Neurophysiological Measures of Efficacy and Safety for Botulinum Toxin Injection in Facial and Bulbar Muscles: Special Considerations
Abstract
:1. Introduction
2. Indications and Special Considerations of Safety Regarding BoNT Applications in Facial and Bulbar Muscles
2.1. General Indications and Mode of Action
2.2. Spreading of the Toxin’s Effect Can Give Rise to Undesired Side Effects
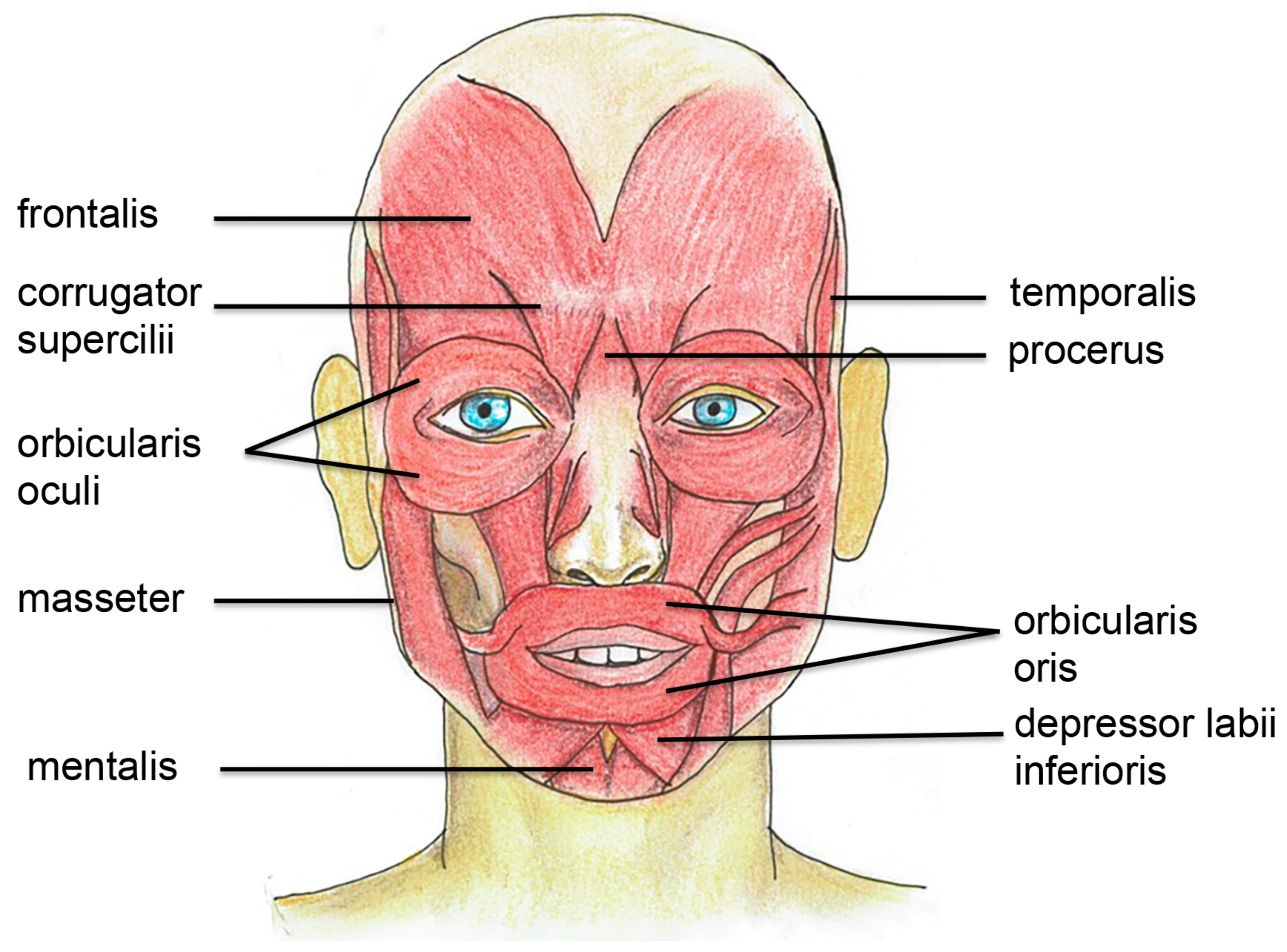
3. Muscle Anatomy and Physiology in the Facial and Bulbar Area
3.1. Facial Muscles are Extra Sensitive to Denervation
3.2. Specific Target Treatment in Facial Muscles
3.2.1. Glabellar Muscle Complex
3.2.2. Frontalis Muscle and Horizontal Rhytids
3.2.3. Orbicularis Oculi Muscle
3.2.4. Brow Lift
3.2.5. Orbicularis Oris, Mentalis Muscle, and Lip Levator
3.2.6. Masseter Hypertrophy
4. Neurophysiological Measures of BoNT Effect in Facial Muscles: Important Objective Parameters and Guiding Techniques
4.1. Application of Neurophysiological Measures
4.2. CMAP and EMG as Indicators of BoNT Effect
4.3. Ultrastructural Muscle Changes Indicating Denervation after BoNT Injection
4.4. Terminal Sprouting Causes Reinnervation
5. Considerations to “Split Face” Design: Spread of BoNT in the Facial and Bulbar Area
5.1. Possible Diffusion and Migration Mechanisms of BoNT
5.2. Glabellar and Frontalis Muscle Area
5.3. Orbicularis Oculi
Acknowledgements
Conflicts of Interest
References
- Rossetto, O.; Pirazzini, M.; Montecucco, C. Botulinum neurotoxins: Genetic, structural and mechanistic insights. Nat. Rev. Microbiol. 2014, 12, 535–549. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Masuyer, G.; Zhang, J.; Shen, Y.; Lundin, D.; Henriksson, L.; Miyashita, S.-I.; Martínez-Carranza, M.; Dong, M.; Stenmark, P. Identification and characterization of a novel botulinum neurotoxin. Nat. Commun. 2017, 8, 14130. [Google Scholar]
- Zornetta, I.; Azarnia Tehran, D.; Arrigoni, G.; Anniballi, F.; Bano, L.; Leka, O.; Zanotti, G.; Binz, T.; Montecucco, C. The first non Clostridial botulinum-like toxin cleaves VAMP within the juxtamembrane domain. Sci. Rep. 2016, 6, 30257. [Google Scholar] [CrossRef] [PubMed]
- Pirazzini, M.; Rossetto, O.; Eleopra, R.; Montecucco, C. Botulinum Neurotoxins: Biology, Pharmacology, and Toxicology. Pharmacol. Rev. 2017, 69, 200–235. [Google Scholar] [CrossRef] [PubMed]
- Azarnia Tehran, D.; Pirazzini, M.; Leka, O.; Mattarei, A.; Lista, F.; Binz, T.; Rossetto, O.; Montecucco, C. Hsp90 is involved in the entry of clostridial neurotoxins into the cytosol of nerve terminals. Cell Microbiol. 2017, 19. [Google Scholar] [CrossRef] [PubMed]
- Pirazzini, M.; Azarnia Tehran, D.; Zanetti, G.; Megighian, A.; Scorzeto, M.; Fillo, S.; Shone, C.C.; Binz, T.; Rossetto, O.; Lista, F.; et al. Thioredoxin and its reductase are present on synaptic vesicles, and their inhibition prevents the paralysis induced by botulinum neurotoxins. Cell Rep. 2014, 8, 1870–1878. [Google Scholar] [CrossRef] [PubMed]
- Zanetti, G.; Azarnia Tehran, D.; Pirazzini, M.; Binz, T.; Shone, C.C.; Fillo, S.; Lista, F.; Rossetto, O.; Montecucco, C. Inhibition of botulinum neurotoxins interchain disulfide bond reduction prevents the peripheral neuroparalysis of botulism. Biochem. Pharmacol. 2015, 98, 522–530. [Google Scholar] [CrossRef] [PubMed]
- Blasi, J.; Chapman, E.R.; Link, E.; Binz, T.; Yamasaki, S.; De Camilli, P.; Südhof, T.C.; Niemann, H.; Jahn, R. Botulinum neurotoxin A selectively cleaves the synaptic protein SNAP-25. Nature 1993, 365, 160–163. [Google Scholar]
- Lucioni, A.; Bales, G.T.; Lotan, T.L.; McGehee, D.S.; Cook, S.P.; Rapp, D.E. Botulinum toxin type A inhibits sensory neuropeptide release in rat bladder models of acute injury and chronic inflammation. BJU Int. 2008, 101, 366–370. [Google Scholar] [CrossRef] [PubMed]
- Erbguth, F.; Brittner, W.; Fogel, W.; Hefter, H.; Herting, B.; von Lindern, J.J.; Umstadt, H.E. Botulinum toxin in migraine. J. Neurol. 2004, 251 (Suppl. 1), i31–i32. [Google Scholar] [CrossRef] [PubMed]
- Erbguth, F.J. Historical notes on botulism, Clostridium botulinum, botulinum toxin, and the idea of the therapeutic use of the toxin. Mov. Disord. Off. J. Mov. Disord. Soc. 2004, 19 (Suppl. S8), S2–S6. [Google Scholar] [CrossRef] [PubMed]
- Jankovic, J.; Brin, M.F. Botulinum toxin: Historical perspective and potential new indications. Muscle Nerve Suppl. 1997, 6, S129–S145. [Google Scholar] [CrossRef]
- Wu, D.C.; Fabi, S.G.; Goldman, M.P. Neurotoxins: Current Concepts in Cosmetic Use on the Face and Neck--Lower Face. Plast. Reconstr. Surg. 2015, 136, 76S–79S. [Google Scholar] [CrossRef] [PubMed]
- Cote, T.R.; Mohan, A.K.; Polder, J.A.; Walton, M.K.; Braun, M.M. Botulinum toxin type A injections: Adverse events reported to the US Food and Drug Administration in therapeutic and cosmetic cases. J. Am. Acad. Dermatol. 2005, 53, 407–415. [Google Scholar] [CrossRef] [PubMed]
- Albavera-Hernandez, C.; Rodriguez, J.M.; Idrovo, A.J. Safety of botulinum toxin type A among children with spasticity secondary to cerebral palsy: A systematic review of randomized clinical trials. Clin. Rehabil. 2009, 23, 394–407. [Google Scholar] [CrossRef] [PubMed]
- Apkon, S.D.; Cassidy, D. Safety considerations in the use of botulinum toxins in children with cerebral palsy. PM R 2010, 2, 282–284. [Google Scholar] [CrossRef] [PubMed]
- Nong, L.B.; He, W.Q.; Xu, Y.H.; Liu, X.Q.; Li, Y.M.; Xiao, Z.L.; Zhong, N.S. Severe respiratory failure after injection of botulinum toxin: Case report and review of the literature. Zhonghua Jie He He Hu Xi Za Zhi 2008, 31, 369–371. [Google Scholar] [PubMed]
- Yiannakopoulou, E. Serious and long-term adverse events associated with the therapeutic and cosmetic use of botulinum toxin. Pharmacology 2015, 95, 65–69. [Google Scholar] [CrossRef] [PubMed]
- Cavallini, M.; Cirillo, P.; Fundaro, S.P.; Quartucci, S.; Sciuto, C.; Sito, G.; Tonini, D.; Trocchi, G.; Signorini, M. Safety of botulinum toxin A in aesthetic treatments: A systematic review of clinical studies. Dermatol. Surg. Off. Publ. Am. Soc. Dermatol. Surg. 2014, 40, 525–536. [Google Scholar] [CrossRef] [PubMed]
- Eleopra, R.; Tugnoli, V.; Caniatti, L.; De Grandis, D. Botulinum toxin treatment in the facial muscles of humans: Evidence of an action in untreated near muscles by peripheral local diffusion. Neurology 1996, 46, 1158–1160. [Google Scholar] [CrossRef] [PubMed]
- Duregotti, E.; Zanetti, G.; Scorzeto, M.; Megighian, A.; Montecucco, C.; Pirazzini, M.; Rigoni, M. Snake and Spider Toxins Induce a Rapid Recovery of Function of Botulinum Neurotoxin Paralysed Neuromuscular Junction. Toxins (Basel) 2015, 7, 5322–5336. [Google Scholar] [CrossRef] [PubMed]
- Morbiato, L.; Carli, L.; Johnson, E.A.; Montecucco, C.; Molgo, J.; Rossetto, O. Neuromuscular paralysis and recovery in mice injected with botulinum neurotoxins A and C. Eur. J. Neurosci. 2007, 25, 2697–2704. [Google Scholar] [CrossRef] [PubMed]
- Pun, S.; Sigrist, M.; Santos, A.F.; Ruegg, M.A.; Sanes, J.R.; Jessell, T.M.; Arber, S.; Caroni, P. An intrinsic distinction in neuromuscular junction assembly and maintenance in different skeletal muscles. Neuron 2002, 34, 357–370. [Google Scholar] [CrossRef]
- Punga, A.R.; Maj, M.; Lin, S.; Meinen, S.; Ruegg, M.A. MuSK levels differ between adult skeletal muscles and influence postsynaptic plasticity. Eur. J. Neurosci. 2011, 33, 890–898. [Google Scholar] [CrossRef] [PubMed]
- Punga, A.R.; Lin, S.; Oliveri, F.; Meinen, S.; Ruegg, M.A. Muscle-selective synaptic disassembly and reorganization in MuSK antibody positive MG mice. Exp. Neurol. 2011, 230, 207–217. [Google Scholar] [CrossRef] [PubMed]
- Kane, M.A.; Gold, M.H.; Coleman, W.P., 3rd; Jones, D.H.; Tanghetti, E.A.; Alster, T.S.; Rohrer, T.E.; Burgess, C.M.; Shamban, A.T.; Finn, E. A Randomized, Double-Blind Trial to Investigate the Equivalence of IncobotulinumtoxinA and OnabotulinumtoxinA for Glabellar Frown Lines. Dermatol. Surg. Off. Publ. Am. Soc. Dermatol. Surg. 2015, 41, 1310–1319. [Google Scholar]
- Solish, N.; Rivers, J.K.; Humphrey, S.; Muhn, C.; Somogyi, C.; Lei, X.; Bhogal, M.; Caulkins, C. Efficacy and Safety of OnabotulinumtoxinA Treatment of Forehead Lines: A Multicenter, Randomized, Dose-Ranging Controlled Trial. Dermatol. Surg. Off. Publ. Am. Soc. Dermatol. Surg. 2016, 42, 410–419. [Google Scholar] [CrossRef] [PubMed]
- Pierard, G.E.; Lapiere, C.M. The microanatomical basis of facial frown lines. Arch. Dermatol. 1989, 125, 1090–1092. [Google Scholar] [CrossRef] [PubMed]
- Carruthers, A.; Carruthers, J.; Cohen, J. A prospective, double-blind, randomized, parallel-group, dose-ranging study of botulinum toxin type a in female subjects with horizontal forehead rhytides. Dermatol. Surg. Off. Publ. Am. Soc. Dermatol. Surg. 2003, 29, 461–467. [Google Scholar]
- Spiegel, J.H.; Goerig, R.C.; Lufler, R.S.; Hoagland, T.M. Frontalis midline dehiscence: An anatomical study and discussion of clinical relevance. J. Plast. Reconstr. Aesthet. Surg. 2009, 62, 950–954. [Google Scholar] [CrossRef] [PubMed]
- Blitzer, A.; Binder, W.J. Current practices in the use of botulinum toxin A in the management of facial lines and wrinkles. Facial Plast. Surg. Clin. N. Am. 2001, 9, 395–404. [Google Scholar]
- Isik, D.; Tekin, H.; Karadag, R.; Bilgili, S.G.; Atik, B. Effect of brow lifting using botulinum a toxin on upper eyelid height in patients with ptosis undergoing the frontal sling technique. Ann. Plast. Surg. 2013, 70, 175–179. [Google Scholar] [CrossRef] [PubMed]
- Dinker, S.; Anitha, A.; Sorake, A.; Kumar, K. Management of gummy smile with Botulinum Toxin Type-A: A case report. J. Int. Oral Health 2014, 6, 111–115. [Google Scholar] [PubMed]
- Park, M.Y.; Ahn, K.Y.; Jung, D.S. Botulinum toxin type A treatment for contouring of the lower face. Dermatol. Surg. Off. Publ. Am. Soc. Dermatol. Surg. 2003, 29, 477–483. [Google Scholar]
- Wu, W.T. Botox facial slimming/facial sculpting: The role of botulinum toxin-A in the treatment of hypertrophic masseteric muscle and parotid enlargement to narrow the lower facial width. Facial Plast. Surg. Clin. N. Am. 2010, 18, 133–140. [Google Scholar] [CrossRef] [PubMed]
- Bas, B.; Ozan, B.; Muglali, M.; Celebi, N. Treatment of masseteric hypertrophy with botulinum toxin: A report of two cases. Med. Oral Patol. Oral Cir. Bucal 2010, 15, e649–e652. [Google Scholar] [CrossRef] [PubMed]
- Baker, J.S.; Nolan, P.J. Effectiveness of botulinum toxin type A for the treatment of chronic masticatory myofascial pain: A case series. J. Am. Dent. Assoc. 2017, 148, 33–39. [Google Scholar] [CrossRef] [PubMed]
- Comella, C.L.; Buchman, A.S.; Tanner, C.M.; Brown-Toms, N.C.; Goetz, C.G. Botulinum toxin injection for spasmodic torticollis: Increased magnitude of benefit with electromyographic assistance. Neurology 1992, 42, 878–882. [Google Scholar] [CrossRef] [PubMed]
- Werdelin, L.; Dalager, T.; Fuglsang-Frederiksen, A.; Regeur, L.; Karlsborg, M.; Korbo, L.; Munck, O.; Winge, K. The utility of EMG interference pattern analysis in botulinum toxin treatment of torticollis: A randomised, controlled and blinded study. Clin. Neurophysiol. 2011, 122, 2305–2309. [Google Scholar] [CrossRef] [PubMed]
- Flynn, T.C.; Carruthers, A.; Carruthers, J.; Geister, T.L.; Gortelmeyer, R.; Hardas, B.; Himmrich, S.; Kerscher, M.; de Maio, M.; Mohrmann, C.; et al. Validated assessment scales for the upper face. Dermatol. Surg. Off. Publ. Am. Soc. Dermatol. Surg. 2012, 38, 309–319. [Google Scholar] [CrossRef] [PubMed]
- Honeck, P.; Weiss, C.; Sterry, W.; Rzany, B. Reproducibility of a four-point clinical severity score for glabellar frown lines. Br. J. Dermatol. 2003, 149, 306–310. [Google Scholar] [CrossRef] [PubMed]
- De Almeida, A.R.; da Costa Marques, E.R.; Banegas, R.; Kadunc, B.V. Glabellar Contraction Patterns: A Tool to Optimize Botulinum Toxin Treatment. Dermatol. Surg. Off. Publ. Am. Soc. Dermatol. Surg. 2012, 38, 1506–1515. [Google Scholar] [CrossRef] [PubMed]
- Nestor, M.S.; Ablon, G.R. The frontalis activity measurement standard: A novel contralateral method for assessing botulinum neurotoxin type-A activity. J. Drugs Dermatol. 2011, 10, 968–972. [Google Scholar] [PubMed]
- Wabbels, B.; Roggenkamper, P. Botulinum toxin in hemifacial spasm: The challenge to assess the effect of treatment. J. Neural Transm. 2012, 119, 963–980. [Google Scholar] [CrossRef] [PubMed]
- Arimura, K.; Arimura, Y.; Takata, Y.; Nakamura, T.; Kaji, R. Comparative electrophysiological study of response to botulinum toxin type B in Japanese and Caucasians. Mov. Disord. Off. J. Mov. Disord. Soc. 2008, 23, 240–245. [Google Scholar] [CrossRef] [PubMed]
- Wohlfarth, K.; Muller, C.; Sassin, I.; Comes, G.; Grafe, S. Neurophysiological double-blind trial of a botulinum neurotoxin type a free of complexing proteins. Clin. Neuropharmacol. 2007, 30, 86–94. [Google Scholar] [CrossRef] [PubMed]
- Wohlfarth, K.; Schwandt, I.; Wegner, F.; Jurgens, T.; Gelbrich, G.; Wagner, A.; Bogdahn, U.; Schulte-Mattler, W. Biological activity of two botulinum toxin type A complexes (Dysport and Botox) in volunteers: A double-blind, randomized, dose-ranging study. J. Neurol. 2008, 255, 1932–1939. [Google Scholar] [CrossRef] [PubMed]
- Hamjian, J.A.; Walker, F.O. Serial neurophysiological studies of intramuscular botulinum-A toxin in humans. Muscle Nerve 1994, 17, 1385–1392. [Google Scholar] [CrossRef] [PubMed]
- Sloop, R.R.; Escutin, R.O.; Matus, J.A.; Cole, B.A.; Peterson, G.W. Dose-response curve of human extensor digitorum brevis muscle function to intramuscularly injected botulinum toxin type A. Neurology 1996, 46, 1382–1386. [Google Scholar] [CrossRef] [PubMed]
- Alimohammadi, M.; Andersson, M.; Punga, A.R. Correlation of Botulinum Toxin Dose with Neurophysiological Parameters of Efficacy and Safety in the Glabellar Muscles: A Double-blind, Placebo-controlled, Randomized Study. Acta Derm. Venereol. 2013, 94, 32–37. [Google Scholar] [CrossRef] [PubMed]
- Saybel, A.; Artemenko, A.; Nikitin, S.; Kurenkov, A. A Prospective, Neurophysiologic Comparative Study to Assess the Efficacy and Duration of Effect of IncobotulinumtoxinA and AbobotulinumtoxinA in the Treatment of Crow’s Feet. J. Drugs Dermatol. 2015, 14, 1291–1296. [Google Scholar] [PubMed]
- Kim, J.H.; Shin, J.H.; Kim, S.T.; Kim, C.Y. Effects of two different units of botulinum toxin type a evaluated by computed tomography and electromyographic measurements of human masseter muscle. Plast. Reconstr. Surg. 2007, 119, 711–717. [Google Scholar] [CrossRef] [PubMed]
- Ma, F.; Zhai, Z.; Zhu, S.; Tang, S. Ultrastructural changes in human masseter muscles after botulinum neurotoxin a injection. Muscle Nerve 2017. [Google Scholar] [CrossRef] [PubMed]
- Dengis, C.A.; Steinbach, M.J.; Kraft, S.P. Registered eye position: Short- and long-term effects of botulinum toxin injected into eye muscle. Exp. Brain Res. 1998, 119, 475–482. [Google Scholar] [CrossRef] [PubMed]
- Suh, Y.W.; Uhm, C.S.; Cho, Y.A. Ultrastructural changes in myotendinous nerve endings induced by injection of botulinum toxin into the extraocular muscle. Graefes Arch. Clin. Exp. Ophthalmol. 2010, 248, 1795–1801. [Google Scholar]
- Elmas, C.; Ayhan, S.; Tuncer, S.; Erdogan, D.; Calguner, E.; Basterzi, Y.; Gozil, R.; Bahcelioglu, M. Effect of fresh and stored botulinum toxin a on muscle and nerve ultrastructure: An electron microscopic study. Ann. Plast. Surg. 2007, 59, 316–322. [Google Scholar] [CrossRef] [PubMed]
- De Paiva, A.; Meunier, F.A.; Molgo, J.; Aoki, K.R.; Dolly, J.O. Functional repair of motor endplates after botulinum neurotoxin type A poisoning: Biphasic switch of synaptic activity between nerve sprouts and their parent terminals. Proc. Natl. Acad. Sci. USA 1999, 96, 3200–3205. [Google Scholar] [CrossRef] [PubMed]
- Thesleff, S. Supersensitivity of skeletal muscle produced by botulinum toxin. J. Physiol. 1960, 151, 598–607. [Google Scholar] [CrossRef] [PubMed]
- Wright, M.C.; Cho, W.J.; Son, Y.J. Distinct patterns of motor nerve terminal sprouting induced by ciliary neurotrophic factor vs. botulinum toxin. J. Comp. Neurol. 2007, 504, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Eleopra, R.; Tugnoli, V.; Rossetto, O.; De Grandis, D.; Montecucco, C. Different time courses of recovery after poisoning with botulinum neurotoxin serotypes A and E in humans. Neurosci. Lett. 1998, 256, 135–138. [Google Scholar] [CrossRef]
- Punga, A.R.; Alimohammadi, M.; Fagrell, D.; Nyberg, F.; Rees, D.; Wong, C. A Randomized, Comparative Study to Evaluate Efficacy and Safety of Two Injection Volumes of AbobotulinumtoxinA in Treatment of Glabellar Lines. Dermatol. Surg. Off. Publ. Am. Soc. Dermatol. Surg. 2016, 42, 967–976. [Google Scholar] [CrossRef] [PubMed]
- Allen, S.B.; Goldenberg, N.A. Pain difference associated with injection of abobotulinumtoxinA reconstituted with preserved saline and preservative-free saline: A prospective, randomized, side-by-side, double-blind study. Dermatol. Surg. Off. Publ. Am. Soc. Dermatol. Surg. 2012, 38, 867–870. [Google Scholar] [CrossRef] [PubMed]
- Choi, J.W.; Youn, C.S.; An, H.T.; Yoo, J.Y.; Na, J.I.; Park, K.C.; Youn, S.W.; Huh, C.H. Combined use of botulinum toxin type A and B for forehead rhytides: A randomized, double-blind, split-face study. J. Dermatol. Treat. 2013, 24, 126–132. [Google Scholar] [CrossRef] [PubMed]
- Yeilding, R.H.; Fezza, J.P. A Prospective, Split-Face, Randomized, Double-Blind Study Comparing OnabotulinumtoxinA to IncobotulinumtoxinA for Upper Face Wrinkles. Plast. Reconstr. Surg. 2015, 135, 1328–1335. [Google Scholar] [CrossRef] [PubMed]
- Hallett, M. Explanation of timing of botulinum neurotoxin effects, onset and duration, and clinical ways of influencing them. Toxicon 2015, 107, 64–67. [Google Scholar] [CrossRef] [PubMed]
- Ramirez-Castaneda, J.; Jankovic, J.; Comella, C.; Dashtipour, K.; Fernandez, H.H.; Mari, Z. Diffusion, spread, and migration of botulinum toxin. Mov. Disord. Off. J. Mov. Disord. Soc. 2013, 28, 1775–1783. [Google Scholar] [CrossRef] [PubMed]
- Curra, A.; Berardelli, A. Do the unintended actions of botulinum toxin at distant sites have clinical implications? Neurology 2009, 72, 1095–1099. [Google Scholar] [CrossRef] [PubMed]
- Roche, N.; Schnitzler, A.; Genet, F.F.; Durand, M.C.; Bensmail, D. Undesirable distant effects following botulinum toxin type a injection. Clin. Neuropharmacol. 2008, 31, 272–280. [Google Scholar] [CrossRef] [PubMed]
- Carli, L.; Montecucco, C.; Rossetto, O. Assay of diffusion of different botulinum neurotoxin type a formulations injected in the mouse leg. Muscle Nerve 2009, 40, 374–380. [Google Scholar] [CrossRef] [PubMed]
- Dodd, S.L.; Rowell, B.A.; Vrabas, I.S.; Arrowsmith, R.J.; Weatherill, P.J. A comparison of the spread of three formulations of botulinum neurotoxin A as determined by effects on muscle function. Eur. J. Neurol. 1998, 5, 181–186. [Google Scholar] [CrossRef] [PubMed]
- Borodic, G.E.; Joseph, M.; Fay, L.; Cozzolino, D.; Ferrante, R.J. Botulinum A toxin for the treatment of spasmodic torticollis: dysphagia and regional toxin spread. Head Neck 1990, 12, 392–399. [Google Scholar] [CrossRef] [PubMed]
- Yaraskavitch, M.; Leonard, T.; Herzog, W. Botox produces functional weakness in non-injected muscles adjacent to the target muscle. J. Biomech. 2008, 41, 897–902. [Google Scholar] [CrossRef] [PubMed]
- Shaari, C.M.; George, E.; Wu, B.L.; Biller, H.F.; Sanders, I. Quantifying the spread of botulinum toxin through muscle fascia. Laryngoscope 1991, 101, 960–964. [Google Scholar] [CrossRef] [PubMed]
- Girlanda, P.; Quartarone, A.; Sinicropi, S.; Nicolosi, C.; Messina, C. Unilateral injection of botulinum toxin in blepharospasm: Single fiber electromyography and blink reflex study. Mov. Disord. Off. J. Mov. Disord. Soc. 1996, 11, 27–31. [Google Scholar] [CrossRef] [PubMed]
- Punga, A.R.; Eriksson, A.; Alimohammadi, M. Regional diffusion of botulinum toxin in facial muscles: A randomised double-blind study and a consideration for clinical studies with split-face design. Acta Derm. Venereol. 2015, 95, 948–951. [Google Scholar] [CrossRef] [PubMed]
- Sanders, D.B.; Massey, E.W.; Buckley, E.G. Botulinum toxin for blepharospasm: Single-fiber EMG studies. Neurology 1986, 36, 545–547. [Google Scholar] [CrossRef] [PubMed]
- Lorenzano, C.; Bagnato, S.; Gilio, F.; Fabbrini, G.; Berardelli, A. No clinical or neurophysiological evidence of botulinum toxin diffusion to non-injected muscles in patients with hemifacial spasm. Neurotox. Res. 2006, 9, 141–144. [Google Scholar] [CrossRef] [PubMed]
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alimohammadi, M.; Punga, A.R. Neurophysiological Measures of Efficacy and Safety for Botulinum Toxin Injection in Facial and Bulbar Muscles: Special Considerations. Toxins 2017, 9, 352. https://doi.org/10.3390/toxins9110352
Alimohammadi M, Punga AR. Neurophysiological Measures of Efficacy and Safety for Botulinum Toxin Injection in Facial and Bulbar Muscles: Special Considerations. Toxins. 2017; 9(11):352. https://doi.org/10.3390/toxins9110352
Chicago/Turabian StyleAlimohammadi, Mohammad, and Anna Rostedt Punga. 2017. "Neurophysiological Measures of Efficacy and Safety for Botulinum Toxin Injection in Facial and Bulbar Muscles: Special Considerations" Toxins 9, no. 11: 352. https://doi.org/10.3390/toxins9110352




