Diabetogenic Effects of Ochratoxin A in Female Rats
Abstract
:1. Introduction
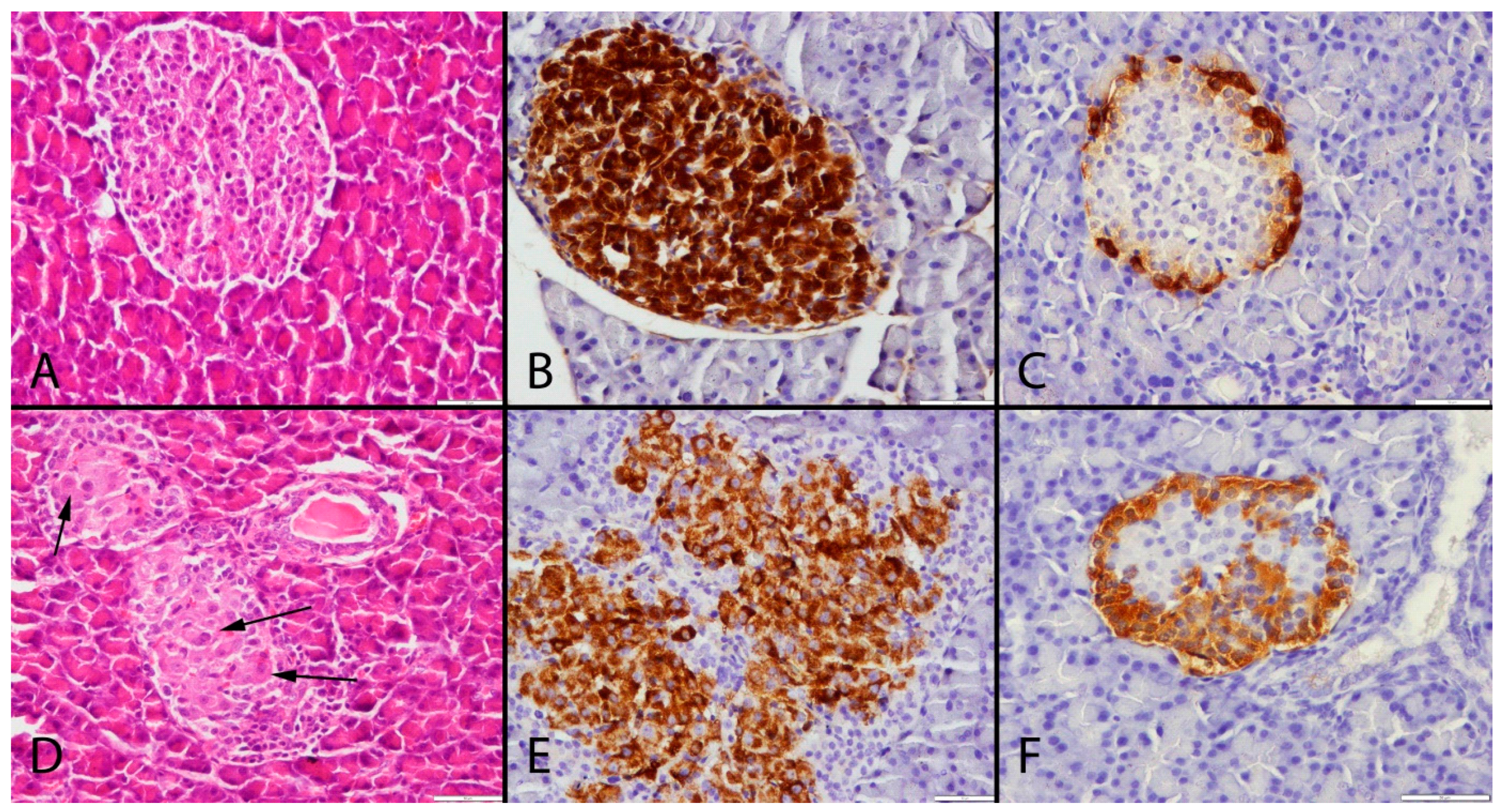
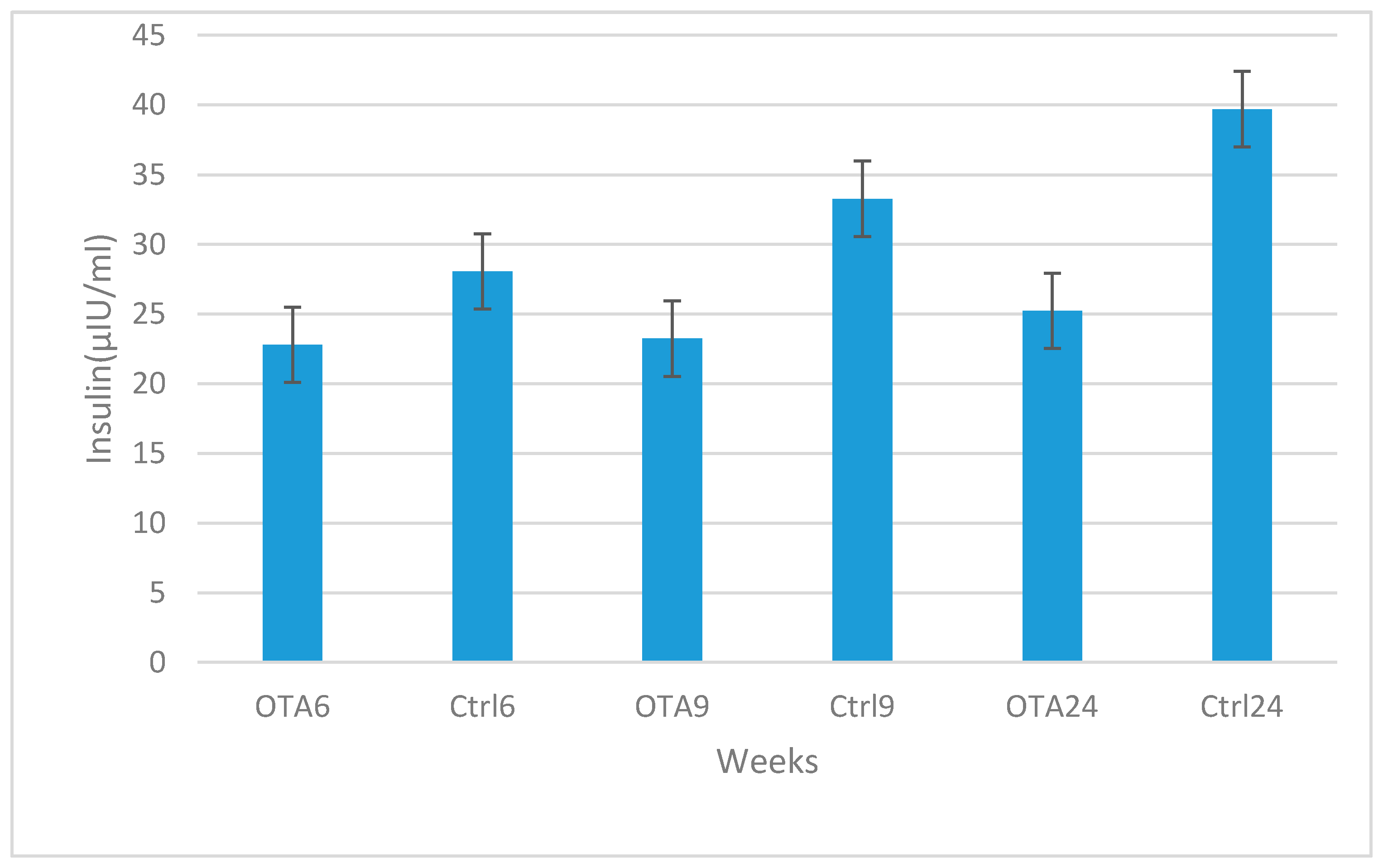
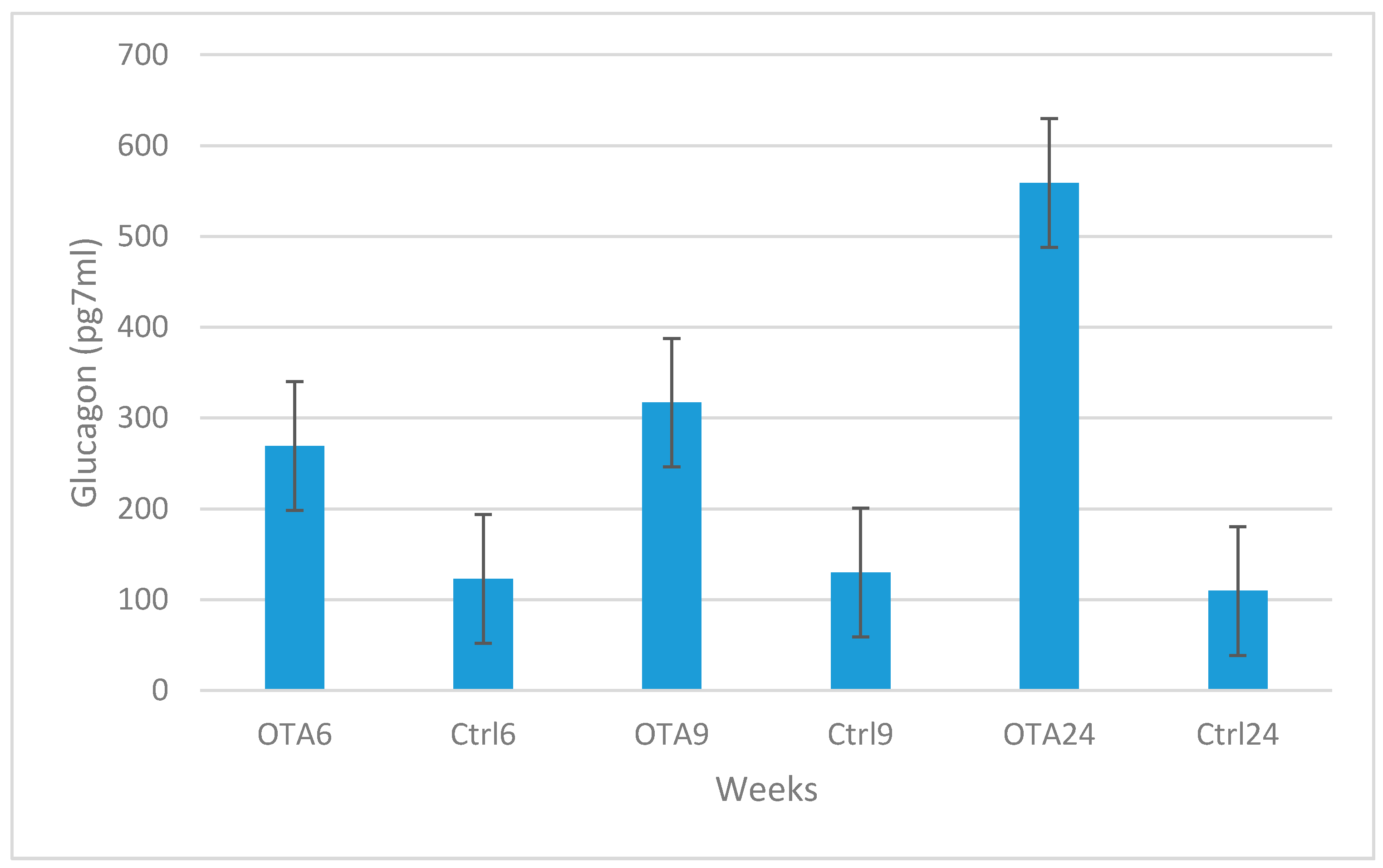
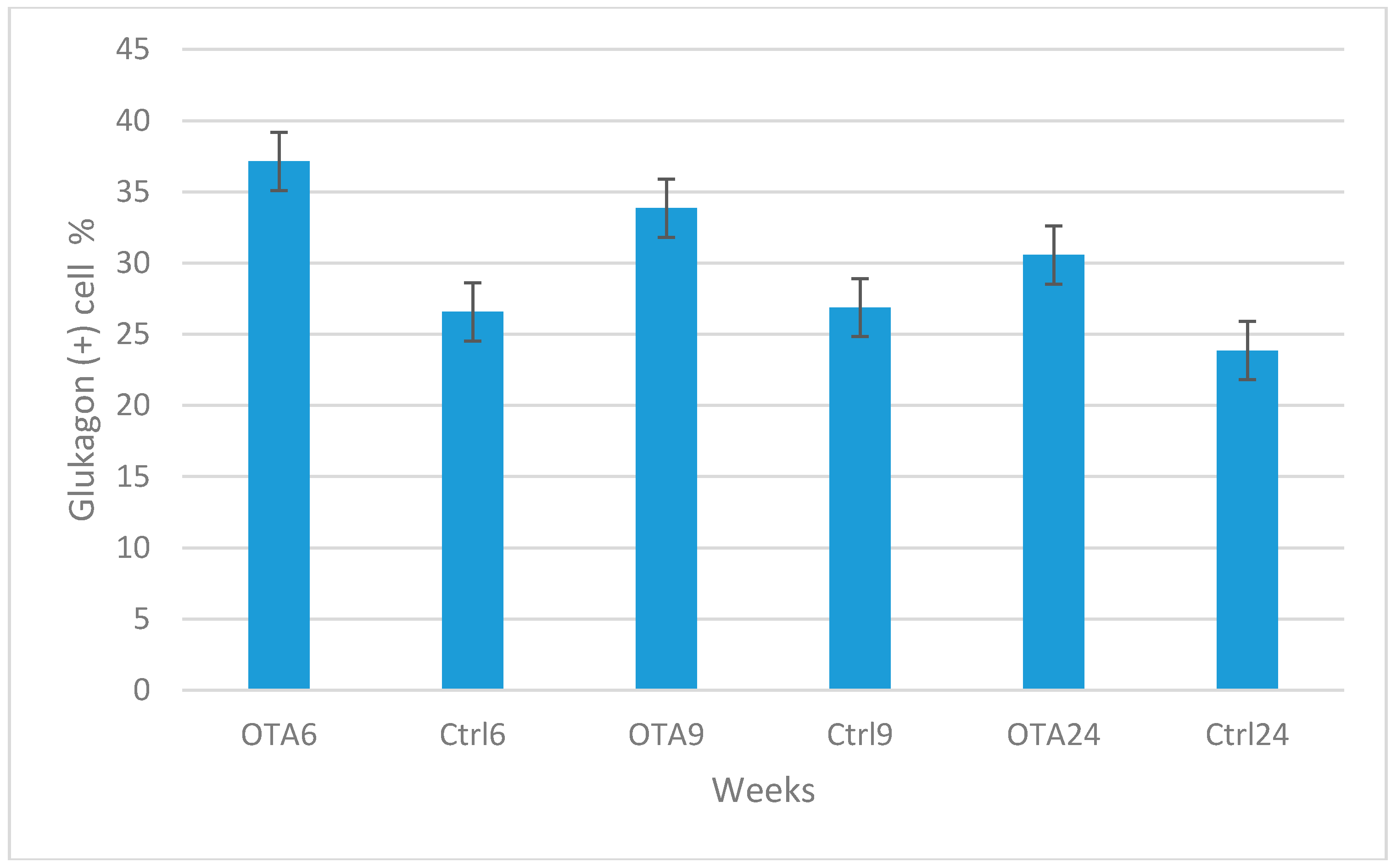
2. Results
3. Discussion
4. Materials and Methods
4.1. Animals, Housing Conditions and Experimental Procedures
4.2. Plasma Insulin, Glucagon and Glucose Determination
4.3. Histological Examination
4.4. Statistical Analysis
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Larsen, T.O.; Svendsen, A.; Smedsgaard, J. Biochemical characterization of ochratoxin A-producing strains of the genus Penicillium. Appl. Environ. Microbiol. 2001, 67, 3630–3635. [Google Scholar] [CrossRef] [PubMed]
- Fink-Gremmels, J. Ochratoxin A in food: Recent developments and significance. Food Addit. Contam. 2005, 22 (Suppl. S1), 1–5. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization (WHO). Selected Mycotoxins: Ochratoxins, Trichothecenes, Ergot (Environmental Health Criteria 105); WHO: Geneva, Switzerland, 1990. [Google Scholar]
- O’Brien, E.; Dietrich, D.R. Ochratoxin A: The continuing enigma. Crit. Rev. Toxicol. 2005, 35, 33–60. [Google Scholar] [CrossRef] [PubMed]
- EFSA (European Food Safety Authority). Opinion of the Scientific Committee on a request from EFSA related to Exposure Assessments. EFSA J. 2005, 249, 1–26. [Google Scholar]
- Szkudelska, K.; Drzymała, H.; Szkudelski, T.; Bukowska, K.; Nogowski, L. Lack of the effect of mycotoxins–aflatoxin B1 and ochratoxin A on some functions of rat adipocytes. Toxicol. In Vitro 2005, 19, 771–777. [Google Scholar] [CrossRef] [PubMed]
- Kuiper-Goodman, T. Risk assessment of ochratoxin A: An update. Food Addit. Contam. 1996, 13, 53–57. [Google Scholar] [PubMed]
- Speijers, G.J.A.; Van Egmond, H.P. Worldwide ochratoxin A levels in food and feeds. In Human Ochratoxicosis and Its Pathologies; Creppy, E.E., Castegnaro, M., Dirheimer, G., Eds.; John Libbey Eurotext: London, UK, 1993; Volume 231, pp. 85–100. [Google Scholar]
- Krogh, P.; Hald, B.; Plestina, R.; Ceovic, S. Balkan (endemic) nephropathy and food borne ochratoxin A: Preliminary results of a survey in foodstuff. Acta Pathol. Microbiol. Scand. Sect. B 1977, 85, 238–240. [Google Scholar]
- Pavlovic, M.; Plestina, R.; Krogh, P. Ochratoxin A contamination of foodstuffs in an area with Balkan (endemic) nephropathy. Acta Pathol. Microbiol. Scand. Sect. B 1979, 87, 243–246. [Google Scholar] [CrossRef]
- Jurjevic, Z.; Solfrizzo, M.; Cvjetkovic, B.; Avantoggiato, G.; Visconti, A. Ochratoxin A and Fumonisin (B1 and B2) in maize from Balkan nephropathy endemic and non-endemic areas of Croatia. Mycotoxin Res. 1999, 15, 67–80. [Google Scholar] [CrossRef] [PubMed]
- Puntarić, D.; Bosnir, J.; Smit, Z.; Skes, I.; Baklaić, Z. Ochratoxin A in corn and wheat: Geographical association with endemic nephropathy. Croat Med. J. 2001, 42, 175–180. [Google Scholar] [PubMed]
- Akash, M.S.H.; Shen, Q.; Rehman, K.; Chen, S. Interleukin-1 receptor antagonist: A new therapy for type 2 diabetes mellitus. J. Pharm. Sci. 2012, 101, 1647–1658. [Google Scholar] [CrossRef] [PubMed]
- Anjum, R.; Zahra, N.; Rehman, K.; Alam, R.; Parveen, A.; Akash, M.S.H. Comparative analysis of serum lipid profile between normotensive and hypertensive Pakistani pregnant women. J. Mol. Genet. Med. 2013, 7, 64. [Google Scholar] [CrossRef]
- Akash, M.S.H.; Rehman, K.; Chen, S. Role of inflammatory mechanisms in pathogenesis of type 2 diabetes mellitus. J. Cell Biochem. 2013, 114, 525–531. [Google Scholar] [CrossRef] [PubMed]
- Akash, M.S.H.; Rehman, K.; Chen, S. An overview of valuable scientific models for diabetes mellitus. Curr. Diabetes Rev. 2013, 9, 286–293. [Google Scholar] [CrossRef] [PubMed]
- Nayak, B.S.; Roberts, L. Relationship between inflammatory markers, metabolic and anthropometric variables in the Caribbean type 2 diabetic patients with and without microvascular complications. J. Inflamm. 2006, 3. [Google Scholar] [CrossRef] [PubMed]
- ADA (American Diabetes Association). Evidence-based nutrition principles and recommendations for the treatment and prevention of diabetes and related complications (Position Statement). Diabetes Care 2003, 26 (Suppl. S1), S51–S61. [Google Scholar]
- Yajnik, C.S. The insulin resistance epidemic in India: fetal origins, later lifestyle, or both? Nutr. Rev. 2001, 59, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Molitch, M.E. Diabetes mellitus. In Clinical Methods: The History, Physical, and Laboratory Examinations, 3rd ed.; Walker, H.K., Hall, W.D., Hurst, J.W., Eds.; Butterworths: Boston, MA, USA, 1990; Chapter 136; pp. 2808–2809. [Google Scholar]
- Colditz, G.A.; Manson, J.E.; Stampfer, M.J.; Rosner, B.; Willett, W.C.; Speizer, F.E. Diet and risk of clinical diabetes in women. Am. J. Clin. Nutr. 1992, 55, 1018–1023. [Google Scholar] [PubMed]
- Knowler, W.C.; Barrett-Connor, E.; Fowler, S.E.; Hamman, R.F.; Lachin, J.M.; Walker, E.A.; Nathan, D.M. Diabetes Prevention Program Research Group. Reduction in the incidence of type 2 diabetes with lifestyle intervention or metformin. N. Engl. J. Med. 2002, 346, 393–403. [Google Scholar] [PubMed]
- Pfohl-Leszkowicz, A.; Manderville, R.A. Ochratoxin A: An overview on toxicity and carcinogenicity in animals and humans. Mol. Nutr. Food Res. 2007, 51, 61–99. [Google Scholar] [CrossRef] [PubMed]
- Shanmugasundaram, E.R.B.; Parameswari, C.S.; Shanmugasundaram, K.R. Tereic Acid-A diabetogenic mycotoxin in rats. Curr. Sci. 1984, 53, 1290–1292. [Google Scholar]
- Subramanian, S.; Govindasamy, S.A. ochraceus toxicity on carbohydrate metabolism in chicks. Curr. Sci. India 1985, 54, 860–861. [Google Scholar]
- Suseela, R.; Shanmugasundaram, E.R.B.; Shanmugasundaram, K.R. Effect of Penitrem A on glucose tolerance studied in rats. Curr. Sci. 1986, 55, 98–100. [Google Scholar]
- Kumar, S.N.; Gopal, A. Toxic manifestation of endosulfan and ochratoxin-A in adult male rats. MOJ Toxicol. 2015, 1, 1–6. [Google Scholar]
- Stoev, S.D.; Stoeva, J.K.; Anguelov, G.; Hald, B.; Creppy, E.E.; Radic, B. Haematological, Biochemical and toxicological investigations in spontaneous cases with different frequency of porcine nephropathy in Bulgaria. J. Vet. Med. Ser. A 1998, 45, 229–236. [Google Scholar] [CrossRef]
- Stoev, S.D.; Gundasheva, D.; Zarkov, I.; Mircheva, T.; Zapryanova, D.; Denev, S.; Mitev, Y.; Daskalov, H.; Dutton, M.; Mwanza, M.; et al. Experimental mycotoxic nephropathy in pigs provoked by a mouldy diet containing ochratoxin A and fumonisin B1. Exp. Toxicol. Pathol. 2012, 64, 733–741. [Google Scholar] [CrossRef] [PubMed]
- Stoev, S.D.; Vitanov, S.; Anguelov, G.; Petkova-Bocharova, T.; Creppy, E.E. Experimental mycotoxic nephropathy in pigs provoked by a diet containing ochratoxin A and penicillic acid. Vet. Res. Commun. 2001, 25, 205–223. [Google Scholar] [CrossRef] [PubMed]
- Pleadin, J.; Perši, N.; Mitak, M.; Terzić, S.; Milić, D.; Vulić, A.; Brstilo, M. Biochemical changes in pig serum after ochratoxin A exposure. Bull. Environ. Contam. Toxicol. 2012, 88, 1043–1047. [Google Scholar] [CrossRef] [PubMed]
- Mir, M.S.; Dwıvedi, P. Ochratoxin A-induced serum biochemical altertions in New Zealand White rabbits (Oryctolagus cuniculus). Turk. J. Vet. Anim. Sci. 2010, 34, 525–531. [Google Scholar]
- Subramanian, S.; Kanthasamy, A.; Balasubramanian, N.; Sekar, N.; Govindasamy, S. Ochratoxin A toxicity on carbohydrate metabolism in rats. Bull. Environ. Contam. Toxicol. 1989, 43, 180–184. [Google Scholar] [CrossRef] [PubMed]
- Ozmen, O.; Topsakal, S.; Sahinduran, S.; Ozcelik, M. Effect of insufficient insulin treatment in streptozotocin-induced Diabetes mellitus. Pancreas 2007, 34, 354–358. [Google Scholar] [CrossRef] [PubMed]
- Mor, F.; Kilic, M.A.; Ozmen, O.; Yilmaz, M.; Eker, I.; Uran, K. The effects of orchidectomy on toxicological responses to dietaryochratoxin A in Wistar rats. Exp. Toxicol. Pathol. 2014, 66, 267–275. [Google Scholar] [CrossRef] [PubMed]
- Shaw, E.; Sicree, A.; Zimmet, Z. Global estimates of the prevalence of diabetes for 2010 and 2030. Diabetes Res. Clin. Pract. 2010, 87, 4–14. [Google Scholar] [CrossRef] [PubMed]
- Topsakal, S.; Ozmen, O. Diabetes mellitus in human and animals. MAE Vet. Fak. Derg. 2016, 1, 47–57. [Google Scholar]

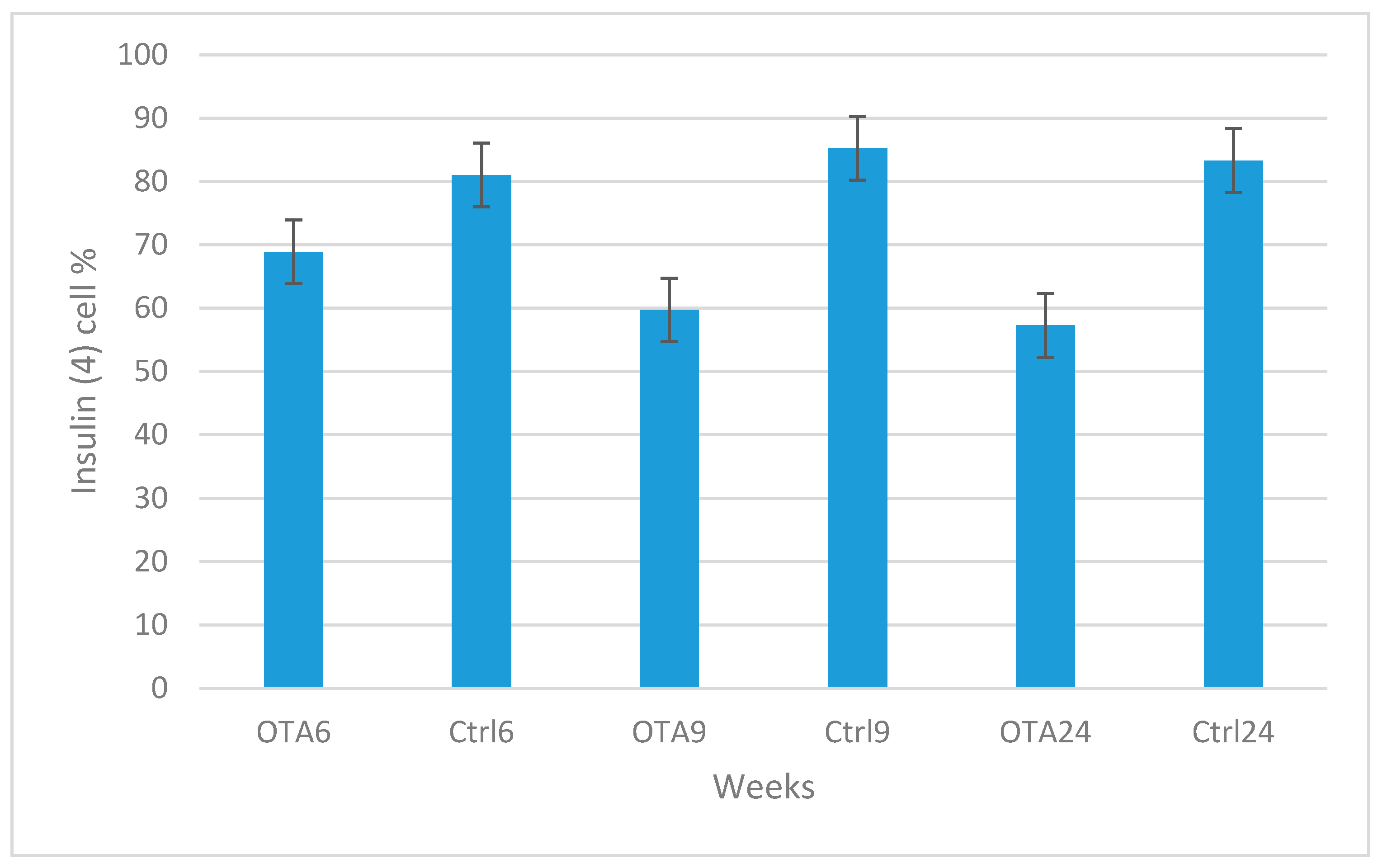
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mor, F.; Sengul, O.; Topsakal, S.; Kilic, M.A.; Ozmen, O. Diabetogenic Effects of Ochratoxin A in Female Rats. Toxins 2017, 9, 144. https://doi.org/10.3390/toxins9040144
Mor F, Sengul O, Topsakal S, Kilic MA, Ozmen O. Diabetogenic Effects of Ochratoxin A in Female Rats. Toxins. 2017; 9(4):144. https://doi.org/10.3390/toxins9040144
Chicago/Turabian StyleMor, Firdevs, Omur Sengul, Senay Topsakal, Mehmet Akif Kilic, and Ozlem Ozmen. 2017. "Diabetogenic Effects of Ochratoxin A in Female Rats" Toxins 9, no. 4: 144. https://doi.org/10.3390/toxins9040144




