] [CrossRef]
- Van Vliet, T.; Vanschaik, F.; Schreurs, W.H.P.; van Den Berg, H. In vitro measurement of beta-carotene cleavage activity: Methodological considerations and the effect of other carotenoids on beta-carotene cleavage. Int. J. Vitam. Nutr. Res. 1996, 66, 77–85. [Google Scholar]
- You, C.S.; Parker, R.S.; Goodman, K.J.; Swanson, J.E.; Corso, T.N. Evidence of cis-trans isomerization of 9-cis-beta-carotene during absorption in humans. Am. J. Clin. Nutr. 1996, 64, 177–183. [Google Scholar]
- Noy, N. Retinoid-binding proteins: Mediators of retinoid action. Biochem. J. 2000, 348, 481–495. [Google Scholar] [CrossRef]
- Napoli, J.L. Retinoic acid: Its biosynthesis and metabolism. Prog. Nucleic Acid Res. Mol. Biol. 1999, 63, 139–188. [Google Scholar] [CrossRef]
- Crow, J.A.; Ong, D.E. Cell-specific immunohistochemical localization of a cellular retinol-binding protein (type two) in the small intestine of rat. Proc. Natl. Acad. Sci. USA 1985, 82, 4707–4711. [Google Scholar] [CrossRef]
- Suruga, K.; Suzuki, R.; Goda, T.; Takase, S. Unsaturated fatty acids regulate gene expression of cellular retinol-binding protein, type II in rat jejunum. J. Nutr. 1995, 125, 2039–2044. [Google Scholar]
- E, X.; Zhang, L.; Lu, J.; Tso, P.; Blaner, W.S.; Levin, M.S.; Li, E. Increased neonatal mortality in mice lacking cellular retinol-binding protein II. J. Biol. Chem. 2002, 277, 36617–36623. [Google Scholar]
- Wongsiriroj, N.; Piantedosi, R.; Palczewski, K.; Goldberg, I.J.; Johnston, T.P.; Li, E.; Blaner, W.S. The molecular basis of retinoid absorption: A genetic dissection. J. Biol. Chem. 2008, 283, 13510–13519. [Google Scholar] [CrossRef]
- Uchio, K.; Tuchweber, B.; Manabe, N.; Gabbiani, G.; Rosenbaum, J.; Desmouliere, A. Cellular retinol-binding protein-1 expression and modulation during in vivo and in vitro myofibroblastic differentiation of rat hepatic stellate cells and portal fibroblasts. Lab. Investig. 2002, 82, 619–628. [Google Scholar] [CrossRef]
- Tabunoki, H.; Sugiyama, H.; Tanaka, Y.; Fujii, H.; Banno, Y.; Jouni, Z.E.; Kobayashi, M.; Sato, R.; Maekawa, H.; Tsuchida, K. Isolation, characterization, and cDNA sequence of a carotenoid binding protein from the silk gland of Bombyx mori larvae. J. Biol. Chem. 2002, 277, 32133–32140. [Google Scholar] [CrossRef]
- Yu, L.; Bharadwaj, S.; Brown, J.M.; Ma, Y.; Du, W.; Davis, M.A.; Michaely, P.; Liu, P.; Willingham, M.C.; Rudel, L.L. Cholesterol-regulated translocation of NPC1L1 to the cell surface facilitates free cholesterol uptake. J. Biol. Chem. 2006, 281, 6616–6624. [Google Scholar] [CrossRef]
- Sane, A.T.; Sinnett, D.; Delvin, E.; Bendayan, M.; Marcil, V.; Menard, D.; Menard, D.; Beaulieu, J.F.; Levy, E. Localization and role of NPC1L1 in cholesterol absorption in human intestine. J. Lipid Res. 2006, 47, 2112–2120. [Google Scholar] [CrossRef]
- Pohl, J.; Ring, A.; Korkmaz, U.; Ehehalt, R.; Stremmel, W. FAT/CD36-mediated long-chain fatty acid uptake in adipocytes requires plasma membrane rafts. Mol. Biol. Cell 2005, 16, 24–31. [Google Scholar]
- Hansen, G.H.; Niels-Christiansen, L.L.; Immerdal, L.; Danielsen, E.M. Scavenger receptor class B type I (SR-BI) in pig enterocytes: Trafficking from the brush border to lipid droplets during fat absorption. Gut 2003, 52, 1424–1431. [Google Scholar] [CrossRef]
- Velkov, T.; Lim, M.L.; Horne, J.; Simpson, J.S.; Porter, C.J.; Scanlon, M.J. Characterization of lipophilic drug binding to rat intestinal fatty acid binding protein. Mol. Cell. Biochem. 2009, 326, 87–95. [Google Scholar] [CrossRef]
- Chuang, S.; Velkov, T.; Horne, J.; Porter, C.J.; Scanlon, M.J. Characterization of the drug binding specificity of rat liver fatty acid binding protein. J. Med. Chem. 2008, 51, 3755–3764. [Google Scholar] [CrossRef]
- Besnard, P.; Niot, I.; Poirier, H.; Clement, L.; Bernard, A. New insights into the fatty acid-binding protein (FABP) family in the small intestine. Mol. Cell. Biochem. 2002, 239, 139–147. [Google Scholar] [CrossRef]
- Hanhoff, T.; Lucke, C.; Spener, F. Insights into binding of fatty acids by fatty acid binding proteins. Mol. Cell. Biochem. 2002, 239, 45–54. [Google Scholar] [CrossRef]
- Borel, P.; Moussa, M.; Reboul, E.; Lyan, B.; Defoort, C.; Vincent-Baudry, S.; Maillot, M.; Gastaldi, M.; Darmon, M.; Portugal, H.; et al. Human fasting plasma concentrations of vitamin E and carotenoids, and their association with genetic variants in apo C-III, cholesteryl ester transfer protein, hepatic lipase, intestinal fatty acid binding protein and microsomal triacylglycerol transfer protein. Br. J. Nutr. 2009, 101, 680–687. [Google Scholar] [CrossRef]
- Nayak, N.; Harrison, E.H.; Hussain, M.M. Retinyl ester secretion by intestinal cells: A specific and regulated process dependent on assembly and secretion of chylomicrons. J. Lipid Res. 2001, 42, 272–280. [Google Scholar]
- Hussain, M.M.; Fatma, S.; Pan, X.; Iqbal, J. Intestinal lipoprotein assembly. Curr. Opin. Lipidol. 2005, 16, 281–285. [Google Scholar] [CrossRef]
- Huang, H.S.; Goodman, D.S. Intestinal absorbtion and metabolism of c-labeled viatamin a alcohol and b-carotene in the rat. J. Biol. Chem. 1965, 240, 2839–2844. [Google Scholar]
- Gouras, P.; Carr, R.E.; Gunkel, R.D. Retinitis pigmentosa in abetalipoproteinemia: Effects of vitamin A. Investig. Ophthalmol. 1971, 10, 784–793. [Google Scholar]
- Brunham, L.R.; Kruit, J.K.; Iqbal, J.; Fievet, C.; Timmins, J.M.; Pape, T.D.; Coburn, B.A.; Bissada, N.; Staels, B.; Groen, A.K.; et al. Intestinal ABCA1 directly contributes to HDL biogenesis in vivo. J. Clin. Investig. 2006, 116, 1052–1062. [Google Scholar] [CrossRef]
- Reboul, E.; Dyka, F.M.; Quazi, F.; Molday, R.S. Cholesterol transport via ABCA1: New insights from solid-phase binding assay. Biochimie 2013, 95, 957–961. [Google Scholar] [CrossRef]
- Drobnik, W.; Lindenthal, B.; Lieser, B.; Ritter, M.; Weber, T.C.; Liebisch, G.; Giesa, U.; Igel, M.; Borsukova, H.; Buchler, C.; et al. ATP-Binding Cassette transporter A1 (ABCA1) affects total body sterol metabolism. Gastroenterology 2001, 120, 1203–1211. [Google Scholar] [CrossRef]
- Mulligan, J.D.; Flowers, M.T.; Tebon, A.; Bitgood, J.J.; Wellington, C.; Hayden, M.R.; Attie, A.D. ABCA1 is essential for efficient basolateral cholesterol efflux during the absorption of dietary cholesterol in chickens. J. Biol. Chem. 2003, 278, 13356–13366. [Google Scholar]
- Nieland, T.J.; Chroni, A.; Fitzgerald, M.L.; Maliga, Z.; Zannis, V.I.; Kirchhausen, T.; Krieger, M. Cross-inhibition of SR-BI- and ABCA1-mediated cholesterol transport by the small molecules BLT-4 and glyburide. J. Lipid Res. 2004, 45, 1256–1265. [Google Scholar] [CrossRef]
- Lobo, G.P.; Hessel, S.; Eichinger, A.; Noy, N.; Moise, A.R.; Wyss, A.; Palczewski, K.; von Lintig, J. ISX is a retinoic acid-sensitive gatekeeper that controls intestinal beta,beta-carotene absorption and vitamin A production. FASEB J. 2010, 24, 1656–1666. [Google Scholar] [CrossRef]
- Levin, M.S.; Davis, A.E. Retinoic acid increases cellular retinol binding protein II mRNA and retinol uptake in the human intestinal Caco-2 cell line. J. Nutr. 1997, 127, 13–17. [Google Scholar]
- De Vogel-Van Den Bosch, H.M.; de Wit, N.J.; Hooiveld, G.J.; Vermeulen, H.; van Der Veen, J.N.; Houten, S.M.; Kuipers, F.; Muller, M.; van der Meer, R. A cholesterol-free, high-fat diet suppresses gene expression of cholesterol transporters in murine small intestine. Am. J. Physiol. Gastrointest. Liver Physiol. 2008, 294, G1171–G1180. [Google Scholar] [CrossRef]
- Greenwalt, D.E.; Scheck, S.H.; Rhinehart-Jones, T. Heart CD36 expression is increased in murine models of diabetes and in mice fed a high fat diet. J. Clin. Investig. 1995, 96, 1382–1388. [Google Scholar] [CrossRef]
- Spady, D.K.; Kearney, D.M.; Hobb, H.H. Polyunsaturated fatty acids up-regulate hepatic scavenger receptor B1 (SR-BI) expression and HDL cholesteryl ester uptake in the hamster. J. Lipid Res. 1999, 40, 1384–1394. [Google Scholar]
- Borel, P.; Moussa, M.; Reboul, E.; Lyan, B.; Defoort, C.; Vincent-Baudry, S.; Maillot, M.; Gastaldi, M.; Darmon, M.; Portugal, H.; et al. Human plasma levels of vitamin E and carotenoids are associated with genetic polymorphisms in genes involved in lipid metabolism. J. Nutr. 2007, 137, 2653–2659. [Google Scholar]
- Borel, P.; de Edelenyi, F.S.; Vincent-Baudry, S.; Malezet-Desmoulin, C.; Margotat, A.; Lyan, B.; Gorrand, J.M.; Meunier, N.; Drouault-Holowacz, S.; Bieuvelet, S. Genetic variants in BCMO1 and CD36 are associated with plasma lutein concentrations and macular pigment optical density in humans. Ann. Med. 2010, 43, 47–59. [Google Scholar]
© 2013 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Reboul, E. Absorption of Vitamin A and Carotenoids by the Enterocyte: Focus on Transport Proteins. Nutrients 2013, 5, 3563-3581. https://doi.org/10.3390/nu5093563
Reboul E. Absorption of Vitamin A and Carotenoids by the Enterocyte: Focus on Transport Proteins. Nutrients. 2013; 5(9):3563-3581. https://doi.org/10.3390/nu5093563
Chicago/Turabian StyleReboul, Emmanuelle. 2013. "Absorption of Vitamin A and Carotenoids by the Enterocyte: Focus on Transport Proteins" Nutrients 5, no. 9: 3563-3581. https://doi.org/10.3390/nu5093563





