Is the Proportion of Carbohydrate Intake Associated with the Incidence of Diabetes Complications?—An Analysis of the Japan Diabetes Complications Study
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Cohort
2.2. Outcome Measures
2.3. Dietary Assessment
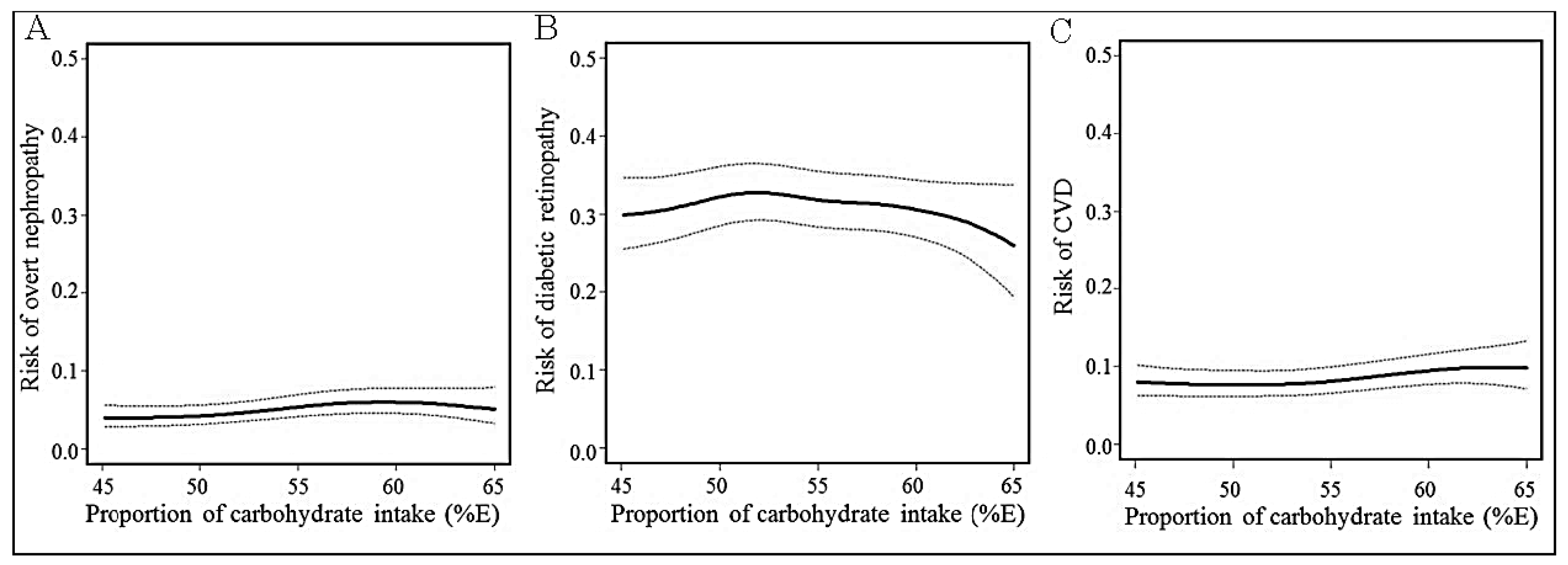
2.4. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Kodama, S.; Saito, K.; Tanaka, S.; Maki, M.; Yachi, Y.; Sato, M.; Sugawara, A.; Totsuka, K.; Shimano, H.; Ohashi, Y.; et al. Influence of fat and carbohydrate proportions on the metabolic profile in patients with type 2 diabetes: A meta-analysis. Diabetes Care 2009, 32, 959–965. [Google Scholar] [CrossRef] [PubMed]
- Noto, H.; Goto, A.; Tsujimoto, T.; Noda, M. Low-carbohydrate diets and all-cause mortality: A systematic review and meta-analysis of observational studies. PLoS ONE 2013, 8, e55030. [Google Scholar] [CrossRef] [PubMed]
- American Diabetes Association. 3. Foundations of Care and Comprehensive Medical Evaluation. Diabetes Care 2016, 39, S23–S35. [Google Scholar]
- Mann, J.I.; De Leeuw, I.; Hermansen, K.; Karamanos, B.; Karlstrom, B.; Katsilambros, N.; Riccardi, G.; Rivellese, A.A.; Rizkalla, S.; Slama, G.; et al. Diabetes and Nutrition Study Group (DNSG) of the European Association. Evidence-based nutritional approaches to the treatment and prevention of diabetes mellitus. Nutr. Metab. Cardiovasc. Dis. 2004, 14, 373–394. [Google Scholar] [CrossRef]
- Guideline Committee of the Japan Diabetes Society. Japan Diabetes Society Evidence-Based Practice Guidelines for the Treatment of Diabetes in Japan; Nankodo: Tokyo, Japan, 2013. [Google Scholar]
- National Academy of Sciences. Institute of Medicine. Food and Nutrition Board. Dietary Reference Intakes for Energy, Carbohydrate, Fiber, Fat, Fatty Acids, Cholesterol, Protein, and Amino Acids (Macronutrients); The National Academies Press: Wasington, DC, USA, 2005. [Google Scholar]
- National Institute of Health and Nutrition. Overview of Dietary Reference Intakes for Japanese (2015). Available online: http://www.mhlw.go.jp/file/06-Seisakujouhou-10900000-Kenkoukyoku/overview.pdf (accessed on 5 November 2016).
- Sone, H.; Tanaka, S.; Iimuro, S.; Tanaka, S.; Oida, K.; Yamasaki, Y.; Oikawa, S.; Ishibashi, S.; Katayama, S.; Yamashita, H.; et al. Long-term lifestyle intervention lowers the incidence of stroke in Japanese patients with type 2 diabetes: A nationwide multicentre randomized controlled trial (the Japan Diabetes Complications Study). Diabetologia 2010, 53, 419–428. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Report of a World Health Organization Consultation. Use of glycated haemoglobin (HbA1c) in the diagnosis of diabetes mellitus. Diabetes Res. Clin. Pract. 2011, 93, 299–309. [Google Scholar]
- The committee of Japan Diabetes Society on the Diagnostic Criteria of Diabetes Mellitus. Report of the committee on the classification and diagnostic criteria of diabetes mellitus. J. Diabetes Investig. 2010, 1, 212–228. [Google Scholar]
- Tanaka, S.; Tanaka, S.; Iimuro, S.; Yamashita, H.; Katayama, S.; Ohashi, Y.; Akanuma, Y.; Yamada, N.; Sone, H.; Japan Diabetes Complications Study Group. Cohort Profile: The Japan Diabetes Complications Study: A long-term follow-up of a randomised lifestyle intervention study of type 2 diabetes. Int. J. Epidemiol. 2014, 43, 1054–1062. [Google Scholar] [CrossRef] [PubMed]
- Sone, H.; Katagiri, A.; Ishibashi, S.; Abe, R.; Saito, Y.; Murase, T.; Yamashita, H.; Yajima, Y.; Ito, H.; Ohashi, Y.; et al. Effects of lifestyle modifications on patients with type 2 diabetes: The Japan Diabetes Complications Study (JDCS) study design, baseline analysis and three-year interim report. Horm. Metab. Res. 2002, 34, 509–515. [Google Scholar] [CrossRef] [PubMed]
- Takahashi, K.; Yoshimura, Y.; Kaimoto, T.; Kunii, D.; Komatsu, T.; Yamamoto, S. Validation of a Food Frequency Questionnaire Based on Food Groups for Estimating Individual Nutrient Intake. Jpn. J. Nutr. 2001, 59, 221–232. [Google Scholar] [CrossRef]
- Ministry of Education, Culture, Sports, Science and Technology, Japan. Standard Tables of Food Composition in Japan 2005. Available online: http://www.mext.go.jp/b_menu/shingi/gijyutu/gijyutu3/toushin/05031802.htm (accessed on 5 November 2016).
- Forbes, J.M.; Cooper, M.E. Mechanisms of diabetic complications. Physiol. Rev. 2013, 93, 137–188. [Google Scholar] [CrossRef] [PubMed]
- Coulston, A.M.; Liu, G.C.; Reaven, G.M. Plasma glucose, insulin and lipid responses to high-carbohydrate low-fat diets in normal humans. Metabolism 1983, 32, 52–56. [Google Scholar] [CrossRef]
- Boden, G. Free fatty acids as target for therapy. Curr. Opin. Endocrinol. Diabetes Obes. 2004, 11, 258–263. [Google Scholar] [CrossRef]
- Ma, Y.; Olendzki, B.C.; Hafner, A.R.; Chiriboga, D.E.; Culver, A.L.; Andersen, V.A.; Merriam, P.A.; Pagoto, S.L. Low-carbohydrate and high-fat intake among adult patients with poorly controlled type 2 diabetes mellitus. Nutrition 2006, 22, 1129–1136. [Google Scholar] [CrossRef] [PubMed]
- Wilkinson, C.P.; Ferris, F.L., III; Klein, R.E.; Lee, P.P.; Agardh, C.D.; Davis, M.; Dills, D.; Kampik, A.; Pararajasegaram, R.; Verdaguer, J.T.; et al. Proposed international clinical diabetic retinopathy and diabetic macular edema disease severity scales. Ophthalmology 2003, 110, 1677–1682. [Google Scholar] [CrossRef]
- Horikawa, C.; Yoshimura, Y.; Kamada, C.; Tanaka, S.; Tanaka, S.; Takahashi, A.; Hanyu, O.; Araki, A.; Ito, H.; Tanaka, A.; et al. Dietary intake in Japanese patients with type 2 diabetes: Analysis from Japan Diabetes Complications Study. J. Diabetes Investig. 2014, 5, 176–187. [Google Scholar] [CrossRef] [PubMed]
- Sone, H.; Ito, H.; Ohashi, Y.; Akanuma, Y.; Yamada, N.; Japan Diabetes Complication Study Group. Obesity and type 2 diabetes in Japanese patients. Lancet 2003, 361, 85. [Google Scholar] [CrossRef]
- Karter, A.J.; Ferrara, A.; Liu, J.Y.; Moffet, H.H.; Ackerson, L.M.; Selby, J.V. Ethnic disparities in diabetic complications in an insured population. JAMA 2002, 287, 2519–2527. [Google Scholar] [CrossRef] [PubMed]
| First Tertile | Second Tertile | Third Tertile | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Total | <50.9% | 51.0%–56.4% | ≥56.5% | ||||||
| (n = 1516) | (n = 496) | (n = 518) | (n = 502) | Trend | |||||
| Mean | ±SD | Mean | ±SD | Mean | ±SD | Mean | ±SD | p | |
| Carbohydrate (% energy) | 53.6 | ±6.6 | 46.4 | ±4.0 | 53.6 | ±1.6 | 60.7 | ±3.4 | <0.01 |
| Age (year) | 58.7 | ±6.9 | 57.8 | ±7.0 | 58.7 | ±7.0 | 59.6 | ±6.5 | <0.01 |
| Women (%) | 46.8% | 39.5% | 51.0% | 49.6% | <0.01 | ||||
| Years after diagnosis (year) | 11.0 | ±7.1 | 11.1 | ±7.2 | 11.0 | ±7.2 | 10.7 | ±6.9 | 0.55 |
| HbA1c (% in NGSP value) | 8.3 | ±1.3 | 8.2 | ±1.2 | 8.4 | ±1.4 | 8.3 | ±1.4 | 0.01 |
| HbA1c (mmol/mol) | 67.3 | ±14.5 | 66.0 | ±13.2 | 68.5 | ±15.2 | 67.3 | ±14.9 | 0.01 |
| Fasting blood glucose (mg/dL) | 160.7 | ±43.6 | 160.0 | ±39.6 | 162.1 | ±46.0 | 159.9 | ±44.7 | 0.62 |
| Diabetic retinopathy (%) | 22.2% | 22.0% | 22.6% | 22.1% | 0.85 | ||||
| BMI (kg/m2) | 22.9 | ±3.0 | 23.1 | ±2.9 | 22.8 | ±3.0 | 22.9 | ±3.0 | 0.19 |
| Waist circumference (cm) | 79.4 | ±9.0 | 80.3 | ±8.8 | 78.8 | ±9.4 | 79.2 | ±8.7 | 0.01 |
| SBP (mmHg) | 131.4 | ±16.0 | 132.2 | ±16.3 | 131.3 | ±15.9 | 130.8 | ±15.8 | 0.20 |
| LDL-cholesterol (mg/dL) | 122.4 | ±32.5 | 121.0 | ±35.0 | 123.1 | ±30.3 | 123.1 | ±32.0 | 0.24 |
| HDL-cholesterol (mg/dL) | 54.5 | ±17.0 | 54.8 | ±17.0 | 55.6 | ±17.5 | 53.1 | ±16.3 | 0.86 |
| Triglycerides a (mg/dL) | 102.0 | ±71.0 | 101.0 | ±73.0 | 98.0 | ±69.0 | 104.0 | ±71.0 | 0.94 |
| Urine ACR a (mg/gCre) | 16.9 | ±30.3 | 16.8 | ±29.1 | 17.5 | ±31.8 | 16.5 | ±30.3 | 0.76 |
| eGFR (mL/min/1.73 m2) | 87.1 | ±30.1 | 87.3 | ±28.6 | 87.2 | ±30.6 | 86.9 | ±31.0 | 0.92 |
| Current smoker (%) | 28.7% | 30.9% | 26.4% | 28.8% | 0.17 | ||||
| Physical activity (kJ/day) a | 589.4 | ±1097.8 | 575 | ±1148 | 630 | ±1167 | 582 | ±967 | 0.53 |
| Treated by OHA without insulin (%) | 65.8% | 64.5% | 68.1% | 64.9% | 0.53 | ||||
| Treated by insulin (%) | 20.0% | 17.9% | 20.7% | 21.4% | 0.17 | ||||
| Treated by antihypertensive agents (%) | 26.4% | 27.5% | 26.6% | 25.2% | 0.57 | ||||
| Treated by lipid-lowering agents (%) | 24.0% | 19.8% | 26.1% | 25.9% | <0.01 | ||||
| First Tertile | Second Tertile | Third Tertile | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Total | <50.9% | 51.0%–56.4% | ≥56.5% | ||||||
| (n = 1516) | (n = 496) | (n = 518) | (n = 502) | Trend | |||||
| Mean | ±SD | Mean | ±SD | Mean | ±SD | Mean | ±SD | p | |
| Carbohydrate (% energy) | 53.6 | ±6.6 | 46.4 | ±4.0 | 53.6 | ±1.6 | 60.7 | ±3.4 | <0.01 |
| Nutritional intake | |||||||||
| Energy (kcal/day) | 1736.9 | ±411.9 | 1910.3 | ±436.7 | 1706.2 | ±371.0 | 1597.2 | ±363.6 | <0.01 |
| Protein (%energy/day) | 15.7 | ±2.4 | 17.0 | ±2.6 | 15.8 | ±1.9 | 14.3 | ±1.8 | <0.01 |
| Fat (%energy/day) | 27.6 | ±5.0 | 31.7 | ±4.6 | 27.8 | ±3.1 | 23.4 | ±3.2 | <0.01 |
| Fiber, total (g/day) | 14.7 | ±5.3 | 15.2 | ±5.9 | 14.5 | ±4.8 | 14.4 | ±5.2 | 0.01 |
| Retinol equivalent (g/day) | 1320.2 | ±532.8 | 1420.6 | ±572.2 | 1308.1 | ±472.4 | 1233.6 | ±535.7 | <0.01 |
| Vitamin B1 (g/day) | 0.9 | ±0.3 | 1.1 | ±0.3 | 0.9 | ±0.2 | 0.8 | ±0.2 | <0.01 |
| Vitamin B2 (g/day) | 1.1 | ±0.3 | 1.3 | ±0.4 | 1.1 | ±0.3 | 0.9 | ±0.3 | <0.01 |
| Vitamin C (g/day) | 134.3 | ±60.7 | 136.7 | ±65.3 | 131.8 | ±53.6 | 134.4 | ±62.8 | 0.25 |
| Vitamin D (g/day) | 11.5 | ±6.7 | 14.6 | ±8.0 | 11.4 | ±5.9 | 8.6 | ±4.5 | <0.01 |
| Sodium (g/day) | 4.2 | ±1.5 | 4.5 | ±1.7 | 4.2 | ±1.4 | 3.9 | ±1.5 | <0.01 |
| Calcium (g/day) | 639.0 | ±229.7 | 721.6 | ±262.5 | 645.9 | ±202.7 | 550.3 | ±185.4 | <0.01 |
| Iron (g/day) | 8.1 | ±2.6 | 9.1 | ±3.0 | 8.0 | ±2.2 | 7.3 | ±2.1 | <0.01 |
| Intake of food groups | |||||||||
| Grain (g/day) | 191.4 | ±53.1 | 174.0 | ±43.4 | 190.1 | ±47.0 | 210.0 | ±61.1 | <0.01 |
| Potato/Aroid (g/day) | 53.5 | ±45.2 | 55.6 | ±51.0 | 53.8 | ±41.1 | 51.1 | ±43.0 | 0.25 |
| Soybeans/Soy products (g/day) | 71.3 | ±51.5 | 90.5 | ±63.6 | 69.7 | ±45.5 | 54.1 | ±34.8 | <0.01 |
| Fruits (g/day) | 133.2 | ±105.1 | 120.4 | ±100.0 | 128.7 | ±93.6 | 150.5 | ±118.2 | <0.01 |
| Green-yellow vegetables (g/day) | 138.0 | ±67.7 | 146.3 | ±71.1 | 136.5 | ±61.2 | 131.4 | ±70.0 | <0.01 |
| Other vegetables (g/day) | 186.1 | ±101.9 | 197.9 | ±107.0 | 184.2 | ±92.9 | 176.3 | ±104.4 | <0.01 |
| Seaweed (g/day) | 2.0 | ±1.6 | 2.3 | ±1.9 | 2.0 | ±1.4 | 1.8 | ±1.4 | <0.01 |
| Meat/Processed meat (g/day) | 49.6 | ±38.3 | 75.9 | ±46.5 | 44.8 | ±26.1 | 28.5 | ±21.3 | <0.01 |
| Fish/Processed fish (g/day) | 100.1 | ±60.3 | 129.6 | ±71.8 | 97.6 | ±50.0 | 73.7 | ±42.0 | <0.01 |
| Eggs (g/day) | 29.0 | ±16.8 | 33.1 | ±16.9 | 29.6 | ±16.9 | 24.3 | ±15.4 | <0.01 |
| Milk/Dairy products (g/day) | 170.4 | ±102.5 | 189.6 | ±110.0 | 180.2 | ±104.2 | 141.4 | ±85.6 | <0.01 |
| Sweets/Snacks (g/day) | 17.8 | ±20.5 | 16.0 | ±19.3 | 18.8 | ±21.8 | 18.5 | ±20.3 | 0.02 |
| Oil (g/day) | 16.9 | ±8.8 | 21.1 | ±9.9 | 16.6 | ±7.6 | 12.9 | ±6.6 | <0.01 |
| Alcoholic beverages (g/day) | 89.3 | ±162.2 | 163.7 | ±224.0 | 71.9 | ±120.0 | 33.7 | ±78.8 | <0.01 |
| First Tertile | Second Tertile | Third Tertile | ||||||
|---|---|---|---|---|---|---|---|---|
| <50.9% | 51.0%–56.4% | ≥56.5% | ||||||
| HR | 95% CI | HR | 95% CI | p | HR | 95% CI | p | |
| Carbohydrate intake at baseline (% Energy) | 52.5 | ±1.4 | 57.3 | ±1.5 | 63.4 | ±2.9 | ||
| Overt nephropathy (n = 1275) | ||||||||
| Events/Patients | 23/411 | 29/439 | 29/425 | |||||
| Not adjusted | ref | 1.21 | (0.70 to 2.10 ) | 0.50 | 1.23 | (0.71 to 2.12 ) | 0.46 | |
| Adjusted a | ref | 1.13 | (0.63 to 2.02 ) | 0.68 | 1.31 | (0.72 to 2.37 ) | 0.38 | |
| Adjusted b | ref | 1.05 | (0.54 to 2.06 ) | 0.89 | 0.98 | (0.40 to 2.44 ) | 0.97 | |
| Adjusted c | ref | 1.19 | (0.66 to 2.15 ) | 0.56 | 1.32 | (0.71 to 2.44 ) | 0.37 | |
| Diabetic retinopathy (n = 936) | ||||||||
| Events/Patients | 88/305 | 100/321 | 89/310 | |||||
| Not adjusted | ref | 1.13 | (0.86 to 1.50 ) | 0.41 | 1.00 | (0.74 to 1.34 ) | 0.99 | |
| Adjusted a | ref | 1.12 | (0.82 to 1.51 ) | 0.48 | 1.00 | (0.72 to 1.38 ) | 1.00 | |
| Adjusted b | ref | 1.30 | (0.90 to 1.88 ) | 0.17 | 1.30 | (0.78 to 2.15 ) | 0.31 | |
| Adjusted c | ref | 1.14 | (0.84 to 1.55 ) | 0.41 | 1.06 | (0.76 to 1.48 ) | 0.73 | |
| CVD (n = 1353) | ||||||||
| Events/Patients | 40/443 | 38/458 | 51/452 | |||||
| Not adjusted | ref | 0.90 | (0.57 to 1.40 ) | 0.63 | 1.29 | (0.85 to 1.95 ) | 0.23 | |
| Adjusted a | ref | 0.90 | (0.57 to 1.44 ) | 0.67 | 1.24 | (0.79 to 1.96 ) | 0.35 | |
| Adjusted b | ref | 0.95 | (0.55 to 1.63 ) | 0.84 | 1.37 | (0.69 to 2.72 ) | 0.36 | |
| Adjusted c | ref | 0.88 | (0.55 to 1.41 ) | 0.58 | 1.21 | (0.76 to 1.93 ) | 0.42 | |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Horikawa, C.; Yoshimura, Y.; Kamada, C.; Tanaka, S.; Tanaka, S.; Matsunaga, S.; Hanyu, O.; Araki, A.; Ito, H.; Tanaka, A.; et al. Is the Proportion of Carbohydrate Intake Associated with the Incidence of Diabetes Complications?—An Analysis of the Japan Diabetes Complications Study. Nutrients 2017, 9, 113. https://doi.org/10.3390/nu9020113
Horikawa C, Yoshimura Y, Kamada C, Tanaka S, Tanaka S, Matsunaga S, Hanyu O, Araki A, Ito H, Tanaka A, et al. Is the Proportion of Carbohydrate Intake Associated with the Incidence of Diabetes Complications?—An Analysis of the Japan Diabetes Complications Study. Nutrients. 2017; 9(2):113. https://doi.org/10.3390/nu9020113
Chicago/Turabian StyleHorikawa, Chika, Yukio Yoshimura, Chiemi Kamada, Shiro Tanaka, Sachiko Tanaka, Satoshi Matsunaga, Osamu Hanyu, Atsushi Araki, Hideki Ito, Akira Tanaka, and et al. 2017. "Is the Proportion of Carbohydrate Intake Associated with the Incidence of Diabetes Complications?—An Analysis of the Japan Diabetes Complications Study" Nutrients 9, no. 2: 113. https://doi.org/10.3390/nu9020113





