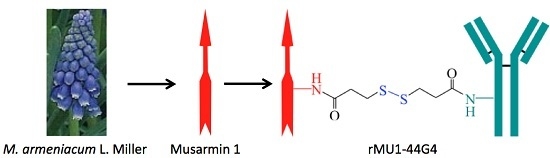
Anti-Human Endoglin (hCD105) Immunotoxin—Containing Recombinant Single Chain Ribosome-Inactivating Protein Musarmin 1
Abstract
:1. Introduction
2. Results and Discussion
3. Materials and Methods
3.1. Chemicals, Reagents and Biological Materials
3.2. Recombinant Musarmin 1 (rMU1)
3.3. Activation of the 44G4 Monoclonal Antibody
3.4. Preparation of Activated rMU1
3.5. Preparation and Purification of rMU1-44G4 IT
3.6. Assay of Cell-Free Protein Synthesis
3.7. Cytotoxicity of the rMU1-44G4 IT on L929-hCD105+ Cells
3.8. Other Procedures
Acknowledgments
Author Contributions
Conflicts of Interest
Abbreviations
| 2-ME | 2-mercaptoethanol |
| mAb | monoclonal antibody |
| RIP | ribosome inactivating protein |
| rMU1 | recombinant musarmin 1 |
| rNTP‘s | ribonucleotides |
| SDS-PAGE | sodium dodecyl sulfate polyacrylamide gel electrophoresis |
| SPDP | N-succinimidyl 3-(2-pyridyldithio)propionate |
References
- Boehm, T.; Folkman, J.; Browder, T.; O’Reilly, M.S. Antiangiogenic therapy of experimental cancer does not induce acquired drug resistance. Nature 1997, 390, 404–407. [Google Scholar] [CrossRef] [PubMed]
- Pastan, I.; Hassan, R.; FitzGerald, D.J.; Kreitman, R.J. Immunotoxin therapy of cancer. Nat. Rev. Cancer 2006, 6, 559–565. [Google Scholar] [CrossRef] [PubMed]
- Ferreras, J.M.; Citores, L.; Iglesias, R.; Jiménez, P.; Girbés, T. Use of ribosome-inactivating proteins from Sambucus for the construction of immunotoxins and conjugates for cancer therapy. Toxins 2011, 3, 420–441. [Google Scholar] [CrossRef] [PubMed]
- Weidle, U.H.; Tiefenthaler, G.; Schiller, C.; Weiss, E.H.; Georges, G.; Brinkmann, U. Prospects of bacterial and plant protein-based immunotoxins for treatment of cancer. Cancer Genom. Proteom. 2014, 11, 25–38. [Google Scholar]
- Gilabert-Oriol, R.; Weng, A.; Mallinckrodt, B.; Melzig, M.F.; Fuchs, H.; Thakur, M. Immunotoxins constructed with ribosome-inactivating proteins and their enhancers: A lethal cocktail with tumor specific efficacy. Curr. Pharm. Des. 2014, 20, 6584–6643. [Google Scholar] [CrossRef] [PubMed]
- FitzGerald, D.J.; Kreitman, R.; Wilson, W.; Squires, D.; Pastan, I. Recombinant immunotoxins for treating cancer. Int. J. Med. Microbiol. 2004, 293, 577–582. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.M.; Vallera, D.A.; Hall, W.A. Diphtheria toxin-based targeted toxin therapy for brain tumors. J. Neurooncol. 2013, 114, 155–164. [Google Scholar] [CrossRef] [PubMed]
- Polito, L.; Bortolotti, M.; Mercatelli, D.; Battelli, M.G.; Bolognesi, A. Saporin-S6: A useful tool in cancer therapy. Toxins 2013, 5, 1698–1722. [Google Scholar] [CrossRef] [PubMed]
- Bernabeu, C.; Lopez-Novoa, J.M.; Quintanilla, M. The emerging role of TGF-beta superfamily coreceptors in cancer. Biochim. Biophys. Acta 2009, 1792, 954–973. [Google Scholar] [CrossRef] [PubMed]
- Sakurai, T.; Okumura, H.; Matsumoto, M.; Uchikado, Y.; Owaki, T.; Kita, Y.; Setoyama, T.; Omoto, I.; Kijima, Y.; Ishigami, S.; et al. Endoglin (CD105) is a useful marker for evaluating microvessel density and predicting prognosis in esophageal squamous cell carcinoma. Anticancer Res. 2014, 34, 3431–3438. [Google Scholar] [PubMed]
- Rosen, L.S.; Gordon, M.S.; Robert, F.; Matei, D.E. Endoglin for targeted cancer treatment. Curr. Oncol. Rep. 2014, 16, 365. [Google Scholar] [CrossRef] [PubMed]
- Muñoz, R.; Arias, Y.; Ferreras, J.M.; Jiménez, P.; Langa, C.; Rojo, M.A.; Gayoso, M.J.; Córdoba-Díaz, D.; Bernabéu, C.; Girbés, T. In vitro and in vivo effects of an anti-mouse endoglin (CD105)-immunotoxin on the early stages of mouse B16MEL4A5 melanoma tumours. Cancer Immunol. Immunother. 2013, 62, 541–551. [Google Scholar] [CrossRef] [PubMed]
- Seon, B.K.; Haba, A.; Matsuno, F.; Takahashi, N.; Tsujie, M.; She, X.; Harada, N.; Uneda, S.; Tsujie, T.; Toi, H.; et al. Endoglin-targeted cancer therapy. Curr. Drug Deliv. 2011, 8, 135–143. [Google Scholar] [CrossRef] [PubMed]
- Fonsatti, E.; Nicolay, H.J.; Altomonte, M.; Covre, A.; Maio, M. Targeting cancer vasculature via endoglin/CD105: A novel antibody-based diagnostic and therapeutic strategy in solid tumours. Cardiovasc. Res. 2010, 86, 12–19. [Google Scholar] [CrossRef] [PubMed]
- Dolinsek, T.; Markelc, B.; Bosnjak, M.; Blagus, T.; Prosen, L.; Kranjc, S.; Stimac, M.; Lampreht, U.; Sersa, G.; Cemazar, M. Endoglin silencing has significant antitumor effect on murine mammary adenocarcinoma mediated by vascular targeted effect. Curr. Gene Ther. 2015, 15, 228–244. [Google Scholar] [CrossRef] [PubMed]
- Matsuno, F.; Haruta, Y.; Kondo, M.; Tsai, H.; Barcos, M.; Seon, B.K. Induction of lasting complete regression of preformed distinct solid tumors by targeting the tumor vasculature using two new anti-endoglin monoclonal antibodies. Clin. Cancer Res. 1999, 5, 371–382. [Google Scholar] [PubMed]
- Duan, C.L.; Hou, G.H.; Liu, Y.P.; Liang, T.; Song, J.; Han, J.K.; Zhang, C. Tumor vascular homing endoglin-targeted radioimmunotherapy in hepatocellular carcinoma. Tumor Biol. 2014, 35, 12205–12215. [Google Scholar] [CrossRef] [PubMed]
- Thorpe, P.E. Vascular targeting agents as cancer therapeutics. Clin. Cancer Res. 2004, 10, 415–427. [Google Scholar] [CrossRef] [PubMed]
- Ghetie, V.; Vitetta, E.S. Chemical construction of immunotoxins. Mol. Biotechnol. 2001, 18, 351–368. [Google Scholar] [CrossRef]
- Muñoz, R.; Arias, Y.; Ferreras, J.M.; Rojo, M.A.; Gayoso, M.J.; Nocito, M.; Benitez, J.; Bernabéu, C.; Girbés, T. Targeting a marker of the tumour neovasculature using a novel anti-human CD105-immunotoxin containing the non-toxic type 2 ribosome-inactivating protein nigrin b. Cancer Lett. 2007, 256, 73–80. [Google Scholar] [CrossRef] [PubMed]
- Benitez, J.; Ferreras, J.M.; Muñoz, R.; Arias, Y.; Iglesias, R.; Córdoba-Díaz, M.; del Villar, R.; Girbés, T. Cytotoxicity of an Ebulin l-anti-human CD105 Immunotoxin on mouse fibroblasts (L929) and rat myoblasts (L6E9) cells expressing human CD105. Med. Chem. 2005, 1, 65–70. [Google Scholar] [CrossRef] [PubMed]
- Raab, U.; Velasco, B.; Lastres, P.; Letamendia, A.; Cales, C.; Langa, C.; Tapia, E.; Lopez-Bote, J.P.; Paez, E.; Bernabeu, C. Expression of normal and truncated forms of human endoglin. Biochem. J. 1999, 339, 579–588. [Google Scholar] [CrossRef] [PubMed]
- Antolín, P.; Girotti, A.; Arias, F.J.; Barriuso, B.; Jiménez, P.; Rojo, M.A.; Girbés, T. Bacterial expression of biologically active recombinant musarmin 1 from bulbs of Muscari armeniacum L. and Miller. J. Biotechnol. 2004, 112, 313–322. [Google Scholar] [CrossRef] [PubMed]
- Ferreras, J.M.; Barbieri, L.; Girbés, T.; Battelli, M.G.; Rojo, M.A.; Arias, F.J.; Rocher, M.A.; Soriano, F.; Mendéz, E.; Stirpe, F. Distribution and properties of major ribosome-inactivating proteins (28 S rRNA N-glycosidases) of the plant Saponaria officinalis L. (Caryophyllaceae). Biochim. Biophys. Acta 1993, 1216, 31–42. [Google Scholar] [CrossRef]
- Zeng, M.; Zheng, M.; Lu, D.; Wang, J.; Jiang, W.; Sha, O. Anti-tumor activities and apoptotic mechanism of ribosome-inactivating proteins. Chin. J. Cancer 2015, 34, 325–334. [Google Scholar] [CrossRef] [PubMed]
- Uneda, S.; Toi, H.; Tsujie, T.; Tsujie, M.; Harada, N.; Tsai, H.; Seon, B.K. Anti-endoglin monoclonal antibodies are effective for suppressing metastasis and the primary tumors by targeting tumor vasculature. Int. J. Cancer 2009, 125, 1446–1453. [Google Scholar] [CrossRef] [PubMed]
- Kays, S.K.; Kaufmann, K.B.; Abel, T.; Brendel, C.; Bonig, H.; Grez, M.; Buchholz, C.J.; Kneissl, S. CD105 is a surface marker for receptor-targeted gene transfer into human long-term repopulating hematopoietic stem cells. Stem Cells Dev. 2015, 24, 714–723. [Google Scholar] [CrossRef] [PubMed]
- Bachanova, V.; Frankel, A.E.; Cao, Q.; Lewis, D.; Grzywacz, B.; Verneris, M.R.; Ustun, C.; Lazaryan, A.; McClune, B.; Warlick, E.D.; et al. Phase I study of a bispecific ligand-directed toxin targeting CD22 and CD19 (DT2219) for refractory B-cell malignancies. Clin. Cancer Res. 2015, 21, 1267–1272. [Google Scholar] [CrossRef] [PubMed]
- Dinndorf, P.; Krailo, M.; Liu-Mares, W.; Frierdich, S.; Sondel, P.; Reaman, G. Phase I trial of anti-B4-blocked ricin in pediatric patients with leukemia and lymphoma. J. Immunother. 2001, 24, 511–516. [Google Scholar] [CrossRef] [PubMed]
- Herrera, L.; Bostrom, B.; Gore, L.; Sandler, E.; Lew, G.; Schlegel, P.G.; Aquino, V.; Ghetie, V.; Vitetta, E.S.; Schindler, J. A phase 1 study of Combotox in pediatric patients with refractory B-lineage acute lymphoblastic leukemia. J. Pedriatr. Hematol. Oncol. 2009, 31, 936–941. [Google Scholar] [CrossRef] [PubMed]
- Baluna, R.; Vitetta, E.S. Vascular leak syndrome: A side effect of immunotherapy. Immunopharmacology 1997, 37, 117–132. [Google Scholar] [CrossRef]
- Furman, R.R.; Grossbard, M.L.; Johnson, J.L.; Pecora, A.L.; Cassileth, P.A.; Jung, S.H.; Peterson, B.A.; Nadler, L.M.; Freedman, A.; Bayer, R.L.; et al. A phase III study of anti-B4-blocked ricin as adjuvant therapy post-autologous bone marrow transplant: CALGB 9254. Leuk. Lymphoma 2011, 52, 587–596. [Google Scholar] [CrossRef] [PubMed]
- Mazor, R.; Onda, M.; Pastan, I. Immunogenicity of therapeutic recombinant immunotoxins. Immunol. Rev. 2016, 270, 152–164. [Google Scholar] [CrossRef] [PubMed]
- Toi, H.; Tsujie, M.; Haruta, Y.; Fujita, K.; Duzen, J.; Seon, B.K. Facilitation of endoglin-targeting cancer therapy by development/utilization of a novel genetically engineered mouse model expressing humanized endoglin (CD105). Int. J. Cancer 2015, 136, 452–461. [Google Scholar] [CrossRef] [PubMed]
- Bellón, T.; Corbí, A.; Lastres, P.; Cales, C.; Cebrián, M.; Vera, S.; Cheifetz, S.; Massague, J.; Letarte, M.; Bernabéu, C. Identification and expression of two forms of the human transforming growth factor-beta-binding protein endoglin with distinct cytoplasmic regions. Eur. J. Immunol. 1993, 23, 2340–2345. [Google Scholar] [CrossRef] [PubMed]
- Shih, N.J.; McDonald, K.A.; Girbés, T.; Iglesias, R.; Kohlhoff, A.J.; Jackman, A.P. Ribosome-inactivating proteins (RIPs) of wild Oregon cucumber (Marah oreganus). Biol. Chem. 1998, 379, 721–725. [Google Scholar] [PubMed]




© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Barriuso, B.; Antolín, P.; Arias, F.J.; Girotti, A.; Jiménez, P.; Cordoba-Diaz, M.; Cordoba-Diaz, D.; Girbés, T. Anti-Human Endoglin (hCD105) Immunotoxin—Containing Recombinant Single Chain Ribosome-Inactivating Protein Musarmin 1. Toxins 2016, 8, 184. https://doi.org/10.3390/toxins8060184
Barriuso B, Antolín P, Arias FJ, Girotti A, Jiménez P, Cordoba-Diaz M, Cordoba-Diaz D, Girbés T. Anti-Human Endoglin (hCD105) Immunotoxin—Containing Recombinant Single Chain Ribosome-Inactivating Protein Musarmin 1. Toxins. 2016; 8(6):184. https://doi.org/10.3390/toxins8060184
Chicago/Turabian StyleBarriuso, Begoña, Pilar Antolín, F. Javier Arias, Alessandra Girotti, Pilar Jiménez, Manuel Cordoba-Diaz, Damián Cordoba-Diaz, and Tomás Girbés. 2016. "Anti-Human Endoglin (hCD105) Immunotoxin—Containing Recombinant Single Chain Ribosome-Inactivating Protein Musarmin 1" Toxins 8, no. 6: 184. https://doi.org/10.3390/toxins8060184