Embryotoxicity Caused by DON-Induced Oxidative Stress Mediated by Nrf2/HO-1 Pathway
Abstract
:1. Introduction
2. Results
2.1. Animal Experiment
2.1.1. Body Weight and Food Consumption of the Maternal Mice
2.1.2. Embryonic Survival, Growth and Development of the Embryos
2.1.3. Visceral and Skeleton Morphology of the Embryos
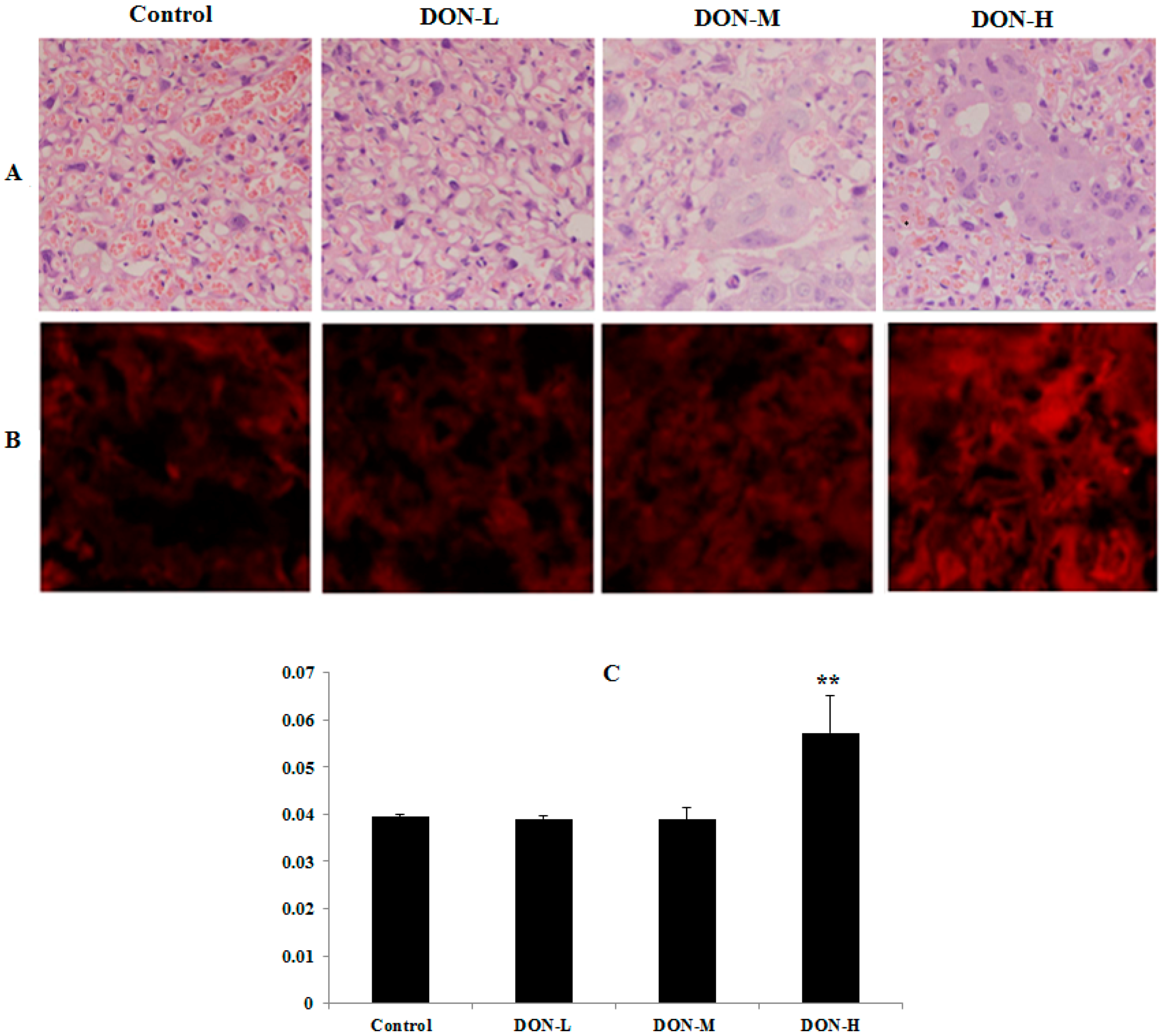
2.1.4. Pathological Observations in the Placenta of the Maternal Mice
2.1.5. ROS Levels and Activity of Anti-Oxidative System in the Placenta of the Maternal Mice
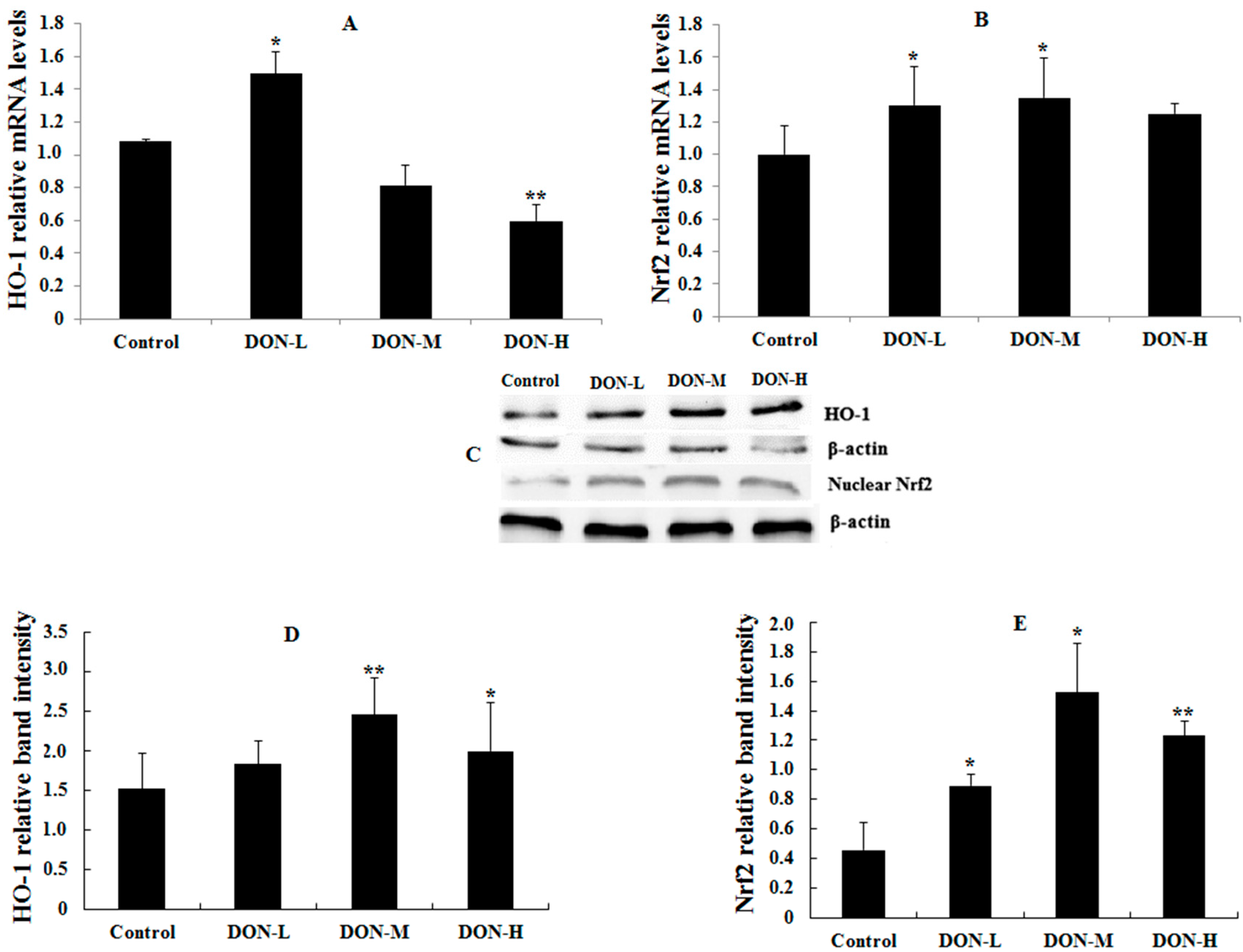
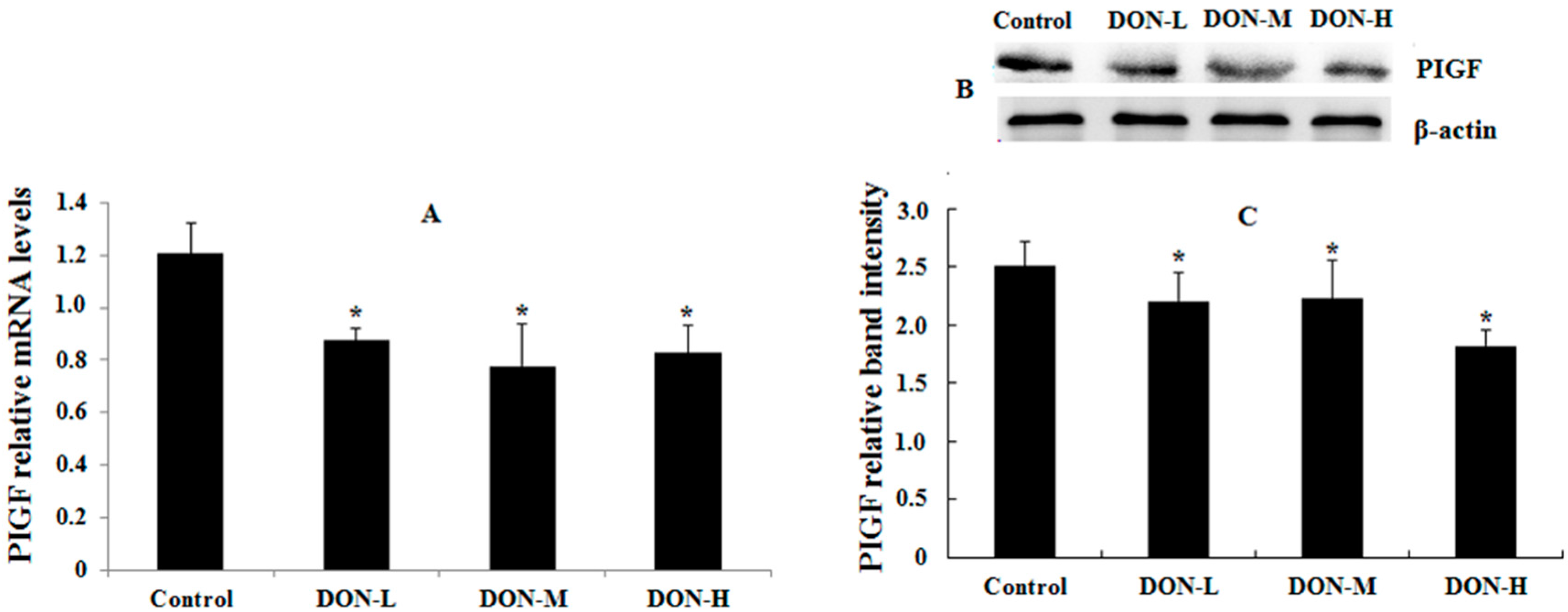
2.1.6. HO-1, Nrf2 and PIGF Expressions in the Placenta of the Maternal Mice
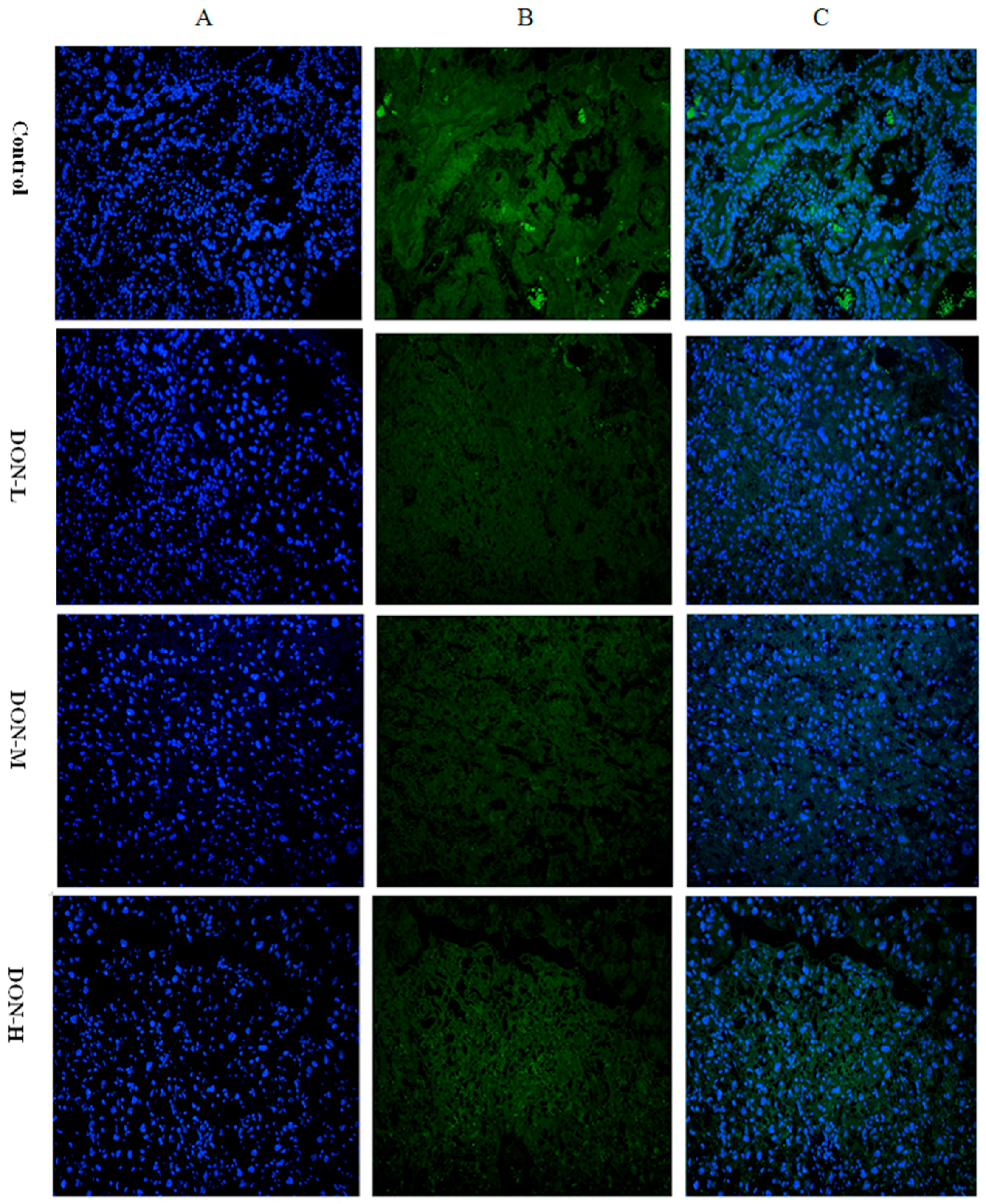
2.1.7. Intracellular Translocation of Nrf2 in the Placenta of the Maternal Mice
2.2. Cell Experiment
2.2.1. Cell Viability with Different Doses of DON
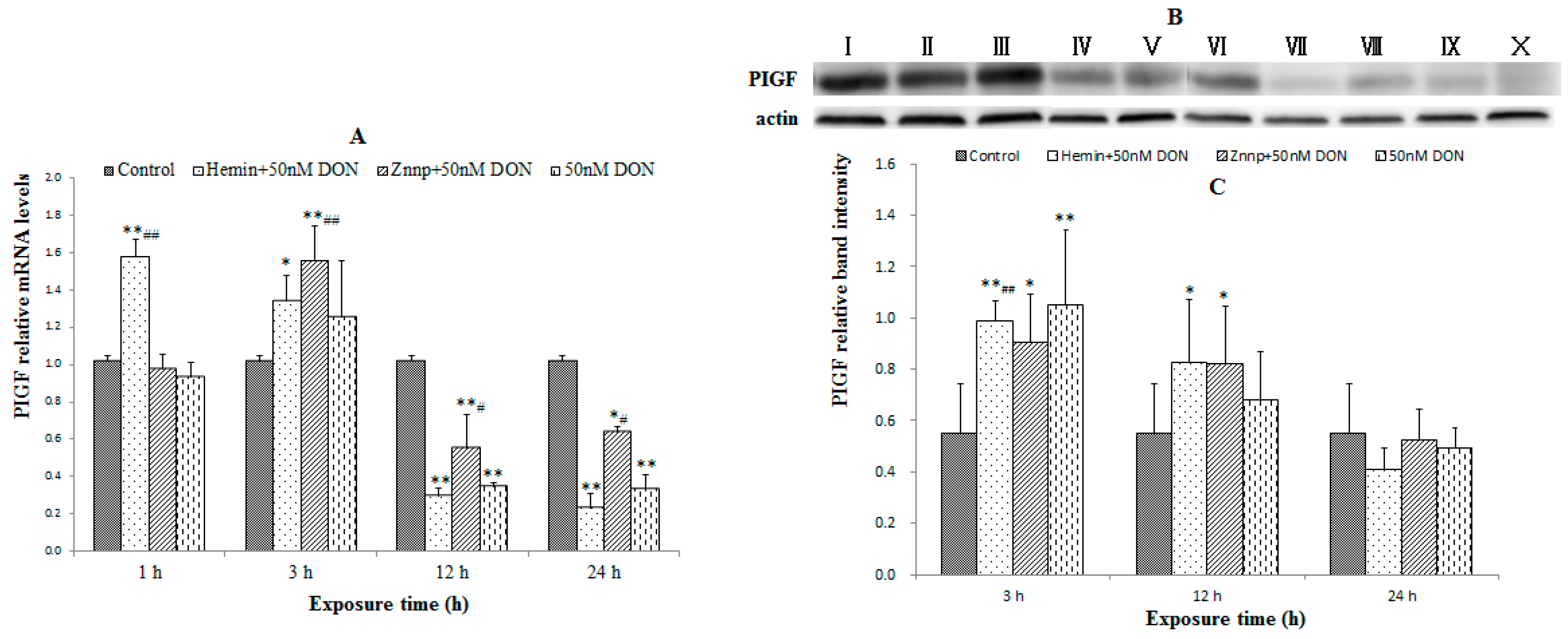
2.2.2. Indexes of Placental Function in BeWo Cell (the Expression of PIGF)
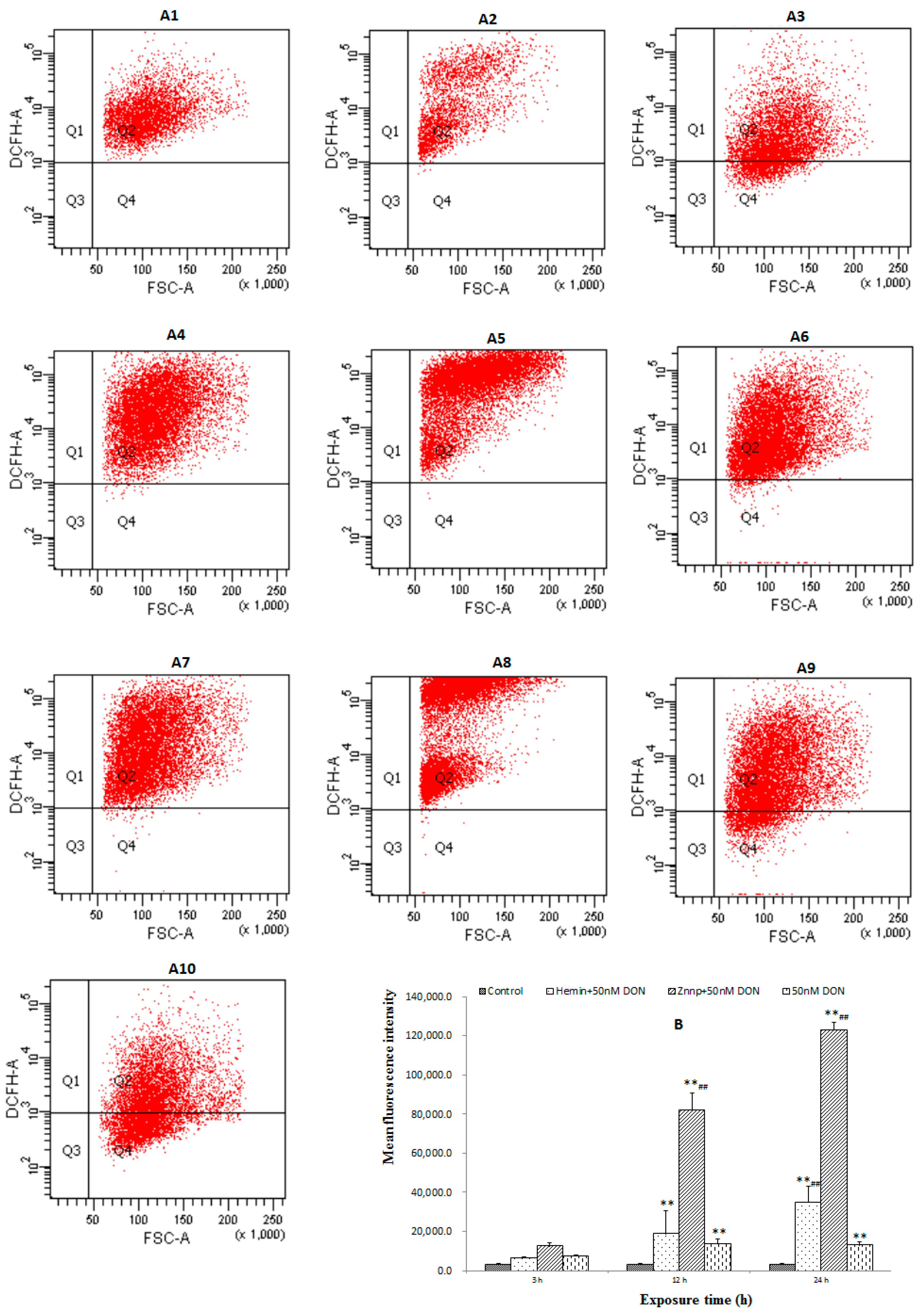
2.2.3. The Level of ROS in BeWo Cell
2.2.4. Activity of Anti-Oxidative System in BeWo Cells
2.2.5. The Expressions of Nrf2 and HO-1 in BeWo Cells
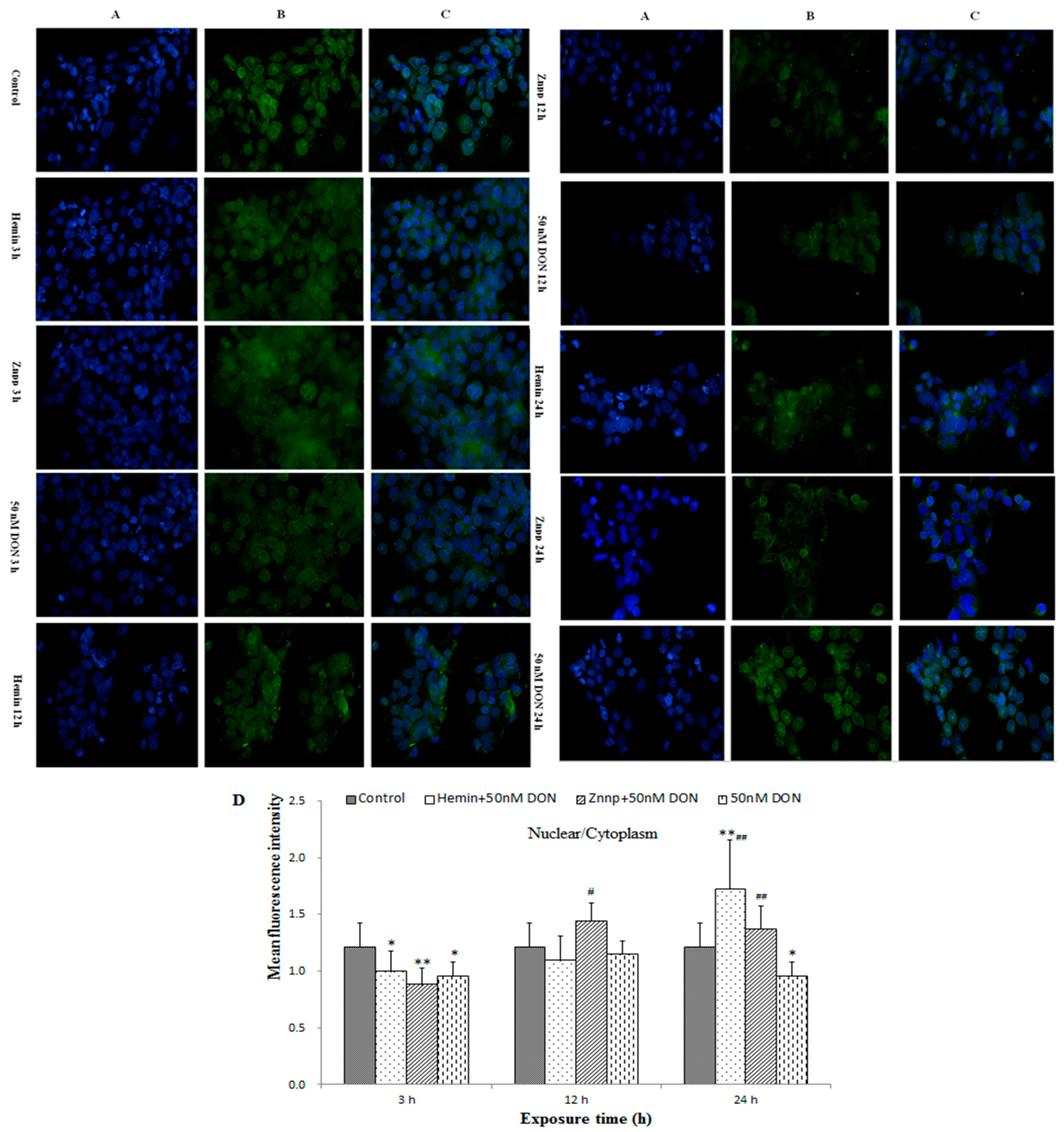
2.2.6. Intracellular Translocation of Nrf2 in BeWo Cell
3. Discussion
4. Conclusions
5. Materials and Methods
5.1. Chemicals
5.2. Animal Experiment
5.2.1. Animals and Treatment
5.2.2. Study Design
5.2.3 ROS in the Maternal Placenta
5.2.4. Superoxide Dismutase (SOD), Malondialdehyde (MDA), GSH and HO-1 in the Maternal Placenta
5.2.5. Real-Time Polymerase Chain Reaction (RT-PCR)
- HO-1, Forward: 5′-TGCCCCACTCTACTTCCCTG-3′;
- Reverse: 5′-GGCGGTCTTAGCCTCTTCTGT-3′;
- Nrf2, Forward: 5′-CCACATTTCCTTCATGGTTTTG-3′;
- Reverse: 5′-GACACTTCCAGGGGCACTATCT-3′;
- PIGF Forward: 5′-TCCTTCTGAGTCGCTGTAGTGG-3′;
- Reverse: 5′-CCTCCTTTCTGCCTTTGTCG-3′;
- β-actin, Forward: 5′-AGAGGGAAATCGTGCGTGAC-3′; and
- Reverse: 5′-CCATACCCAAGAAGGAAGGCT-3′.
5.2.6. Western Blot Analysis
5.2.7. Immunohistochemistry
5.3. Cell Experiment
- HO-1, Forward: 5′-CAGCATGCCCCAGGATTTG-3′;
- Reverse: 5′-AGCTGGATGTTGAGCAGGA-3′; Nrf2,
- Forward: 5′-TCCAGTCAGAAACCAGTGGAT-3′;
- Reverse: 5′-AATGTCTGCGCCAAAAGCTG-3′; PIGF,
- Forward: 5′-TCCCTACTTTGGACAGGAGC-3′;
- Reverse: 5′-CTGCAGAAGGAAAGAAGGGG-3′; β-actin,
- Forward: 5’-TGACGGGGTCACCCACACTGTGCCCATCTA-3′;
- Reverse: 5′-CTAGAAGCATTTGCGGTGGACGATG-3′.
5.4. Statistical Analysis
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
Abbreviations
| ARE | antioxidant response element |
| DON | Deoxynivalenol |
| DON-H, DON | high dose group |
| DON-M, DON | medium dose group; |
| DON-L, DON | low dose group |
| GD | gestation day |
| GSH | glutathione |
| GPx | glutathione peroxidase |
| HSP | heat shock protein |
| H&E | hematoxylin and eosin |
| HO-1 | heme oxygenase-1 |
| H2O2 | hydrogen peroxide |
| •OH | hydroxyl free radical |
| Keap-1 | the inhibitor of Nrf2, Kelch-like ECH-associated protein 1 |
| MDA | Malondialdehyde |
| MFI | Mean fluorescence intensity |
| MAPKs | Mitogen-Activated Protein Kinases |
| Nrf2 | NF-E2-related factor 2 |
| NO | nitric oxide |
| ROS | reactive oxygen species |
| SPF | Specific Pathogen Free |
| SOD | Superoxide Dismutase |
| Znpp | Zinc protoporphyrin |
References
- Grove, J.F. Non-macrocyclic trichothecenes. Nat. Prod. Rep. 1988, 5, 187–209. [Google Scholar] [CrossRef] [PubMed]
- Grove, J.F. Macrocyclic trichothecenes. Nat. Prod. Rep. 1993, 10, 429–448. [Google Scholar] [CrossRef]
- Grove, J.F. Non-macrocyclic trichothecenes part 2. Prog. Chem. Org. Nat. Prod. 2000, 69, 1–70. [Google Scholar]
- Rotter, B.A.; Prelusky, D.B.; Pestka, J.J. Toxicology of deoxynivalenol (vomitoxin). J. Toxicol. Environ. Health 1996, 48, 1–34. [Google Scholar] [CrossRef] [PubMed]
- Evaluation of Certain Mycotoxins in Food. Fifty-Sixth Report of the Joint FAO/WHO Expert Committee on Food Additives; World Health Organization Techical Report Series 906; World Health Organization: Geneva, Switzerland, 2002; Chapter i–viii; pp. 1–62.
- Pestka, J.J. Deoxynivalenol: Mechanisms of action, human exposure, and toxicological relevance. Arch. Toxicol. 2010, 84, 663–679. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Peng, Z.; Nussler, A.K.; Liu, L.; Yang, W. Current and prospective sights in mechanism of deoxynivalenol-induced emesis for future scientific study and clinical treatment. J. Appl. Toxicol. 2017, 37, 784–791. [Google Scholar] [CrossRef] [PubMed]
- Peng, Z.; Chen, L.; Nussler, A.K.; Liu, L.; Yang, W. Current sights for mechanisms of deoxynivalenol-induced hepatotoxicity and prospective views for future scientific research: A mini review. J. Appl. Toxicol. 2017, 37, 518–529. [Google Scholar] [CrossRef] [PubMed]
- Sobrova, P.; Adam, V.; Vasatkova, A.; Beklova, M.; Zeman, L.; Kizek, R. Deoxynivalenol and its toxicity. Interdiscip. Toxicol. 2010, 3, 94–99. [Google Scholar] [CrossRef] [PubMed]
- Yang, W.; Yu, M.; Fu, J.; Bao, W.; Wang, D.; Hao, L.; Yao, P.; Nussler, A.K.; Yan, H.; Liu, L. Deoxynivalenol induced oxidative stress and genotoxicity in human peripheral blood lymphocytes. Food Chem. Toxicol. 2014, 64, 383–396. [Google Scholar] [CrossRef] [PubMed]
- Yu, M.; Chen, L.; Peng, Z.; Nussler, A.K.; Wu, Q.; Liu, L.; Yang, W. Mechanism of deoxynivalenol effects on the reproductive system and fetus malformation: Current status and future challenges. Toxicol. In Vitro 2017, 41, 150–158. [Google Scholar] [CrossRef] [PubMed]
- Khera, K.S.; Whalen, C.; Angers, G.; Vesonder, R.F.; Kuiper-Goodman, T. Embryotoxicity of 4-deoxynivalenol (vomitoxin) in mice. Bull. Environ. Contam. Toxicol. 1982, 29, 487–491. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Zhu, X.; Wu, H.; Zhuang, D.; Yu, G.; Li, X.; Li, F.; Yu, A. Evaluation of fetal skeletal malformations in deoxynivalenol-treated mice using microarray analysis. Arch. Environ. Contam. Toxicol. 2012, 63, 445–452. [Google Scholar] [CrossRef] [PubMed]
- Nielsen, J.K.; Vikstrom, A.C.; Turner, P.; Knudsen, L.E. Deoxynivalenol transport across the human placental barrier. Food Chem. Toxicol. 2011, 49, 2046–2052. [Google Scholar] [CrossRef] [PubMed]
- Collins, T.F.; Sprando, R.L.; Black, T.N.; Olejnik, N.; Eppley, R.M.; Hines, F.A.; Rorie, J.; Ruggles, D.I. Effects of deoxynivalenol (don, vomitoxin) on in utero development in rats. Food Chem. Toxicol. 2006, 44, 747–757. [Google Scholar] [CrossRef] [PubMed]
- Debouck, C.; Haubruge, E.; Bollaerts, P.; van Bignoot, D.; Brostaux, Y.; Werry, A.; Rooze, M. Skeletal deformities induced by the intraperitoneal administration of deoxynivalenol (vomitoxin) in mice. Int. Orthop. 2001, 25, 194–198. [Google Scholar] [CrossRef] [PubMed]
- Tiemann, U.; Brussow, K.P.; Dannenberger, D.; Jonas, L.; Pohland, R.; Jager, K.; Danicke, S.; Hagemann, E. The effect of feeding a diet naturally contaminated with deoxynivalenol (don) and zearalenone (zon) on the spleen and liver of sow and fetus from day 35 to 70 of gestation. Toxicol. Lett. 2008, 179, 113–117. [Google Scholar] [CrossRef] [PubMed]
- Al-Gubory, K.H.; Fowler, P.A.; Garrel, C. The roles of cellular reactive oxygen species, oxidative stress and antioxidants in pregnancy outcomes. Int. J. Biochem. Cell Biol. 2010, 42, 1634–1650. [Google Scholar] [CrossRef] [PubMed]
- Cardoso, H.D.; Cabral, E.V.; Vieira-Filho, L.D.; Vieyra, A.; Paixao, A.D. Fetal development and renal function in adult rats prenatally subjected to sodium overload. Pediatr. Nephrol. 2009, 24, 1959–1965. [Google Scholar] [CrossRef] [PubMed]
- Myatt, L. Review: Reactive oxygen and nitrogen species and functional adaptation of the placenta. Placenta 2010, 31, S66–S69. [Google Scholar] [CrossRef] [PubMed]
- Prater, M.R.; Laudermilch, C.L.; Liang, C.; Holladay, S.D. Placental oxidative stress alters expression of murine osteogenic genes and impairs fetal skeletal formation. Placenta 2008, 29, 802–808. [Google Scholar] [CrossRef] [PubMed]
- Mattison, D.R. Environmental exposures and development. Curr. Opin. Pediatr. 2010, 22, 208–218. [Google Scholar] [CrossRef] [PubMed]
- Krishnaswamy, R.; Devaraj, S.N.; Padma, V.V. Lutein protects ht-29 cells against deoxynivalenol-induced oxidative stress and apoptosis: Prevention of nf-kappab nuclear localization and down regulation of nf-kappab and cyclo-oxygenase-2 expression. Free Radic. Biol. Med. 2010, 49, 50–60. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Jiang, L.; Geng, C.; Cao, J.; Zhong, L. The role of oxidative stress in deoxynivalenol-induced DNA damage in hepg2 cells. Toxicon 2009, 54, 513–518. [Google Scholar] [CrossRef] [PubMed]
- Bae, H.K.; Pestka, J.J. Deoxynivalenol induces p38 interaction with the ribosome in monocytes and macrophages. Toxicol. Sci. 2008, 105, 59–66. [Google Scholar] [CrossRef] [PubMed]
- Zhou, H.R.; Islam, Z.; Pestka, J.J. Rapid, sequential activation of mitogen-activated protein kinases and transcription factors precedes proinflammatory cytokine mrna expression in spleens of mice exposed to the trichothecene vomitoxin. Toxicol. Sci. 2003, 72, 130–142. [Google Scholar] [CrossRef] [PubMed]
- Jaramillo, M.C.; Zhang, D.D. The emerging role of the nrf2-keap1 signaling pathway in cancer. Genes Dev. 2013, 27, 2179–2191. [Google Scholar] [CrossRef] [PubMed]
- Golbidi, S.; Badran, M.; Laher, I. Antioxidant and anti-inflammatory effects of exercise in diabetic patients. Exp. Diabetes Res. 2012, 2012, 941868. [Google Scholar] [CrossRef] [PubMed]
- Alam, J.; Cook, J.L. How many transcription factors does it take to turn on the heme oxygenase-1 gene? Am. J. Respir. Cell Mol. Biol. 2007, 36, 166–174. [Google Scholar] [CrossRef] [PubMed]
- Yu, M.; Xu, M.; Liu, Y.; Yang, W.; Rong, Y.; Yao, P.; Yan, H.; Wang, D.; Liu, L. Nrf2/are is the potential pathway to protect sprague-dawley rats against oxidative stress induced by quinocetone. Regul. Toxicol. Pharmacol. 2013, 66, 279–285. [Google Scholar] [CrossRef] [PubMed]
- Yu, M.; Wang, D.; Xu, M.; Liu, Y.; Wang, X.; Liu, J.; Yang, X.; Yao, P.; Yan, H.; Liu, L. Quinocetone-induced nrf2/ho-1 pathway suppression aggravates hepatocyte damage of sprague-dawley rats. Food Chem. Toxicol. 2014, 69, 210–219. [Google Scholar] [CrossRef] [PubMed]
- Da Silva, J.L.; Morishita, T.; Escalante, B.; Staudinger, R.; Drummond, G.; Goligorsky, M.S.; Lutton, J.D.; Abraham, N.G. Dual role of heme oxygenase in epithelial cell injury: Contrasting effects of short-term and long-term exposure to oxidant stress. J. Lab. Clin. Med. 1996, 128, 290–296. [Google Scholar] [CrossRef]
- Watanabe, S.; Akagi, R.; Mori, M.; Tsuchiya, T.; Sassa, S. Marked developmental changes in heme oxygenase-1 (ho-1) expression in the mouse placenta: Correlation between ho-1 expression and placental development. Placenta 2004, 25, 387–395. [Google Scholar] [CrossRef] [PubMed]
- Zenclussen, M.L.; Casalis, P.A.; El-Mousleh, T.; Rebelo, S.; Langwisch, S.; Linzke, N.; Volk, H.D.; Fest, S.; Soares, M.P.; Zenclussen, A.C. Haem oxygenase-1 dictates intrauterine fetal survival in mice via carbon monoxide. J. Pathol. 2011, 225, 293–304. [Google Scholar] [CrossRef] [PubMed]
- Chaudhary, M.; Rao, P.V. Brain oxidative stress after dermal and subcutaneous exposure of t-2 toxin in mice. Food Chem. Toxicol. 2010, 48, 3436–3442. [Google Scholar] [CrossRef] [PubMed]
- Pedruzzi, L.M.; Stockler-Pinto, M.B.; Leite, M., Jr.; Mafra, D. Nrf2-keap1 system versus nf-kappab: The good and the evil in chronic kidney disease? Biochimie 2012, 94, 2461–2466. [Google Scholar] [CrossRef] [PubMed]
- Khera, K.S.; Arnold, D.L.; Whalen, C.; Angers, G.; Scott, P.M. Vomitoxin (4-deoxynivalenol): Effects on reproduction of mice and rats. Toxicol. Appl. Pharmacol. 1984, 74, 345–356. [Google Scholar] [CrossRef]
- Ye, Z.W.; Zhang, J.; Townsend, D.M.; Tew, K.D. Oxidative stress, redox regulation and diseases of cellular differentiation. Biochim. Biophys. Acta 2014, 1850, 1607–1621. [Google Scholar] [CrossRef] [PubMed]
- Owuor, E.D.; Kong, A.N. Antioxidants and oxidants regulated signal transduction pathways. Biochem. Pharmacol. 2002, 64, 765–770. [Google Scholar] [CrossRef]
- Kim, Y.J.; Ahn, J.Y.; Liang, P.; Ip, C.; Zhang, Y.; Park, Y.M. Human prx1 gene is a target of nrf2 and is up-regulated by hypoxia/reoxygenation: Implication to tumor biology. Cancer Res. 2007, 67, 546–554. [Google Scholar] [CrossRef] [PubMed]
- Khalil, A.; Muttukrishna, S.; Harrington, K.; Jauniaux, E. Effect of antihypertensive therapy with alpha methyldopa on levels of angiogenic factors in pregnancies with hypertensive disorders. PLoS ONE 2008, 3, e2766. [Google Scholar] [CrossRef] [PubMed]
- Dennery, P.A. Regulation and role of heme oxygenase in oxidative injury. Curr. Top. Cell. Regul. 2000, 36, 181–199. [Google Scholar] [PubMed]
- Bauer, M.; Bauer, I. Heme oxygenase-1: Redox regulation and role in the hepatic response to oxidative stress. Antioxid. Redox Signal. 2002, 4, 749–758. [Google Scholar] [CrossRef] [PubMed]
- Suttner, D.M.; Sridhar, K.; Lee, C.S.; Tomura, T.; Hansen, T.N.; Dennery, P.A. Protective effects of transient ho-1 overexpression on susceptibility to oxygen toxicity in lung cells. Am. J. Physiol. 1999, 276, L443–L451. [Google Scholar] [PubMed]
- Dennery, P.A.; Spitz, D.R.; Yang, G.; Tatarov, A.; Lee, C.S.; Shegog, M.L.; Poss, K.D. Oxygen toxicity and iron accumulation in the lungs of mice lacking heme oxygenase-2. J. Clin. Investig. 1998, 101, 1001–1011. [Google Scholar] [CrossRef] [PubMed]
- Ryter, S.W.; Tyrrell, R.M. The heme synthesis and degradation pathways: Role in oxidant sensitivity. Heme oxygenase has both pro- and antioxidant properties. Free Radic. Biol. Med. 2000, 28, 289–309. [Google Scholar] [CrossRef]
- Balla, G.; Jacob, H.S.; Balla, J.; Rosenberg, M.; Nath, K.; Apple, F.; Eaton, J.W.; Vercellotti, G.M. Ferritin: A cytoprotective antioxidant strategem of endothelium. J. Biol. Chem. 1992, 267, 18148–18153. [Google Scholar] [PubMed]
- Park, S.W.; Kang, J.W.; Lee, S.M. The role of heme oxygenase-1 in drug metabolizing dysfunction in the alcoholic fatty liver exposed to ischemic injury. Toxicol. Appl. Pharmacol. 2016, 292, 30–39. [Google Scholar] [CrossRef] [PubMed]
- Lim, H.S.; Jin, S.; Yun, S.J. Modulation of melanogenesis by heme oxygenase-1 via p53 in normal human melanocytes. Chonnam Med. J. 2016, 52, 45–52. [Google Scholar] [CrossRef] [PubMed]
- Carter, W.O.; Narayanan, P.K.; Robinson, J.P. Intracellular hydrogen peroxide and superoxide anion detection in endothelial cells. J. Leukoc. Biol. 1994, 55, 253–258. [Google Scholar] [PubMed]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative pcr and the 2(-delta delta c(t)) method. Methods. 2001, 25, 402–408. [Google Scholar] [CrossRef] [PubMed]
- Carothers, A.M.; Rizvi, H.; Hasson, R.M.; Heit, Y.I.; Davids, J.S.; Bertagnolli, M.M.; Cho, N.L. Mesenchymal stromal cell mutations and wound healing contribute to the etiology of desmoid tumors. Cancer Res. 2012, 72, 346–355. [Google Scholar] [CrossRef] [PubMed]


| Group | SOD | MDA | GSH | HO-1 |
|---|---|---|---|---|
| (units/mg Protein) | (nmol/mg Protein) | (mg/g Protein) | (pg/mL) | |
| Control | 42.71 ± 2.95 | 3.03 ± 0.29 | 0.40 ± 0.06 | 15,167.57 ± 2773.04 |
| DON-L | 46.26 ± 1.09 * | 2.88 ± 0.77 | 0.40 ± 0.02 | 10,246.06 ± 1899.01 * |
| DON-M | 50.88 ± 4.27 * | 1.80 ± 0.54 | 0.40 ± 0.05 | 11,554.24 ± 4038.46 |
| DON-H | 43.05 ± 0.25 | 5.18 ± 0.48 ** | 0.37 ± 0.05 | 13,470.00 ± 1939.74 |
| Group | SOD | MDA | GSH | HO-1 |
|---|---|---|---|---|
| (units/mg Protein) | (nmol/mg Protein) | (μmol/mg Protein) | (ng/mL) | |
| Control | 37.79 ± 2.20 | 3.98 ± 0.29 | 23.60 ± 2.44 | 73.33 ± 4.48 |
| Hemin, 3 h | 35.18 ± 2.21 # | 4.30 ± 0.73 # | 11.08 ± 0.30 **,## | 93.74 ± 2.35 **,## |
| Znpp, 3 h | 34.34 ± 2.48 | 4.18 ± 0.18 # | 12.19 ± 1.77 ** | 52.12 ± 2.59 **,## |
| DON, 3 h | 28.58 ± 4.65 * | 2.99 ± 1.25 | 13.37 ± 2.15 ** | 74.24 ± 5.78 |
| Hemin, 12 h | 17.75 ± 6.95 **,## | 2.50 ± 0.24 | 4.35 ± 2.44 ** | 20.71 ± 10.26 ** |
| Znpp, 12 h | 46.20 ± 5.17 *,## | 4.82 ± 0.71 | 5.17 ± 0.57 ** | 21.31 ± 3.24 ** |
| DON, 12 h | 30.38 ± 3.26 * | 3.79 ± 0.39 | 3.51 ± 0.28 ** | 19.90 ± 2.88 ** |
| Hemin, 24 h | 9.13 ± 6.58 **,# | 3.97 ± 0.46 | 2.36 ± 1.74 ** | 14.55 ± 0.00 **,# |
| Znpp, 24 h | 17.16 ± 3.12 ** | 4.03 ± 0.24 | 4.72 ± 1.32 **,## | 21.21 ± 6.00 **,## |
| DON, 24 h | 20.22 ± 5.36 ** | 4.28 ± 0.81 | 0.16 ± 0.27 ** | 7.68 ± 1.52 ** |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yu, M.; Chen, L.; Peng, Z.; Wang, D.; Song, Y.; Wang, H.; Yao, P.; Yan, H.; Nüssler, A.K.; Liu, L.; et al. Embryotoxicity Caused by DON-Induced Oxidative Stress Mediated by Nrf2/HO-1 Pathway. Toxins 2017, 9, 188. https://doi.org/10.3390/toxins9060188
Yu M, Chen L, Peng Z, Wang D, Song Y, Wang H, Yao P, Yan H, Nüssler AK, Liu L, et al. Embryotoxicity Caused by DON-Induced Oxidative Stress Mediated by Nrf2/HO-1 Pathway. Toxins. 2017; 9(6):188. https://doi.org/10.3390/toxins9060188
Chicago/Turabian StyleYu, Miao, Liangkai Chen, Zhao Peng, Di Wang, Yadong Song, Hanyin Wang, Ping Yao, Hong Yan, Andreas K. Nüssler, Liegang Liu, and et al. 2017. "Embryotoxicity Caused by DON-Induced Oxidative Stress Mediated by Nrf2/HO-1 Pathway" Toxins 9, no. 6: 188. https://doi.org/10.3390/toxins9060188







