Adsorption of Ten Microcystin Congeners to Common Laboratory-Ware Is Solvent and Surface Dependent
Abstract
:1. Introduction
2. Results
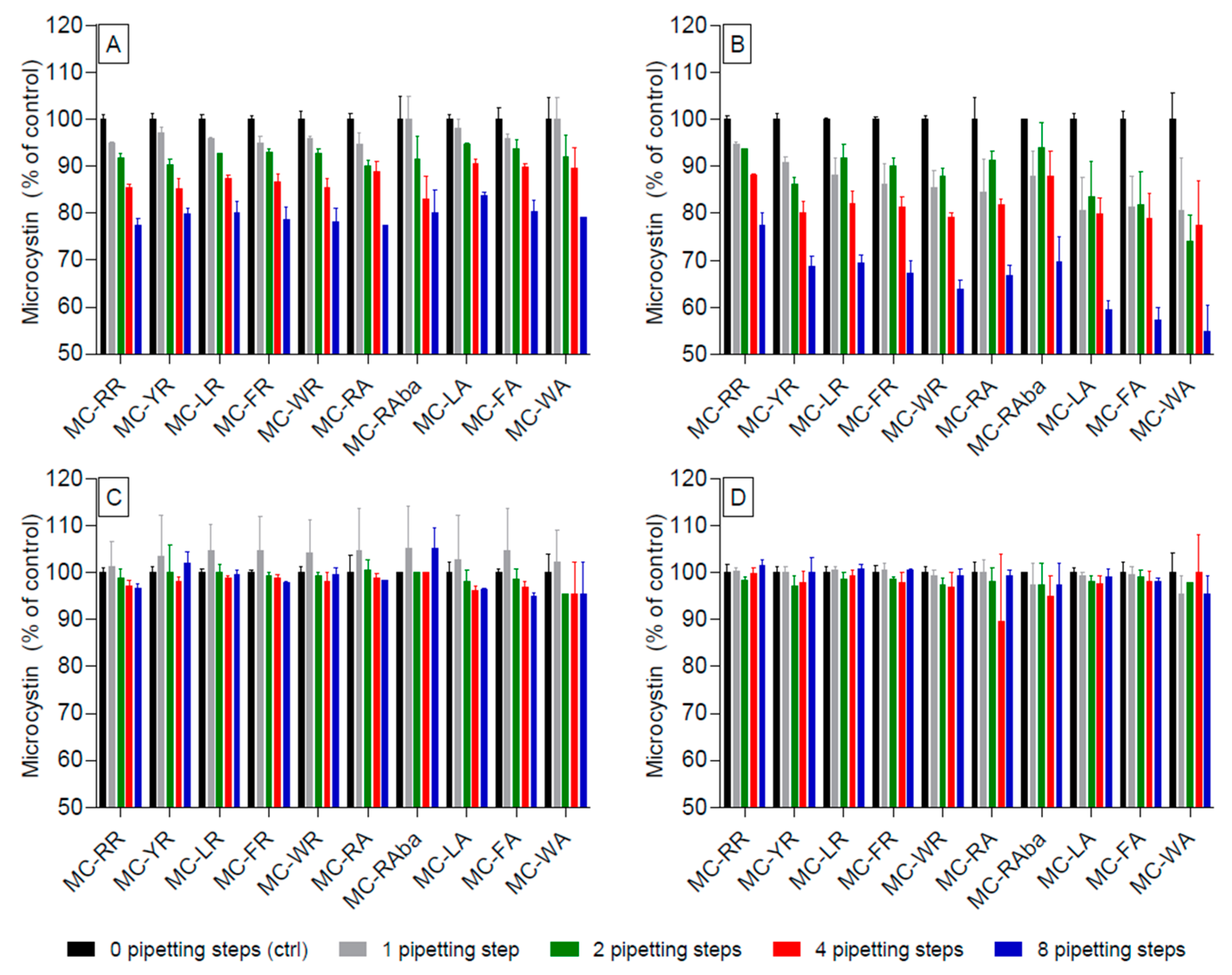
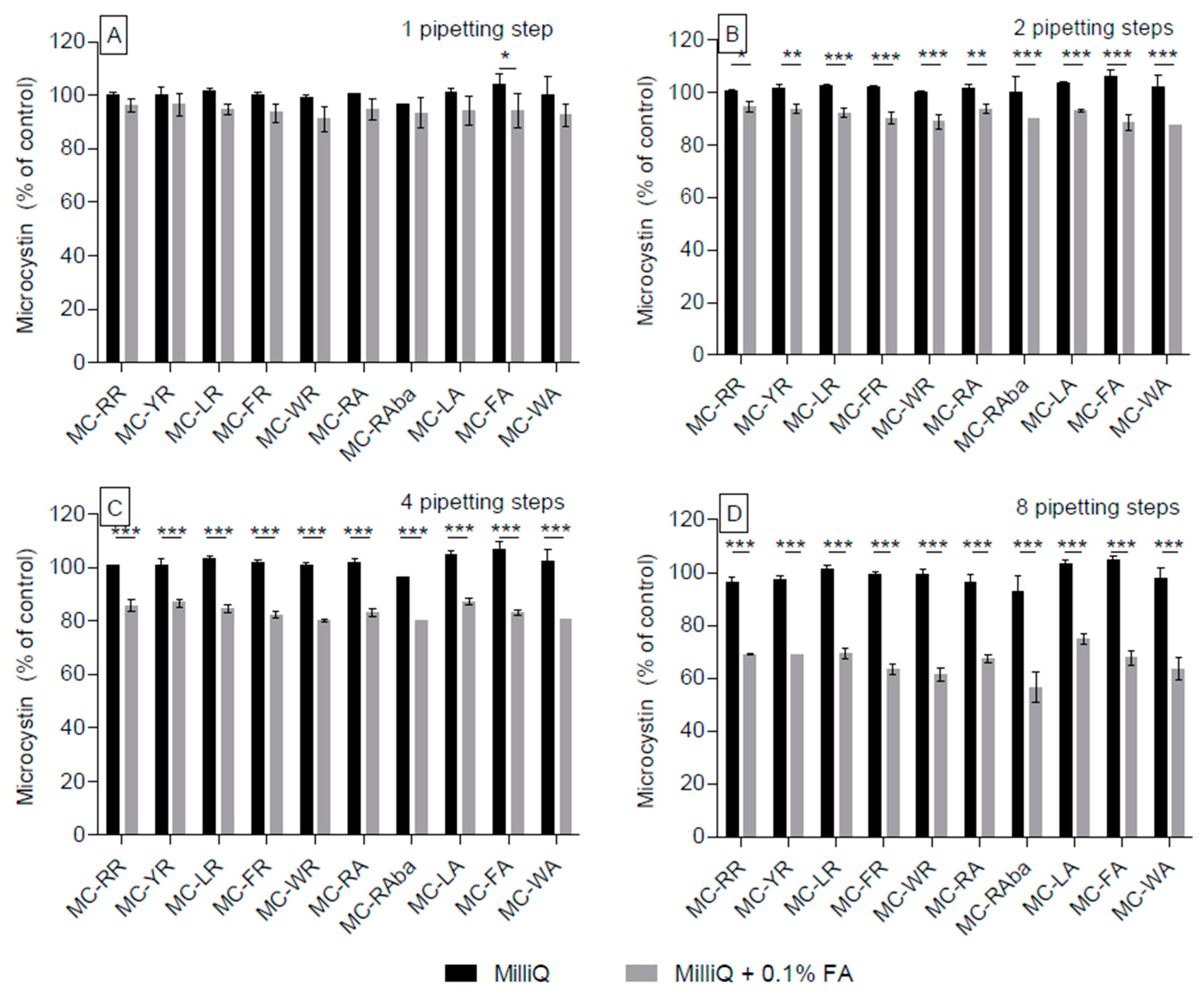
2.1. Adsorption of Microcystin Congeners to Polypropylene Pipette Tips in Aqueous and High-Percentage Methanol Solutions
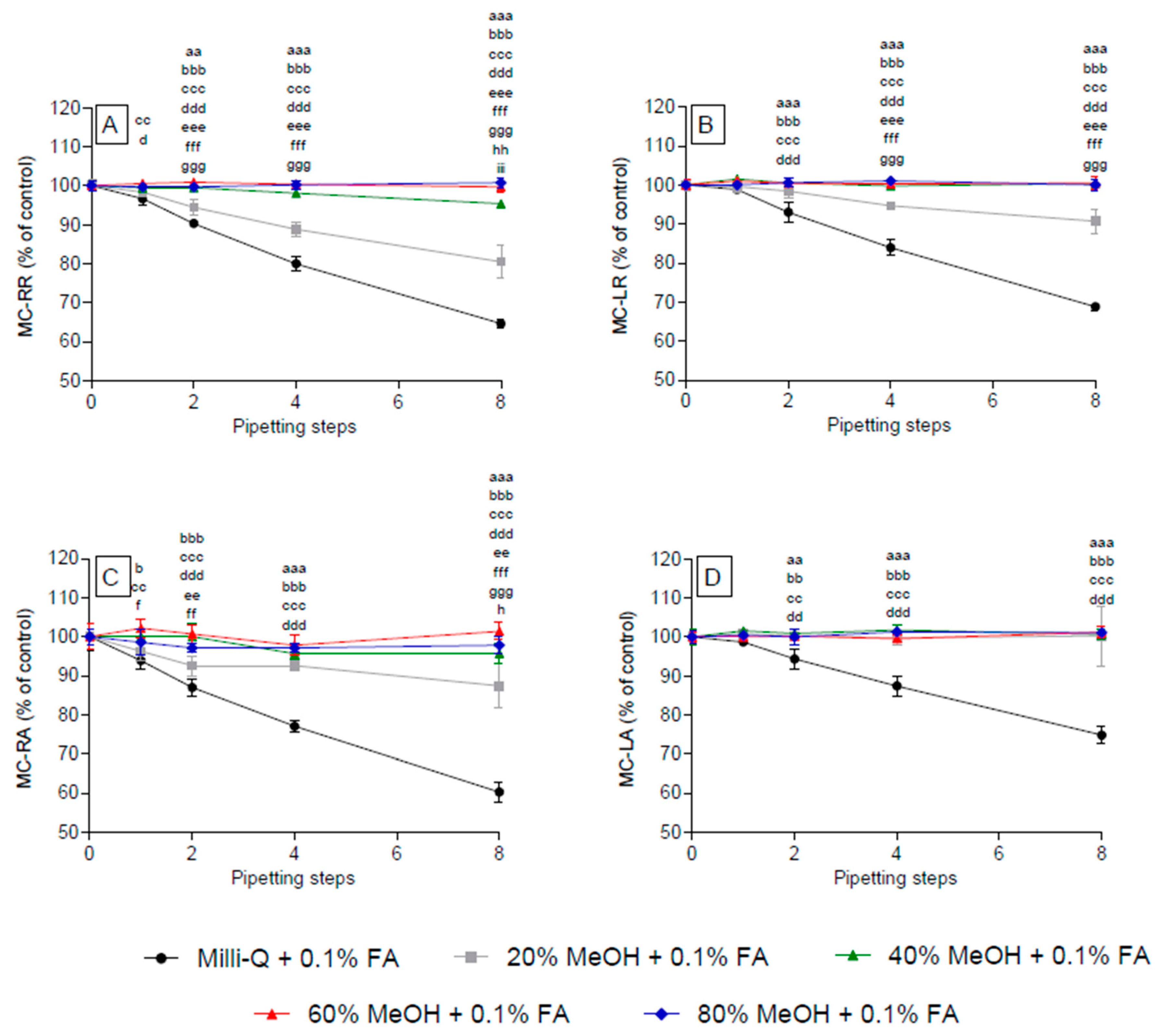
2.2. Effect of Methanol Concentration on the Adsorption of Selected Microcystins to Polypropylene Pipette Tips
2.3. Effect of Acidified Methanol Concentration on the Adsorption of Selected Microcystins (MC) to Polypropylene Pipette Tips
2.4. Adsorption of Selected Microcystins (MC) in Acidified and Non-Acidified Aqueous Solutions to Glass-Ware (Pasteur Pipettes)
2.5. Effect of Acidified Methanol Concentration on the Adsorption of Selected Microcystins to Glass-Ware (Pasteur Pipettes)
2.6. Short Term Storage of MC Solutions in Glass or Polypropylene Vials
3. Discussion
4. Conclusions
5. Materials and Methods
5.1. Reagents and Laboratory-Ware
5.2. Production of Microcystin Congener Stock Solution
5.3. Adsorption of Microcystins to Common Pipetting Laboratory-Ware in Non-Acidified and Acidified Solvents
5.4. Short-Term Storage in Glass or Polypropylene Vials
5.5. Ultra-performance liquid Chromatography-Tandem Mass Spectrometry (UPLC-MS/MS) Detection of Microcystins
5.6. Outlier Analysis
5.7. Data Handling and Statistical Analyses
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Mackintosh, C.; Beattie, K.A.; Klumpp, S.; Cohen, P.; Codd, G.A. Cyanobacterial microcystin-LR is a potent and specific inhibitor of protein phosphatase-1 and phosphatase-2a from both mammals and higher-plants. FEBS Lett. 1990, 264, 187–192. [Google Scholar] [CrossRef]
- Fischer, W.J.; Altheimer, S.; Cattori, V.; Meier, P.J.; Dietrich, D.R.; Hagenbuch, B. Organic anion transporting polypeptides expressed in liver and brain mediate uptake of microcystin. Toxicol. Appl. Pharmacol. 2005, 203, 257–263. [Google Scholar] [CrossRef] [PubMed]
- Falconer, I.R.; Yeung, D.S. Cytoskeletal changes in hepatocytes induced by Microcystis toxins and their relation to hyperphosphorylation of cell proteins. Chem. Biol. Interact. 1992, 81, 181–196. [Google Scholar] [CrossRef]
- Fladmark, K.E.; Brustugun, O.T.; Hovland, R.; Boe, R.; Gjertsen, B.T.; Zhivotovsky, B.; Doskeland, S.O. Ultrarapid caspase-3 dependent apoptosis induction by serine/threonine phosphatase inhibitors. Cell Death Differ. 1999, 6, 1099–1108. [Google Scholar] [CrossRef] [PubMed]
- WHO; UNESCO; UNEP. Chapter 4. Human health aspects. In Toxic Cyanobacteria in Water: A guide to Their Public Health Consequences Monitoring and Management; Chorus, I., Bartram, J., Eds.; E & FN Spon: London, UK, 1999; pp. 125–160. [Google Scholar]
- Fujiki, H.; Suganuma, M. Tumor promotion by inhibitors of protein phosphatases 1 and 2A: The okadaic acid class of compounds. Adv. Cancer Res. 1993, 61, 143–194. [Google Scholar] [PubMed]
- Nishiwaki-Matsushima, R.; Ohta, T.; Nishiwaki, S.; Suganuma, M.; Kohyama, K.; Ishikawa, T.; Carmichael, W.W.; Fujiki, H. Liver tumor promotion by the cyanobacterial cyclic peptide toxin microcystin-LR. J. Cancer Res. Clin. Oncol. 1992, 118, 420–424. [Google Scholar] [CrossRef] [PubMed]
- Carmichael, W.W. The Cyanotoxins. In Advances in Botanical Research; Callow, J.A., Ed.; Academic Press: San Diego, CA, USA, 1997; Volume 27, pp. 211–256. [Google Scholar]
- Codd, G.A.; Bell, S.G.; Kaya, K.; Ward, C.J.; Beattie, K.A.; Metcalf, J.S. Cyanobacterial toxins, exposure routes and human health. Eur. J. Phycol. 1999, 34, 405–415. [Google Scholar] [CrossRef]
- Niedermeyer, T. Microcystin Congeners Described in the Literature, Version 5. Available online: http://dx.doi.org/10.6084/m9.figshare.880756 (accessed on 31 March 2017).
- Paerl, H.W.; Huisman, J. Blooms like it hot. Science 2008, 320, 57–58. [Google Scholar] [CrossRef] [PubMed]
- Harke, M.J.; Steffen, M.M.; Gobler, C.J.; Otten, T.G.; Wilhelm, S.W.; Wood, S.A.; Paerl, H.W. A review of the global ecology, genomics, and biogeography of the toxic cyanobacterium, Microcystis spp. Harmful Algae 2016, 54, 4–20. [Google Scholar] [CrossRef] [PubMed]
- United Nations (UN). Department of Economic and Social Affairs. Population Division, World Population Prospects, the 2015 Revision. Available online: https://esa.un.org/unpd/wpp/ (accessed on 31 March 2017).
- United Nations (UN). Environment Programme, Trends in Global Water Use by Sector. Vital Water Graphics, 2008. Available online: http://new.unep.org/dewa/vitalwater/article43.html (accessed on 31 March 2017).
- Ibelings, B.W.; Backer, L.C.; Kardinaal, W.E.; Chorus, I. Current approaches to cyanotoxin risk assessment and risk management around the globe. Harmful Algae 2015, 49, 63–74. [Google Scholar] [CrossRef] [PubMed]
- Fischer, A.; Hoeger, S.J.; Stemmer, K.; Feurstein, D.J.; Knobeloch, D.; Nussler, A.; Dietrich, D.R. The role of organic anion transporting polypeptides (OATPs/SLCOs) in the toxicity of different microcystin congeners in vitro: A comparison of primary human hepatocytes and OATP-transfected HEK293 cells. Toxicol. Appl. Pharmacol. 2010, 245, 9–20. [Google Scholar] [CrossRef] [PubMed]
- Feurstein, D.; Kleinteich, J.; Heussner, A.H.; Stemmer, K.; Dietrich, D.R. Investigation of Microcystin Congener—Dependent Uptake into Primary Murine Neurons. Environ. Health Perspect. 2010, 118, 1370. [Google Scholar] [CrossRef] [PubMed]
- Feurstein, D.; Stemmer, K.; Kleinteich, J.; Speicher, T.; Dietrich, D.R. Microcystin Congener- and Concentration-Dependent Induction of Murine Neuron Apoptosis and Neurite Degeneration. Toxicol. Sci. 2011, 124, 424–431. [Google Scholar] [CrossRef] [PubMed]
- Heussner, A.H.; Altaner, S.; Kamp, L.; Rubio, F.; Dietrich, D.R. Pitfalls in microcystin extraction and recovery from human blood serum. Chem. Biol. Interact. 2014, 223, 87–94. [Google Scholar] [CrossRef] [PubMed]
- Hyenstrand, P.; Metcalf, J.S.; Beattie, K.A.; Codd, G.A. Effects of adsorption to plastics and solvent conditions in the analysis of the cyanobacterial toxin microcystin-LR by high performance liquid chromatography. Water Res. 2001, 35, 3508–3511. [Google Scholar] [CrossRef]
- Hyenstrand, P.; Metcalf, J.S.; Beattie, K.A.; Codd, G.A. Losses of the cyanobacterial toxin microcystin-LR from aqueous solution by adsorption during laboratory manipulations. Toxicon 2001, 39, 589–594. [Google Scholar] [CrossRef]
- Rogers, S.; Puddick, J.; Wood, S.A.; Dietrich, D.R.; Hamilton, D.P.; Prinsep, M.R. The effect of cyanobacterial biomass enrichment by centrifugation and GF/C filtration on subsequent microcystin measurement. Toxins 2015, 7, 821–834. [Google Scholar] [CrossRef] [PubMed]
- Liang, G.; Xie, P.; Chen, J.; Yu, T. Comparative studies on the pH dependence of DOW of microcystin-RR and -LR using LC-MS. Sci. World J. 2011, 11, 20–26. [Google Scholar] [CrossRef] [PubMed]
- De Maagd, P.G.-J.; Hendriks, A.J.; Seinen, W.; Sijm, D.T. pH-dependent hydrophobicity of the cyanobacteria toxin microcystin-LR. Water Res. 1999, 33, 677–680. [Google Scholar] [CrossRef]
- Rivasseau, C.; Martins, S.; Hennion, M.C. Determination of some physicochemical parameters of microcystins (cyanobacterial toxins) and trace level analysis in environmental samples using liquid chromatography. J. Chromatogr. A 1998, 799, 155–169. [Google Scholar] [CrossRef]
- Snyder, L.R. Classification of the solvent properties of common liquids. J. Chromatogr. Sci. 1978, 16, 223–234. [Google Scholar] [CrossRef]
- Sun, K.H.; Silverman, A. Lewis acid-base theory applied to glass. J. Am. Ceram. Soc. 1945, 28, 8–11. [Google Scholar] [CrossRef]
- Behrens, S.H.; Grier, D.G. The charge of glass and silica surfaces. J. Chem. Phys. 2001, 115, 6716–6721. [Google Scholar] [CrossRef]
- Hiemstra, T.; De Wit, J.C.M.; Van Riemsdijk, W.H. Multisite proton adsorption modeling at the solid/solution interface of (hydr)oxides: A new approach. J. Colloid Interface Sci. 1989, 133, 105–117. [Google Scholar] [CrossRef]
- Kamp, L.; Church, J.L.; Carpino, J.; Faltin-Mara, E.; Rubio, F. The effects of water sample treatment, preparation, and storage prior to cyanotoxin analysis for cylindrospermopsin, microcystin and saxitoxin. Chem. Biol. Interact. 2016, 246, 45–51. [Google Scholar] [CrossRef] [PubMed]
- WHO; UNESCO; UNEP. Chapter 11. Fieldwork: Site inspection and sampling. In Toxic Cyanobacteria in Water: A Guide to Their Public Health Consequences Monitoring and Management; Chorus, I., Bartram, J., Eds.; E & FN Spon: London, UK, 1999; pp. 317–333. [Google Scholar]
- WHO; UNESCO; UNEP. Chapter 12. Determination of cyanobacteria in the laboratory. In Toxic Cyanobacteria in Water: A Guide to Their Public Health Consequences Monitoring and Management; Chorus, I., Bartram, J., Eds.; E & FN Spon: London, UK, 1999; pp. 334–361. [Google Scholar]
- WHO; UNESCO; UNEP. Chapter 13. Laboratory analysis of cyanotoxins. In Toxic Cyanobacteria in Water: A Guide to Their Public Health Consequences Monitoring and Management; Chorus, I., Bartram, J., Eds.; E & FN Spon: London, UK, 1999; pp. 362–400. [Google Scholar]
- Puddick, J.; Prinsep, M.R.; Wood, S.A.; Kaufononga, S.A.; Cary, S.C.; Hamilton, D.P. High levels of structural diversity observed in microcystins from Microcystis CAWBG11 and characterization of six new microcystin congeners. Mar. Drugs 2014, 12, 5372–5395. [Google Scholar] [CrossRef] [PubMed]
- Puddick, J.; Wood, S.A.; Hawes, I.; Hamilton, D.P. Fine-scale cryogenic sampling of planktonic microbial communities: Application to toxic cyanobacterial blooms. Limnol. Oceanogr. Methods 2016, 14, 600–609. [Google Scholar] [CrossRef]
- Grubb’s Test QuickCalculator. Available online: http://graphpad.com/quickcalcs/Grubbs1.cfm (accessed on 31 March 2017).
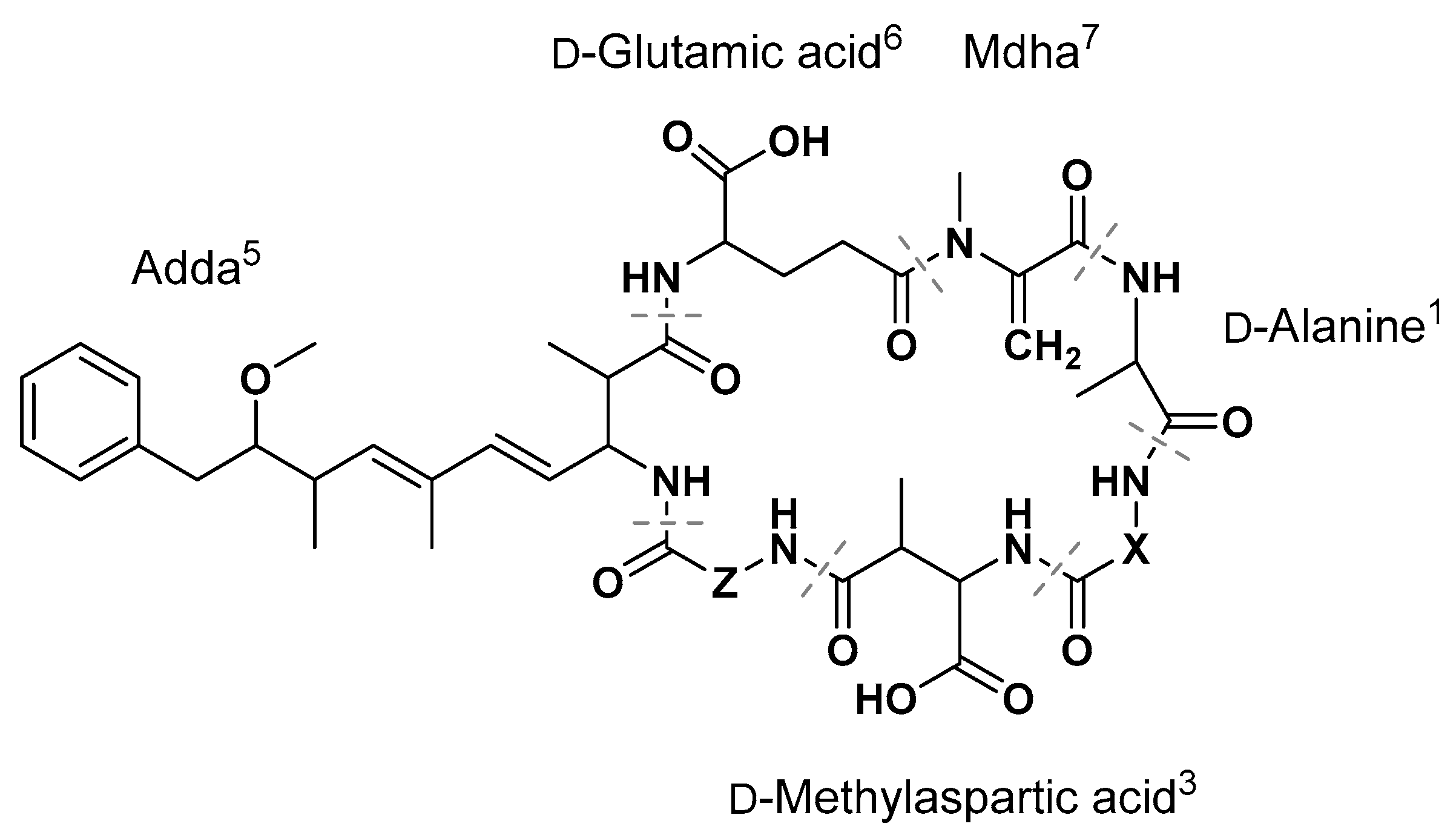

| MC | X2 | Z4 |
|---|---|---|
| MC-RR | l-Arginine | l-Arginine |
| MC-YR | l-Tyrosine | l-Arginine |
| MC-LR | l-Leucine | l-Arginine |
| MC-FR | l-Phenylalanine | l-Arginine |
| MC-WR | l-Tryptophan | l-Arginine |
| MC-RA | l-Arginine | l-Alanine |
| MC-RAba | l-Arginine | l-Aminobutyric acid |
| MC-LA | l-Leucine | l-Alanine |
| MC-FA | l-Phenylalanine | l-Alanine |
| MC-WA | l-Tryptophan | l-Alanine |
| Microcystin Variant | Non-Acidified | Acidified | ||
|---|---|---|---|---|
| Glass | Polypropylene | Glass | Polypropylene | |
| Doubly-arginated (more hydrophilic) | 0 (Milli-Q water) | 20 | 40 | 20 |
| Singly-arginated (amphiphilic) | 0 (Milli-Q water) | 40 | 20 | 40 |
| Non-arginated (more lipophilic) | 0 (Milli-Q water) | 40 | 20 | 40 |
| Congener | Parent | Daughter | Cone Voltage | Collision Energy |
|---|---|---|---|---|
| (m/z) | (m/z) | (V) | (V) | |
| MC-RR | 519.7 | 135.1 | 40 | 27 |
| MC-YR | 1045.5 | 135.1 | 40 | 70 |
| MC-LR | 995.5 | 135.1 | 40 | 65 |
| MC-FR | 1029.5 | 135.1 | 40 | 65 |
| MC-WR | 1068.5 | 135.1 | 40 | 65 |
| MC-RA | 953.5 | 135.1 | 40 | 65 |
| MC-RAba | 967.5 | 135.1 | 40 | 65 |
| MC-LA | 910.6 | 135.1 | 40 | 65 |
| MC-FA | 944.6 | 135.1 | 40 | 65 |
| MC-WA | 983.6 | 135.1 | 40 | 65 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Altaner, S.; Puddick, J.; Wood, S.A.; Dietrich, D.R. Adsorption of Ten Microcystin Congeners to Common Laboratory-Ware Is Solvent and Surface Dependent. Toxins 2017, 9, 129. https://doi.org/10.3390/toxins9040129
Altaner S, Puddick J, Wood SA, Dietrich DR. Adsorption of Ten Microcystin Congeners to Common Laboratory-Ware Is Solvent and Surface Dependent. Toxins. 2017; 9(4):129. https://doi.org/10.3390/toxins9040129
Chicago/Turabian StyleAltaner, Stefan, Jonathan Puddick, Susanna A. Wood, and Daniel R. Dietrich. 2017. "Adsorption of Ten Microcystin Congeners to Common Laboratory-Ware Is Solvent and Surface Dependent" Toxins 9, no. 4: 129. https://doi.org/10.3390/toxins9040129






