Effect of Human Milk Appetite Hormones, Macronutrients, and Infant Characteristics on Gastric Emptying and Breastfeeding Patterns of Term Fully Breastfed Infants
Abstract
:1. Introduction
2. Materials and Methods
2.1. Participants
2.2. Study Design
2.3. Feeding Frequency
2.4. Feed Volume Measurement
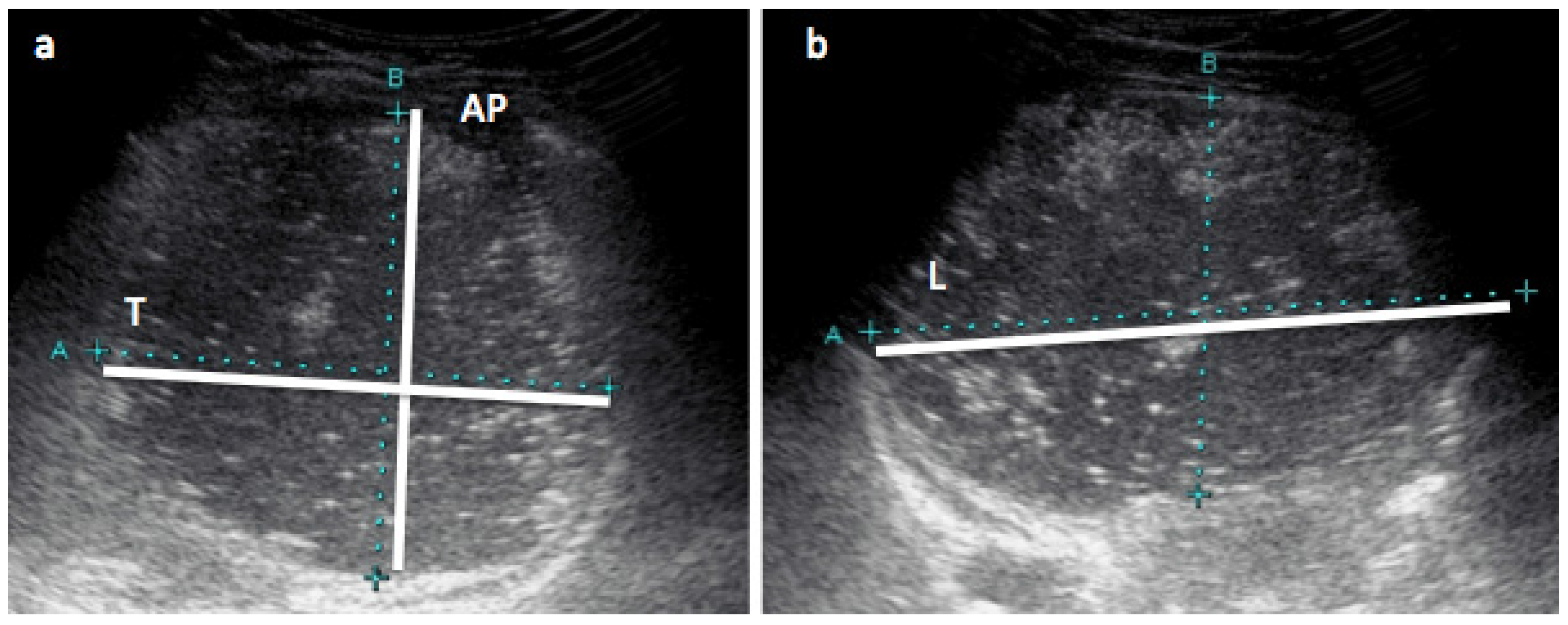
2.5. Stomach Measurements with Ultrasound
2.6. Milk Sample Collection
2.7. Biochemical Analysis
2.7.1. Fat Content
2.7.2. Sample Preparation
2.7.3. Leptin
2.7.4. Adiponectin
2.7.5. Protein
2.7.6. Lysozyme
2.7.7. Carbohydrates
2.8. Hormone and Macronutrient Dose
2.9. Infants’ Anthropometrics and Body Composition
2.9.1. Anthropometric Measurements
2.9.2. Body Composition with Bioelectrical Impedance Spectroscopy
2.9.3. Body Composition with Ultrasound Skinfold Measurements
2.10. Statistical Analysis
3. Results
3.1. Participants
3.2. Influence of Infant Age
3.3. Analyzed Human Milk Components
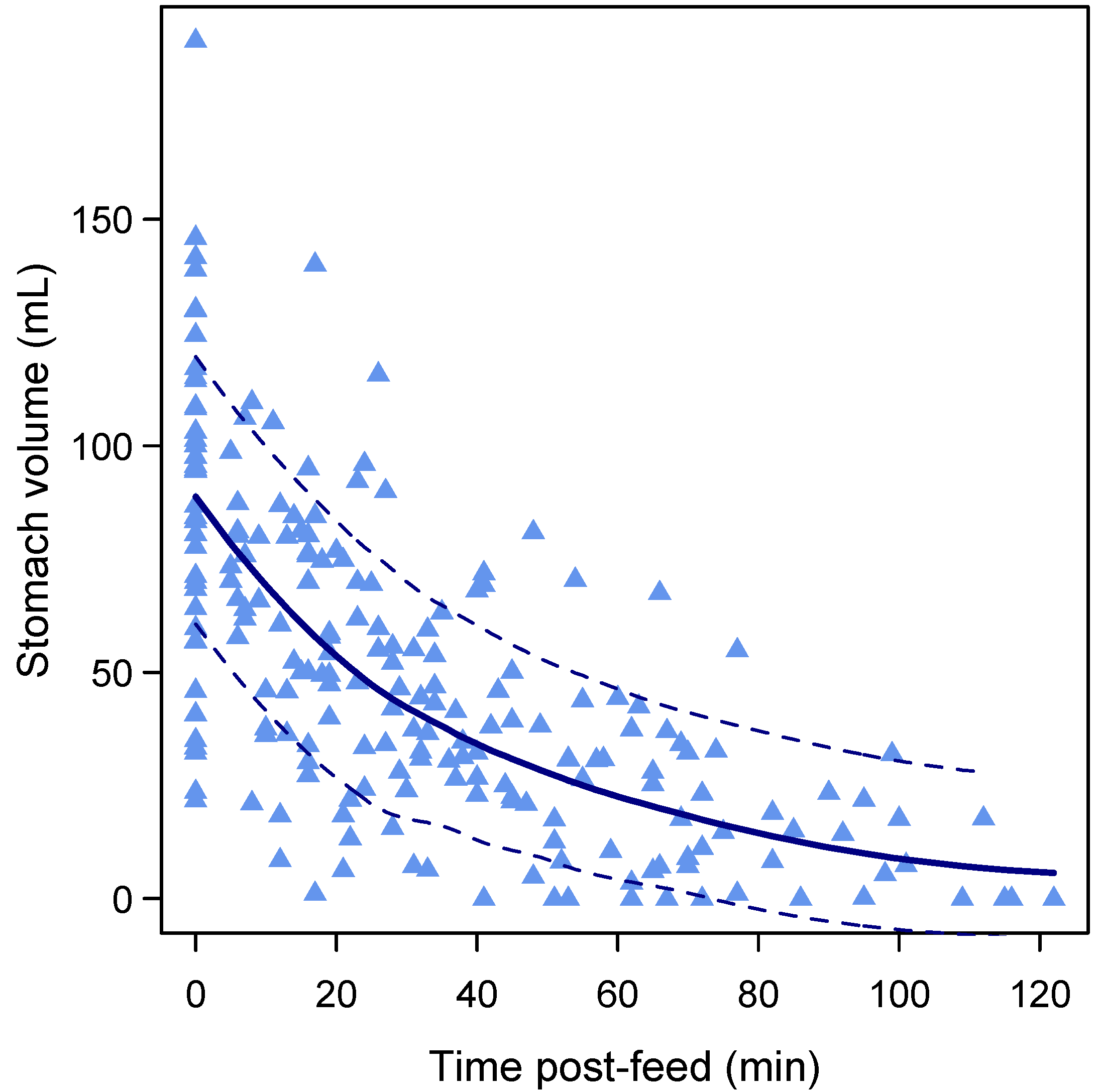
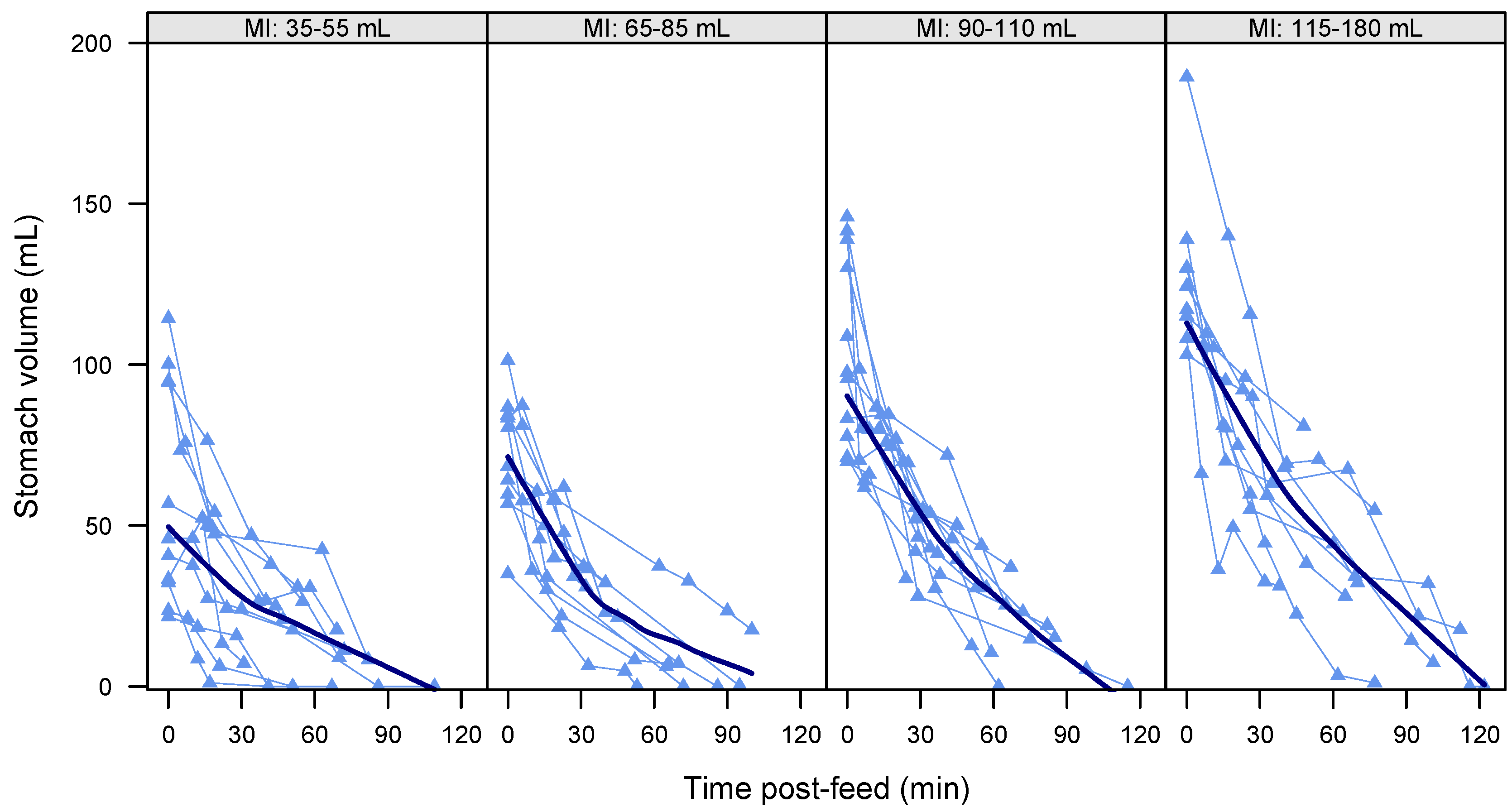
3.4. Gastric Emptying Rate
3.5. Feed Volume, Feed Duration, and Gastric Emptying during Breastfeeding
3.6. Gastric Emptying Time
3.7. Pre-Feed Residuals
3.8. Feeding Frequency
4. Discussion
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Geddes, D.; Prescott, S. Developmental origins of health and disease: The role of human milk in preventing disease in the 21(st) century. J. Hum. Lact. 2013, 29, 123–127. [Google Scholar] [CrossRef] [PubMed]
- Marseglia, L.; Manti, S.; D’Angelo, G.; Cuppari, C.; Salpietro, V.; Filippelli, M.; Trovato, A.; Gitto, E.; Salpietro, C.; Arrigo, T. Obesity and breastfeeding: The strength of association. Women Birth 2015, 28, 81–86. [Google Scholar] [CrossRef] [PubMed]
- Savino, F.; Liguori, S.; Fissore, M.; Oggero, R. Breast milk hormones and their protective effect on obesity. Int. J. Pediatr. Endocrinol. 2009. [Google Scholar] [CrossRef]
- Bartok, C. Babies fed breastmilk by breast versus by bottle: A pilot study evaluating early growth patterns. Breastfeed. Med. 2011, 6, 117–124. [Google Scholar] [CrossRef] [PubMed]
- Sievers, E.; Oldigs, H.D.; Santer, R.; Schaub, J. Feeding patterns in breast-fed and formula-fed infants. Ann. Nutr. Metab. 2001, 46, 243–248. [Google Scholar] [CrossRef]
- Le Huërou-Luron, I.; Bouzerzour, K.; Ferret-Bernard, S.; Ménard, O.; Le Normand, L.; Perrier, C.; Le Bourgot, C.; Jardin, J.; Bourlieu, C.; Carton, T.; et al. A mixture of milk and vegetable lipids in infant formula changes gut digestion, mucosal immunity and microbiota composition in neonatal piglets. Eur. J. Nutr. 2016, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Manti, S.; Lougaris, V.; Cuppari, C.; Tardino, L.; Dipasquale, V.; Arrigo, T.; Salpietro, C.; Leonardi, S. Breastfeeding and il-10 levels in children affected by cow’s milk protein allergy: A restrospective study. Immunobiology 2016. [Google Scholar] [CrossRef] [PubMed]
- Goldman, A.S. Modulation of the gastrointestinal tract of infants by human milk. Interfaces and interactions. An evolutionary perspective. J. Nutr. 2000, 130, S426–S431. [Google Scholar]
- Hanson, C.; Lyden, E.; Furtado, J.; Van Ormer, M.; Anderson-Berry, A. A comparison of nutritional antioxidant content in breast milk, donor milk, and infant formulas. Nutrients 2016, 8, 681. [Google Scholar] [CrossRef] [PubMed]
- Hunt, J. A possible relation between the regulation of gastric emptying and food intake. Am. J. Physiol. 1980, 239, G1–G4. [Google Scholar] [PubMed]
- Hellstrom, P.; Gryback, P.; Jacobsson, H. The physiology of gastric emptying. Best Pract. Res. Clin. Anaesthesiol. 2006, 20, 397–407. [Google Scholar] [CrossRef] [PubMed]
- Gomez, H.; Hornoy, P.; Liehn, J. Ultrasonography and gastric emptying in children: Validation of a sonographic method and determination of physiological and pathological patterns. Pediatr. Radiol. 2003, 33, 522–529. [Google Scholar] [CrossRef] [PubMed]
- Carlos, M.; Babyn, P.; Marcon, M.; Moore, A. Changes in gastric emptying in early postnatal life. J. Pediatr. 1997, 130, 931–937. [Google Scholar] [CrossRef]
- Perrella, S.; Hepworth, A.; Simmer, K.; Geddes, D. Influences of breast milk composition on gastric emptying in preterm infants. J. Paediatr. Gastroenterol. Nutr. 2015, 60, 264–271. [Google Scholar] [CrossRef] [PubMed]
- Cavell, B. Gastric emtying in preterm infants. Acta Paediatr. Scand. 1979, 68, 725–730. [Google Scholar] [CrossRef] [PubMed]
- Armand, M.; Hamosh, M.; Mehta, N.; Angelus, P.; Rhilpott, J.; Henderson, T.; Dwyer, N.; Lairon, D.; Hamosh, P. Effect of human milk or formula on gastric function and fat digestion in the premature infant. Pediatr. Res. 1996, 40, 429–437. [Google Scholar] [CrossRef] [PubMed]
- Cavell, B. Gastric emptying in infants fed human milk or infant formula. Acta Paediatr. Scand. 1981, 70, 639–641. [Google Scholar] [CrossRef] [PubMed]
- Vist, G.; Maughan, R. The effect of osmolality and carbohydrate content on the rate of gastric emptying of liquids in man. J. Physiol. 1995, 486, 523–531. [Google Scholar] [CrossRef] [PubMed]
- Hunt, L.; Antonson, D.; Paxson, C.J.; Vanderhoff, J. Osmolality of carbohydrate solutions and gastric emptying in the newborn. Am. J. Dis. Child. 1982, 136, 448–451. [Google Scholar] [CrossRef] [PubMed]
- Husband, J.; Husband, P.; Mallinson, C. Gastric emptying of starch meals in the newborn. Lancet 1970, 290–292. [Google Scholar] [CrossRef]
- Lonnerdal, B. Bioactive proteins in human milk: Mechanisms of action. J. Pediatr. 2010, 156, S26–S30. [Google Scholar] [CrossRef] [PubMed]
- Michaelsen, K.; Larnkjaer, A.; Molgaard, C. Amount and quality of dietary proteins during the first two years of life in relation to NCD risk in adulthood. Nutr. Metab. Cardiovasc. Dis. 2012, 22, 781–786. [Google Scholar] [CrossRef] [PubMed]
- Billeaud, C.; Guillet, J.; Sandler, B. Gastric emptying in infants with or without gastro-oesophageal reflux according to type of milk. Eur. J. Clin. Nutr. 1990, 44, 577–583. [Google Scholar] [PubMed]
- Wang, G.; Lo, L.; Forsberg, L.; Maier, R. Helicobacter pylori peptidoglycan modifications confer lysozyme resistance and contribute to survival in the host. mBio 2012, 3, e00409-12. [Google Scholar] [CrossRef] [PubMed]
- Artym, J.; Zimecki, M. Milk-derived proteins and peptides in clinical trials. Postepy Hig. Med. Dosw. 2013, 67, 800–816. [Google Scholar] [CrossRef] [Green Version]
- Savino, F.; Sardo, A.; Rossi, L.; Benetti, S.; Savino, A.; Silvestro, L. Mother and infant body mass index, breast milk leptin and their serum leptin values. Nutrients 2016, 8, 383. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.Y.; Zhang, Z.J.; Yao, W.; Morrow, A.; Peng, Y.M. Variation of maternal milk adiponectin and its correlation with infant growth. Zhonghua Er Ke Za Zhi 2011, 49, 338–343. [Google Scholar] [PubMed]
- Miralles, O.; Sanchez, J.; Palou, A.; Pico, C. A physiological role of breast milk leptin in body weight control in developing infants. Obesity 2006, 14, 1371–1377. [Google Scholar] [CrossRef] [PubMed]
- Woo, J.; Guerrero, M.; Altaye, M.; Ruiz-Palacios, G.; Martin, L.; Dubert-Ferrandon, A.; Newburg, D.; Morrow, A. Human milk adiponectin is associated with growth in two independent cohorts. Breastfeed. Med. 2009, 4, 101–109. [Google Scholar] [CrossRef] [PubMed]
- Smedh, U.; Hakansson, M.L.; Meister, B.; Uvnas-Moberg, K. Leptin injected into the fourth ventricle inhibits gastric emptying. Neuroreport 1998, 9, 297–301. [Google Scholar] [CrossRef] [PubMed]
- Sanchez, J.; Oliver, P.; Miralles, O.; Ceresi, E.; Pico, C.; Palou, A. Leptin orally supplied to neonate rats is directly uptaken by the immature stomach and may regulate short-term feeding. Endocrinology 2005, 146, 2575–2582. [Google Scholar] [CrossRef] [PubMed]
- Kugananthan, S.; Lai, C.T.; Gridneva, Z.; Mark, P.J.; Geddes, D.T.; Kakulas, F. Leptin levels are higher in whole compared to skim human milk, supporting a cellular contribution. Nutrients 2016, 8, 711. [Google Scholar] [CrossRef] [PubMed]
- Cannon, A.; Kakulas, F.; Hepworth, A.; Lai, C.; Hartmann, P.; Geddes, D. The effects of leptin on breastfeeding behaviour. Int. J. Environ. Res. Public Helath 2015, 12, 12340–12355. [Google Scholar] [CrossRef] [PubMed]
- Cannon, A.M.; Gridneva, Z.; Hepworth, A.; Lai, C.T.; Tie, W.J.; Khan, S.; Hartmann, P.E.; Geddes, D.T. The relationship of human milk leptin and macronutrients with gastric emptying in term breastfed infants. Pediatr. Res. 2016. under review. [Google Scholar]
- Newburg, D.; Woo, J.; Morrow, A. Characteristics and potential functions of human milk adiponectin. J. Pediatr. 2010, 156, S41–S46. [Google Scholar] [CrossRef] [PubMed]
- Kentish, S.J.; Ratcliff, K.; Li, H.; Wittert, G.A.; Page, A.J. High fat diet induced changes in gastric vagal afferent response to adiponectin. Physiol. Behav. 2015, 152, 354–362. [Google Scholar] [CrossRef] [PubMed]
- Iwase, M.; Iino, K.; Oku, M.; Nohara, S.; Asano, T.; Doi, Y.; Iida, M. Serum high-molecular weight adiponectin is related to early postprandial glycemic increases and gastric emptying in patients with type 2 diabetes mellitus. Diabetes Metab. Res. Rev. 2009, 25, 344–350. [Google Scholar] [CrossRef] [PubMed]
- Kent, J.C.; Mitoulas, L.R.; Cregan, M.D.; Ramsay, D.T.; Doherty, D.A.; Hartmann, P.E. Volume and frequency of breastfeedings and fat content of breast milk throughout the day. Pediatrics 2006, 117, e387–e395. [Google Scholar] [CrossRef] [PubMed]
- Khan, S.; Hepworth, A.R.; Prime, D.K.; Lai, C.T.; Trengove, N.J.; Hartmann, P.E. Variation in fat, lactose, and protein composition in breast milk over 24 h: Associations with infant feeding patterns. J. Hum. Lact. 2013, 29, 81–89. [Google Scholar] [CrossRef] [PubMed]
- Gridneva, Z.; Hepworth, A.; Ward, L.; Lai, C.T.; Hartmann, P.; Geddes, D.T. Bioimpedance spectroscopy in the infant: Effect of milk intake and extracellular fluid reservoirs on resistance measurements in term breastfed infants. Eur. J. Clin. Nutr. 2016, 70, 843–851. [Google Scholar] [CrossRef] [PubMed]
- Gridneva, Z.; Hepworth, A.R.; Ward, L.C.; Lai, C.T.; Hartmann, P.E.; Geddes, D.T. Determinants of body composition in breastfed infants using bioimpedance spectroscopy and ultrasound skinfolds—Methods comparison. Pediatr. Res. 2016, in press. [Google Scholar] [CrossRef] [PubMed]
- Arthur, P.; Hartmann, P.; Smith, M. Measurement of the milk intake of breast-fed infants. J. Paediatr. Gastroenterol. Nutr. 1987, 6, 758–763. [Google Scholar] [CrossRef]
- Neville, M.C.; Keller, R.; Seacat, J.; Lutes, V.; Neifert, M.; Casey, C.; Allen, J.; Archer, P. Studies in human lactation: Milk volumes in lactating women during the onset of lactation and full lactation. Am. J. Clin. Nutr. 1988, 48, 1375–1386. [Google Scholar] [PubMed]
- Perrella, S.; Hepworth, A.; Simmer, K.; Geddes, D. Validation of ultrasound methods to monitor gastric volume changes in preterm infants. J. Paediatr. Gastroenterol. Nutr. 2013, 57, 741–749. [Google Scholar] [CrossRef] [PubMed]
- Fleet, I.; Linzell, J. A rapid method of estimating fat in very small quantities of milk. J. Physiol. 1964, 175, 15–17. [Google Scholar]
- Meier, P.; Engstrom, J.; Zuleger, J.; Motykowski, J.; Vasan, U.; Meier, W.; Hartmann, P.E.; Williams, T.M. Accuracy of a user-friendly centrifuge for measuring creamatocrits on mothers’ milk in the clinical setting. Breastfeed. Med. 2006, 1, 79–87. [Google Scholar] [CrossRef] [PubMed]
- Mitoulas, L.R. Short- and Long-Term Variation in the Production, Content and Composition of Human Milk Fat. Ph.D. Thesis, The University of Western Australia, Crawley, WA, Australia, 2000. [Google Scholar]
- Keller, R.; Neville, M. Determination of total protein in human milk: Comparison of methods. Clin. Chem. 1986, 32, 120–123. [Google Scholar] [PubMed]
- Kunz, C.; Lonnerdal, B. Human milk proteins: Separation of whey proteins and their analysis by polyacrylamide gel electrophoresis, fast protein liquid chromatography (FPLC) gel filtration, and anion-exchange chromatography. Am. J. Clin. Nutr. 1989, 49, 464–470. [Google Scholar] [PubMed]
- Khan, S.; Casadio, Y.; Lai, C.; Prime, D.; Hepworth, A.; Trengove, N.; Hartmann, P. Investigation of short-term variations in casein and whey proteins in breast milk of term mothers. Hepatol. Nutr. 2012, 55, 136–141. [Google Scholar] [CrossRef] [PubMed]
- Mitoulas, L.R.; Kent, J.C.; Cox, D.B.; Owens, R.A.; Sherriff, J.L.; Hartmann, P.E. Variation in fat, lactose and protein in human milk over 24 h and throughout the first year of lactation. Br. J. Nutr. 2002, 88, 29–37. [Google Scholar] [CrossRef] [PubMed]
- Selsted, M.; Martinez, R. A simple and ultrasensitive enzymatic assay for the quantitative determination of lysozyme in the picogram range. Anal. Biochem. 1980, 109, 67–70. [Google Scholar] [CrossRef]
- Euber, J.; Brunner, J. Determination of lactose in milk products by high-performance liquid chromatography. J. Dairy Sci. 1979, 62, 685–690. [Google Scholar] [CrossRef]
- Albalasmeh, A.; Berhe, A.; Ghezzehei, T. A new method for rapid determination of carbohydrate and total carbon concentrations using UV spectrophotometry. Carbohydr. Polym. 2013, 97, 253–261. [Google Scholar] [CrossRef] [PubMed]
- Kuhn, N.; Lowenstein, J. Lactogenesis in the rat. Changes in metabolic parameters at parturition. Biochem. J. 1967, 105, 995–1002. [Google Scholar] [CrossRef] [PubMed]
- Newburg, D.; Neubauer, S. Carbohydrates in milks: Analysis, quantities, and significance. In Handbook of Milk Composition; Jensen, R., Ed.; Academic Press, Inc.: San Diego, CA, USA, 1995; pp. 273–349. [Google Scholar]
- Lingwood, B.; Van Leeuwen, A.; Carberry, A.; Fitzgerald, E.; Callaway, L.; Colditz, P.; Ward, L. Prediction of fat-free mass and percentage of body fat in neonates using biolelectrical impedance analysis and anthropometric measures: Validation against pea pod. Br. J. Nutr. 2012, 107, 1545–1552. [Google Scholar] [CrossRef] [PubMed]
- Brook, C. Determination of body composition of children from skinfold measurements. Arch. Dis. Child. 1971, 46, 182–184. [Google Scholar] [CrossRef] [PubMed]
- Lohman, T. Body composition in children and youth. In Advances in Pediatric Sport Sciences; Boileau, R.A., Ed.; Human Kinetics Publishers: Champaign, IL, USA, 1984; pp. 29–57. [Google Scholar]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2009. [Google Scholar]
- Pinheiro, J.; Bates, D.; DebRoy, S.; Sarkar, D.; Team, T.R.C. nlme: Linear and Nonlinear Mixed Effects Models. R Package Version 3.1-128. 2016. Available online: http://CRAN.R-project.org/package=nlme (accessed on 20 May 2016).
- Sarkar, D. Lattice: Multivariate Data Visualization with R; Springer: New York, NY, USA, 2008. [Google Scholar]
- Sarkar, D.; Andrews, F. LatticeExtra: Extra Graphical Utilities Based on Lattice, R Package Version 0.6-28; 2016. Available online: http://CRAN.R-project.org/package=latticeExtra (accessed on 20 May 2016).
- Fox, J.; Weisberg, S. An R Companion to Applied Regression; Sage: Thousand Oaks, CA, USA, 2011. [Google Scholar]
- Venables, W.N.; Ripley, B.D. Modern Applied Statistics with S, 4th ed.; Springer: New York, NY, USA, 2002. [Google Scholar]
- Maechler, M. Sfsmisc: Utilities from Seminar Fuer Statistik Eth Zurich. R Package Version 1.1-0. 2016. Available online: http://cran.r-project.org/package=sfsmisc (accessed on 20 May 2016).
- Hothorn, T.; Bretz, F.; Westfall, P. Simultaneous inference in general parametric models. Biom. J. 2008, 50, 346–363. [Google Scholar] [CrossRef] [PubMed]
- Cannon, A.M.; Sakalidis, V.S.; Lai, C.T.; Perrella, S.L.; Geddes, D.T. Vacuum characteristics of the sucking cycle and relationships with milk removal from the breast in term infants. Early Hum. Dev. 2016, 96, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Royston, P.; Altman, D.G. Regression using fractional polynomials of continuous covariates: Parsimonious parametric modelling. J. R. Stat. Soc. Ser. C Appl. Stat. 1994, 43, 429–467. [Google Scholar] [CrossRef]
- Curran-Everett, D. Multiple comparisons: Philosophies and illustrations. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2000, 279, R1–R8. [Google Scholar] [PubMed]
- Meyer, R.; Foong, R.; Thapar, N.; Kritas, S.; Shah, N. Systematic review of the impact of feed protein type and degree of hydrolysis on gastric emptying in children. BMC Gastroenterol. 2015, 15, 137. [Google Scholar] [CrossRef] [PubMed]
- Lonnerdal, B.; Havel, P.J. Serum leptin concentrations in infants: Effects of diet, sex, and adiposity. Am. J. Clin. Nutr. 2000, 72, 484–489. [Google Scholar] [PubMed]
- Bouret, S.G.; Draper, S.J.; Simerly, R.B. Trophic action of leptin on hypothalamic neurons that regulate feeding. Science 2004, 304, 108–110. [Google Scholar] [CrossRef] [PubMed]
- Petridou, E.; Mantzoros, C.S.; Belechri, M.; Skalkidou, A.; Dessypris, N.; Papathoma, E.; Salvanos, H.; Lee, J.H.; Kedikoglou, S.; Chrousos, G.; et al. Neonatal leptin levels are strongly associated with female gender, birth length, IGF-I levels and formula feeding. Clin. Endocrinol. 2005, 62, 366–371. [Google Scholar] [CrossRef] [PubMed]
- Savino, F.; Benetti, S.; Liguori, S.; Sorrenti, M.; Cordero Di Montezemolo, L. Advances of human milk hormones and protection against obesity. Cell. Mol. Biol. 2013, 59, 89–98. [Google Scholar] [PubMed]
- Gonzalez, C.R.; Caminos, J.E.; Gallego, R.; Tovar, S.; Vazquez, M.J.; Garces, M.F.; Lopez, M.; Garcia-Caballero, T.; Tena-Sempere, M.; Nogueiras, R.; et al. Adiponectin receptor 2 is regulated by nutritional status, leptin and pregnancy in a tissue-specific manner. Physiol. Behav. 2010, 99, 91–99. [Google Scholar] [CrossRef] [PubMed]
- Perry, B.; Wang, Y. Appetite regulation and weight control: The role of gut hormones. Nutr. Diabetes 2012, 2, e26. [Google Scholar] [CrossRef] [PubMed]
- Chaudhri, O.; Small, C.; Bloom, S. Gastrointestinal hormones regulating appetite. Philos. Trans. R. Soc. Lond. B Biol. Sci. 2006, 361, 1187–1209. [Google Scholar] [CrossRef] [PubMed]
- Maljaars, P.W.J.; Peters, H.P.F.; Mela, D.J.; NMasclee, A.A.M. Ileal brake: A sensible food target for appetite control. A review. Physiol. Behav. 2008, 95, 271–281. [Google Scholar] [CrossRef] [PubMed]
- Van Citters, G.W.; Lin, H.C. Ileal brake: Neuropeptidergic control of intestinal transit. Curr. Gastroenterol. Rep. 2006, 8, 367–373. [Google Scholar] [CrossRef] [PubMed]
- Khoshoo, V.; Brown, S. Gastric emptying of two whey-based formulas of different energy density and its clinical implication in children with volume intolerance. Eur. J. Clin. Nutr. 2002, 56, 656–658. [Google Scholar] [CrossRef] [PubMed]
- Stanstrup, J.; Schou, S.S.; Holmer-Jensen, J.; Hermansen, K.; Dragsted, L.O. Whey protein delays gastric emptying and suppresses plasma fatty acids and their metabolites compared to casein, gluten, and fish protein. J. Proteome Res. 2014, 13, 2396–2408. [Google Scholar] [CrossRef] [PubMed]
- Hale, T.W.; Hartmann, P.E. Hale and Hartmann’s Textbook of Human Lactation, 1st ed.; Hale Publishing, L.P.: Amarillo, TX, USA, 2007. [Google Scholar]
- Van Avesaat, M.; Troost, F.J.; Ripken, D.; Hendriks, H.F.; Masclee, A.A.M. Ileal brake activation: Macronutrient-specific effects on eating behavior? Int. J. Obes. 2015, 39, 235–243. [Google Scholar] [CrossRef] [PubMed]
- Boirie, Y.; Dangin, M.; Gachon, P.; Vasson, M.P.; Maubois, J.L.; Beaufrere, B. Slow and fast dietary proteins differently modulate postprandial protein accretion. Proc. Natl. Acad. Sci. USA 1997, 94, 14930–14935. [Google Scholar] [CrossRef] [PubMed]
- Daniel, H.; Vohwinkel, M.; Rehner, G. Effect of casein and beta-casomorphins on gastrointestinal motility in rats. J. Nutr. 1990, 120, 252–257. [Google Scholar] [PubMed]
- Stromqvist, M.; Falk, F.; Bergstrom, S.; Hansson, L.; Lonnerdal, B.; Normark, S.; Hernell, O. Human milk kappa-casein and inhibition of helicobacter pylori adhesion to human gastric mucosa. J. Pediatr. Gastroenterol. Nutr. 1995, 21, 288–296. [Google Scholar] [CrossRef] [PubMed]
- Francois, F.; Roper, J.; Joseph, N.; Pei, Z.; Chhada, A.; Shal, J.R.; Olivares De Perez, A.Z.; Perez-Perez, G.I.; Blaser, M.J. The effect of H. pylori eradication on meal-associated changes in plasma ghrelin and leptin. BMC Gastroenterol. 2011, 11, 37. [Google Scholar] [CrossRef] [PubMed]
- Montagne, P.; Cuilliere, M.L.; Mole, C.; Bene, M.C.; Faure, G. Changes in lactoferrin and lysozyme levels in human milk during the first twelve weeks of lactation. Adv. Exp. Med. Biol. 2001, 501, 241–247. [Google Scholar] [PubMed]
- Rubio, C.A. The natural antimicrobial enzyme lysozyme is up-regulated in gastrointestinal inflammatory conditions. Pathogens 2014, 3, 73–92. [Google Scholar] [CrossRef] [PubMed]
- Bol’shakova, A.M.; Shcherbakova, E.G.; Ivanova, S.D.; Medvedeva, M.M.; Zhuravleva, T.P. Lysozyme in the feeding of premature infants with mixed pathology. Antibiotiki 1984, 29, 784–790. [Google Scholar] [PubMed]
- Perrella, S.L.; Hepworth, A.R.; Simmer, K.N.; Hartmann, P.E.; Geddes, D.T. Repeatability of gastric volume measurements and intragastric content using ultrasound in preterm infants. J. Pediatr. Gastroenterol. Nutr. 2014, 59, 254–263. [Google Scholar] [CrossRef] [PubMed]
- Hellstrom, P.; Naslund, E. Interactions between gastric emptying and satiety, with special reference to glucagon-like peptide-1. Physiol. Behav. 2001, 74, 735–741. [Google Scholar] [CrossRef]
- Lorenz, D.N. Gastric emptying of milk in rat pups. Am. J. Physiol. 1985, 248, R732–R738. [Google Scholar] [PubMed]
- Kent, J.; Hepworth, A.; Sherriff, J.; Cox, D.; Mitoulas, L.; Hartmann, P. Longitudinal changes in breastfeeding patterns from 1 to 6 months of lactation. Breastfeed. Med. 2013, 8, 401–407. [Google Scholar] [CrossRef] [PubMed]
- Sepple, C.P.; Read, N.W. Gastrointestinal correlates of the development of hunger in man. Appetite 1989, 13, 183–191. [Google Scholar] [CrossRef]
- Lawrence, R.A.; Lawrence, R.M. Practical management of the mother-infant nursing couple. In Breastfeeding: A Guide for Medical Profession, 7th ed.; Lawrence, R.A., Lawrence, R.M., Eds.; Mosby/Elsevier: Maryland Heights, MO, USA, 2011; pp. 232–283. [Google Scholar]
- Natalucci, G.; Riedl, S.; Zidel, T.; Frisch, H. Spontaneous 24-h ghrelin secretion pattern in fasting subjects: Maintenance of a meal-related pattern. Eur. J. Endocrinol. 2005, 152, 845–850. [Google Scholar] [CrossRef] [PubMed]
- Degen, L.P.; Phillips, S.F. Variability of gastrointestinal transit in healthy women and men. Gut 1996, 39, 299–305. [Google Scholar] [CrossRef] [PubMed]
- Butte, N.; Garza, C.; Smith, E.; Nichols, B. Human milk intake and growth in exclusively breast-fed infants. J. Pediatr. 1984, 104, 187–195. [Google Scholar] [CrossRef]
| Characteristics | 2 mo a | 5 mo b | Total c | |||
|---|---|---|---|---|---|---|
| Mean ± SD | Range | Mean ± SD | Range | Mean ± SD | Range | |
| Infant characteristics | ||||||
| Infant age (weeks) | 9 ± 1 | 6–10 | 22 ± 1 | 18–23 | 16 ± 7 | 6–23 |
| Infant length (cm) | 57 ± 2 | 53–61 | 65 ± 2 *** | 62–69 | 61 ± 4 | 53–69 |
| Infant weight (kg) | 5.3 ± 0.8 | 4.2–6.3 | 7.2 ± 1.0 *** | 5.8–9.5 | 6.3 ± 1.3 | 4.2–9.5 |
| Infant BMI | 15.9 ± 1.3 | 13.9–18.1 | 17.6 ± 1.7 *** | 14.9–20.4 | 16.7 ± 1.7 | 13.9–20.4 |
| HC (cm) | 39 ± 1 | 37–42 | 43 ± 2 *** | 40–46 | 41 ± 2 | 37–46 |
| Fat Mass with BIS (%) | 21.4 ± 3.6 | 11.1–27.1 | 28.9 ± 3.2 *** | 21.7–35.8 | 25.3 ± 5.0 | 11.1–35.8 |
| Fat Mass with US (%) | 24.2 ± 3.6 | 17.5–30.5 | 26.6 ± 3.6 | 20.8–35.9 | 25.5 ± 3.8 | 17.5–35.9 |
| BF/GE characteristics | ||||||
| Feed volume (mL) | 86 ± 34 | 35–140 | 85 ± 33 | 36–180 | 86 ± 33 | 35–180 |
| SV after feed 1 (mL) | 87 ± 36 | 32–141 | 93 ± 41 | 22–189 | 90 ± 38 | 22–189 |
| Feed duration (min) | 28 ± 14 | 11–72 | 20 ± 8 | 6–37 | 24 ± 12 | 6–72 |
| SV reduction (mL) d | 5 ± 21 | (−42)–33 | 4 ± 26 | (−57)–56 | 4 ± 24 | (−57)–56 |
| GE time (min) e | 94 ± 29 | 44–153 | 88 ± 18 | 50–140 | 91 ± 24 | 44–140 |
| Residual 1 (mL) | 6 ± 12 | 0–50 | 11 ± 19 | 0–62 | 9 ± 16 | 0–62 |
| Residual 2 (mL) | 20 ± 20 | 0–81 | 15 ± 15 | 0–55 | 18 ± 18 | 0–81 |
| Feeding frequency (h) f | 2.3 ± 0.7 | 1.0–4.0 | 2.7 ± 0.8 | 1.5–4.0 | 2.5 ± 0.7 | 1.0–4.0 |
| Components | Concentration | Dose Per Feed | ||
|---|---|---|---|---|
| Mean ± SD | Range | Mean ± SD | Range | |
| Adiponectin (ng/mL, ng) | 10.02 ± 4.08 | 6.18–22.58 | 868.62 ± 491.32 | 238.60–2536.91 |
| WM leptin (ng/mL, ng) | 0.51 ± 0.18 | 0.23–1.10 | 44.80 ± 24.30 | 10.15–115.03 |
| SM leptin (ng/mL, ng) | 0.28 ± 0.12 | 0.20–0.84 | 24.8 ± 15.0 | 6.91–73.00 |
| Total protein (g/L, g) | 11.29 ± 2.56 | 7.60–24.16 | 0.99 ± 0.39 | 0.35–2.29 |
| Casein (g/L, g) | 1.54 ± 0.53 | 0.69–3.45 | 0.14 ± 0.07 | 0.04–0.29 |
| Whey protein (g/L, g) | 5.97 ± 1.26 | 3.82–9.08 | 0.52 ± 0.19 | 0.17–0.95 |
| Casein:whey ratio | 0.27 ± 0.11 | 0.10–0.73 | n/a a | n/a a |
| Lysozyme (g/L, g) | 0.14 ± 0.12 | 0.05–0.48 | 0.01 ± 0.01 | 0.003–0.030 |
| TCH (g/L, g) | 82.72 ± 7.89 | 67.08–97.49 | 7.28 ± 2.62 | 3.28–15.18 |
| Lactose (g/L, g) | 65.84 ± 5.14 | 53.49–77.94 | 5.86 ± 2.22 | 2.19–12.06 |
| HMO (g/L, g) | 16.88 ± 9.89 | (−10.86) b–35.77 | 1.42 ± 0.94 | (−1.09) b–3.78 |
| Fat (g/L, g) | 42.74 ± 12.10 | 17.42–66.79 | 3.57 ± 1.45 | 0.64–6.40 |
| Predictors | Feed Volume a | Gastric Emptying Time a | Post-Feed Stomach Volumes b | |||
|---|---|---|---|---|---|---|
| Estimate ± SE (95% CI) | p-Value | Estimate ± SE (95% CI) | p-Value | Estimate ± SE (95% CI) | p-Value | |
| Concentrations | ||||||
| Adiponectin (ng/mL) | 1 ± 1.3 (−36.6, 134.5) | 0.44 | 2.3 ± 0.7 (0.9, 3.7) | 0.002 c | 1.3 ± 0.7 (−0.2, 2.7) | 0.081 |
| Whole milk leptin (ng/mL) | 9.9 ± 29.9 (−36.6, 134.5) | 0.74 | 6.8 ± 15.8 (−24.2, 37.8) | 0.67 | −9.5 ± 13.3 (−35.8, 16.9) | 0.48 |
| Skim milk leptin (ng/mL) | 49 ± 43.6 (−36.6, 134.5) | 0.26 | 6.7 ± 24.3 (−41, 54.4) | 0.78 | 39.8 ± 18.4 (−0.8, 80.3) | 0.054 |
| Total protein (g/L) | −2.1 ± 2.1 (−6.2, 1.9) | 0.30 | −0.9 ± 1.1 (−3.1, 1.3) | 0.41 | 1.1 ± 1 (−1.2, 3.4) | 0.30 |
| Whey protein (g/L) | −5.5 ± 4.2 (−13.8, 2.7) | 0.19 | 5.8 ± 2.2 (1.6, 10.1) | 0.011 | 5 ± 1.9 (0.8, 9.1) | 0.023 |
| Casein (g/L) | 2.6 ± 10.2 (−17.4, 22.5) | 0.80 | −12.4 ± 4.7 (−21.5, −3.2) | 0.013 | −2 ± 4.4 (−11.6, 7.6) | 0.66 |
| Casein:whey ratio | 24.5 ± 46.1 (−65.9, 114.9) | 0.59 | −65.9 ± 21 (−107.1, −24.7) | 0.003 | −17.3 ± 20.1 (−61.4, 26.9) | 0.41 |
| Lysozyme (g/L) | −81.4 ± 46.2 (−172, 9.1) | 0.079 | −19.5 ± 28.3 (−75, 36) | 0.49 | 23.3 ± 15.4 (−7.2, 53.8) | 0.13 |
| Total carbohydrates (g/L) | −1.1 ± 0.7 (−2.3, 0.2) | 0.12 | −0.6 ± 0.4 (−1.3, 0.1) | 0.10 | −0.5 ± 0.3 (−1.2, 0.1) | 0.089 |
| Lactose (g/L) | 0.7 ± 1.1 (−1.4, 2.7) | 0.51 | 0.2 ± 0.6 (−0.9, 1.3) | 0.76 | 0.03 ± 0.49 (−1, 1.1) | 0.96 |
| HMO (g/L) | −0.8 ± 0.5 (−1.9, 0.2) | 0.13 | −0.4 ± 0.3 (−1, 0.2) | 0.16 | −0.4 ± 0.2 (−0.9, 0.1) | 0.13 |
| Fat (g/L) | −0.69 ± 0.6 (−1.8, 0.5) | 0.26 | −0.1 ± 0.3 (−0.6, 0.5) | 0.79 | −0.1 ± 0.3 (−0.9, 0.6) | 0.71 |
| Doses | ||||||
| Adiponectin (ng) | n/a d | n/a d | 0.02 ± 0.01 (0.01, 0.03) | 0.005 | 0.01 ± 0.01 (−0.003, 0.03) | 0.094 |
| Whole milk leptin (ng) | n/a | n/a | −0.1 ± 0.2 (−0.4, 0.2) | 0.44 | −0.2 ± 0.2 (−0.5, 0.2) | 0.28 |
| Skim milk leptin (ng) | n/a | n/a | −0.2 ± 0.2 (−0.7, 0.3) | 0.38 | 0.4 ± 0.2 (−0.1, 0.8) | 0.086 |
| Total protein (g) | n/a | n/a | −25.9 ± 12.4 (−50.2, −1.7) | 0.040 | 15 ± 13.1 (−13.7, 43.7) | 0.27 |
| Whey protein (g) | n/a | n/a | 47.6 ± 18.7 (10.8, 84.3) | 0.015 | 50.6 ± 24.3 (−2.8, 104) | 0.061 |
| Casein (g) | n/a | n/a | −119 ± 53.3 (−223.4, −14.6) | 0.030 | 0.4 ± 47.6 (−104.2, 105.1) | 0.99 |
| Lysozyme (g) | n/a | n/a | −276.2 ± 370.7 (−1002.9, 450.4) | 0.46 | 395.5 ± 258.3 (−114.7, 905.6) | 0.13 |
| Total carbohydrates (g) | n/a | n/a | −4.1 ± 1.8 (−7.6, −0.5) | 0.030 | −4.6 ± 3 (−11.2, 2.1) | 0.16 |
| Lactose (g) | n/a | n/a | −5.8 ± 2.7 (−11.1, −0.6) | 0.037 | −3.1 ± 5.4 (−15.1, 8.9) | 0.58 |
| HMO (g) | n/a | n/a | −3.1 ± 3.1 (−9.2, 3) | 0.32 | −3.2 ± 2.7 (−9.1, 2.8) | 0.27 |
| Fat (g) | n/a | n/a | −2.8 ± 2.7 (−8.1, 2.4) | 0.30 | −4.9 ± 2.8 (−12.7, 2.8) | 0.15 |
| Demographics | ||||||
| Infant sex (Male) | −2.2 ± 10.7 (−23.1, 18.8) | 0.84 | −1.5 ± 7.5 (−16.3, 13.2) | 0.84 | −8.4 ± 4.6 (−17.8, 1.1) | 0.081 |
| Infant age (months) | −0.9 ± 3.6 (−7.9, 6) | 0.80 | −1.8 ± 2.5 (−6.6, 3) | 0.47 | −1.5 ± 1.4 (−4.5, 1.6) | 0.32 |
| Anthropometrics | ||||||
| Infant length (cm) | −0.03 ± 1.3 (−2.6, 2.6) | 0.98 | −1.3 ± 0.9 (−3, 0.4) | 0.15 | −0.5 ± 0.6 (−1.8, 0.8) | 0.44 |
| Infant weight (kg) | 0.7 ± 4.1 (−7.4, 8.8) | 0.87 | −2.3 ± 2.9 (−7.9, 3.4) | 0.43 | −2.3 ± 1.8 (−6.3, 1.7) | 0.23 |
| Head circumference (cm) | −2.5 ± 2.6 (−7.5, 2.5) | 0.34 | −1.4 ± 1.8 (−4.9, 2.1) | 0.42 | −1.8 ± 1.2 (−4.5, 0.8) | 0.15 |
| Infant BMI | −0.2 ± 3.2 (−6.5, 6) | 0.94 | −1.5 ± 2.2 (−5.8, 2.8) | 0.48 | −3.2 ± 1.5 (−6.6, 0.2) | 0.062 |
| Body composition | ||||||
| Fat mass with US (%) | 0.6 ± 1.4 (−2.2, 3.4) | 0.67 | −0.3 ± 0.9 (−2.2, 1.5) | 0.71 | −0.6 ± 0.7 (−2.1, 1.0) | 0.42 |
| Fat mass with BIS (%) | 0.4 ± 1.1 (−1.8, 2.5) | 0.74 | −0.4 ± 0.7 (−1.9, 1) | 0.56 | −0.5 ± 0.5 (−1.5, 0.5) | 0.35 |
| Predictors | Feeding Frequency (h) a | |
|---|---|---|
| Estimate ± SE (95% CI) b | p-Value | |
| Concentrations | ||
| Adiponectin (ng/mL) | −0.001 ± 0.03 (−0.06, 0.06) | 0.96 |
| Whole milk leptin (ng/mL) | −1.1 ± 0.7 (−2.5, 0.3) | 0.13 |
| Skim milk leptin (ng/mL) | 0.8 ± 1.6 (−2.3, 4) | 0.60 |
| Total protein (g/L) | −0.05 ± 0.05 (−0.15, 0.04) | 0.28 |
| Whey protein (g/L) | −0.1 ± 0.1 (−0.3, 0.1) | 0.42 |
| Casein (g/L) | 0.04 ± 0.2 (−0.4, 0.5) | 0.86 |
| Casein:whey protein ratio | 0.4 ± 1.1 (−1.7, 2.5) | 0.68 |
| Lysozyme (g/L) | −0.4 ± 1.1 (−2.5, 1.7) | 0.71 |
| Total carbohydrates (g/L) | 0.01 ± 0.02 (−0.03, 0.04) | 0.73 |
| Lactose (g/L) | −0.05 ± 0.02 (−0.1, −0.01) | 0.031 |
| HMO (g/L) | 0.01 ± 0.02 (−0.03, 0.04) | 0.73 |
| Fat (g/L) | −0.02 ± 0.01 (−0.04, 0.01) | 0.19 |
| Doses | ||
| Adiponectin (ng/mL) | 0.0002 ± 0.0003 (−0.0004, 0.0008) | 0.50 |
| Whole milk leptin (ng/mL) | −0.002 ± 0.01 (−0.01, 0.01) | 0.80 |
| Skim milk leptin (ng/mL) | 0.01 ± 0.01 (−0.01, 0.03) | 0.59 |
| Total protein (g/L) | 0.1 ± 0.3 (−0.5, 0.8) | 0.67 |
| Whey protein (g/L) | 0.1 ± 0.7 (−1.3, 1.5) | 0.89 |
| Casein (g/L) | 2.1 ± 2 (−1.7, 5.9) | 0.27 |
| Lysozyme (g/L) | −5.7 ± 17.2 (−39.4, 27.9) | 0.73 |
| Total carbohydrates (g/L) | 0.1 ± 0.1 (0, 0.2) | 0.22 |
| Lactose (g/L) | 0.04 ± 0.06 (−0.08, 0.17) | 0.49 |
| HMO (g/L) | 0.3 ± 0.1 (0, 0.5) | 0.051 |
| Fat (g/L) | −0.2 ± 0.1 (−0.5, 0) | 0.085 |
| Demographics | ||
| Infant sex (Male) | −0.2 ± 0.3 (−0.7, 0.4) | 0.53 |
| Infant age (months) | 0.2 ± 0.1 (0, 0.3) | 0.078 |
| Anthropometrics | ||
| Infant length (cm) | 0.1 ± 0.03 (0.04, 0.15) | 0.004 c |
| Infant weight (kg) | 0.2 ± 0.1 (0.1, 0.4) | 0.010 |
| Head circumference (cm) | 0.1 ± 0.1 (0, 0.2) | 0.23 |
| Infant BMI | 0.13 ± 0.1 (0, 0.3) | 0.10 |
| Body composition | ||
| % fat mass with US | 0.07 ± 0.03 (0, 0.13) | 0.040 |
| % fat mass with BIS | 0.08 ± 0.02 (0.03, 0.12) | 0.002 |
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gridneva, Z.; Kugananthan, S.; Hepworth, A.R.; Tie, W.J.; Lai, C.T.; Ward, L.C.; Hartmann, P.E.; Geddes, D.T. Effect of Human Milk Appetite Hormones, Macronutrients, and Infant Characteristics on Gastric Emptying and Breastfeeding Patterns of Term Fully Breastfed Infants. Nutrients 2017, 9, 15. https://doi.org/10.3390/nu9010015
Gridneva Z, Kugananthan S, Hepworth AR, Tie WJ, Lai CT, Ward LC, Hartmann PE, Geddes DT. Effect of Human Milk Appetite Hormones, Macronutrients, and Infant Characteristics on Gastric Emptying and Breastfeeding Patterns of Term Fully Breastfed Infants. Nutrients. 2017; 9(1):15. https://doi.org/10.3390/nu9010015
Chicago/Turabian StyleGridneva, Zoya, Sambavi Kugananthan, Anna R. Hepworth, Wan J. Tie, Ching T. Lai, Leigh C. Ward, Peter E. Hartmann, and Donna T. Geddes. 2017. "Effect of Human Milk Appetite Hormones, Macronutrients, and Infant Characteristics on Gastric Emptying and Breastfeeding Patterns of Term Fully Breastfed Infants" Nutrients 9, no. 1: 15. https://doi.org/10.3390/nu9010015





