Prevalence, Variability and Bioconcentration of Saxitoxin-Group in Different Marine Species Present in the Food Chain
Abstract
:1. Introduction
2. Results and Discussion
2.1. Prevalence of Alexandrium Catenella in the Study Area
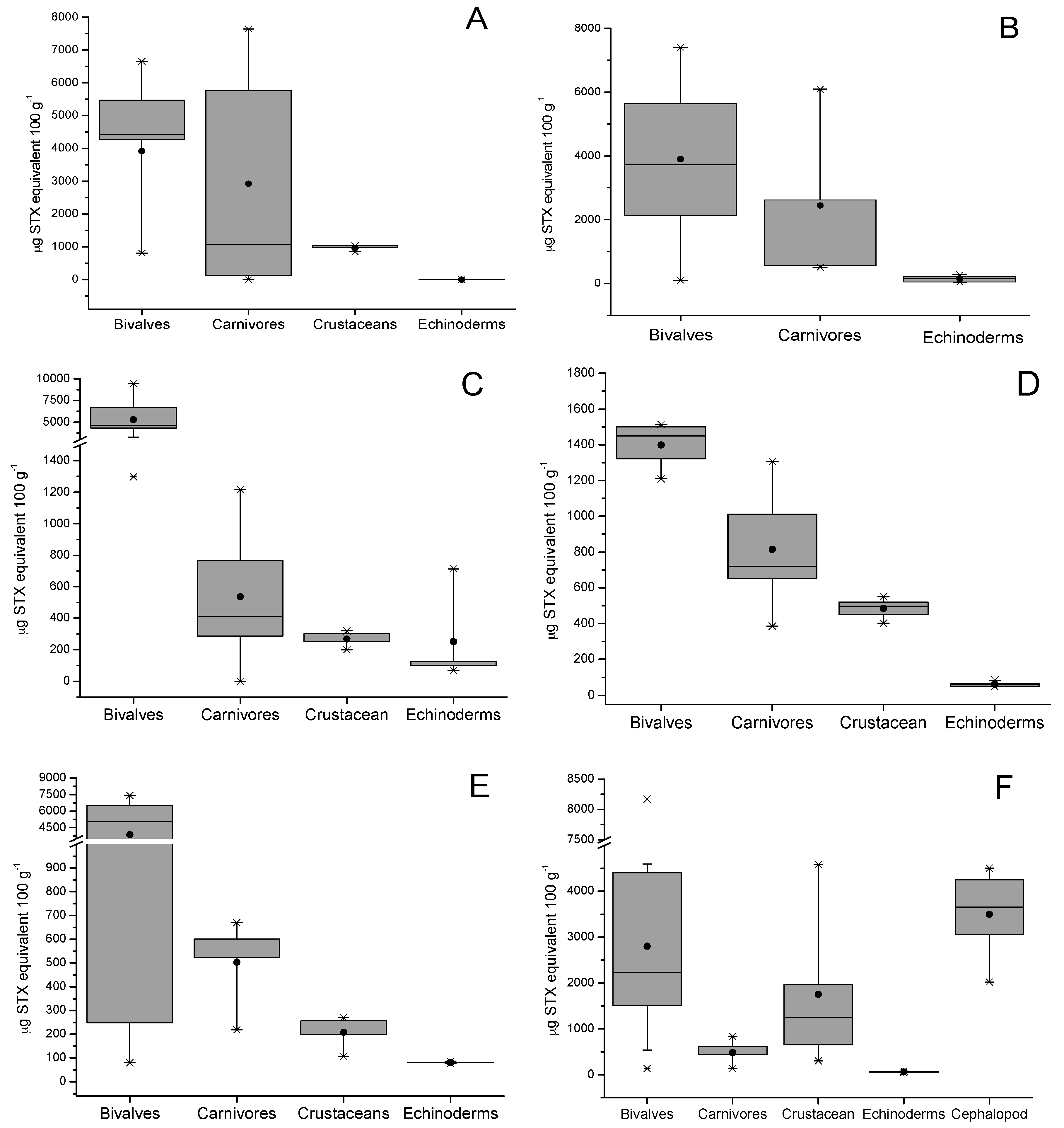
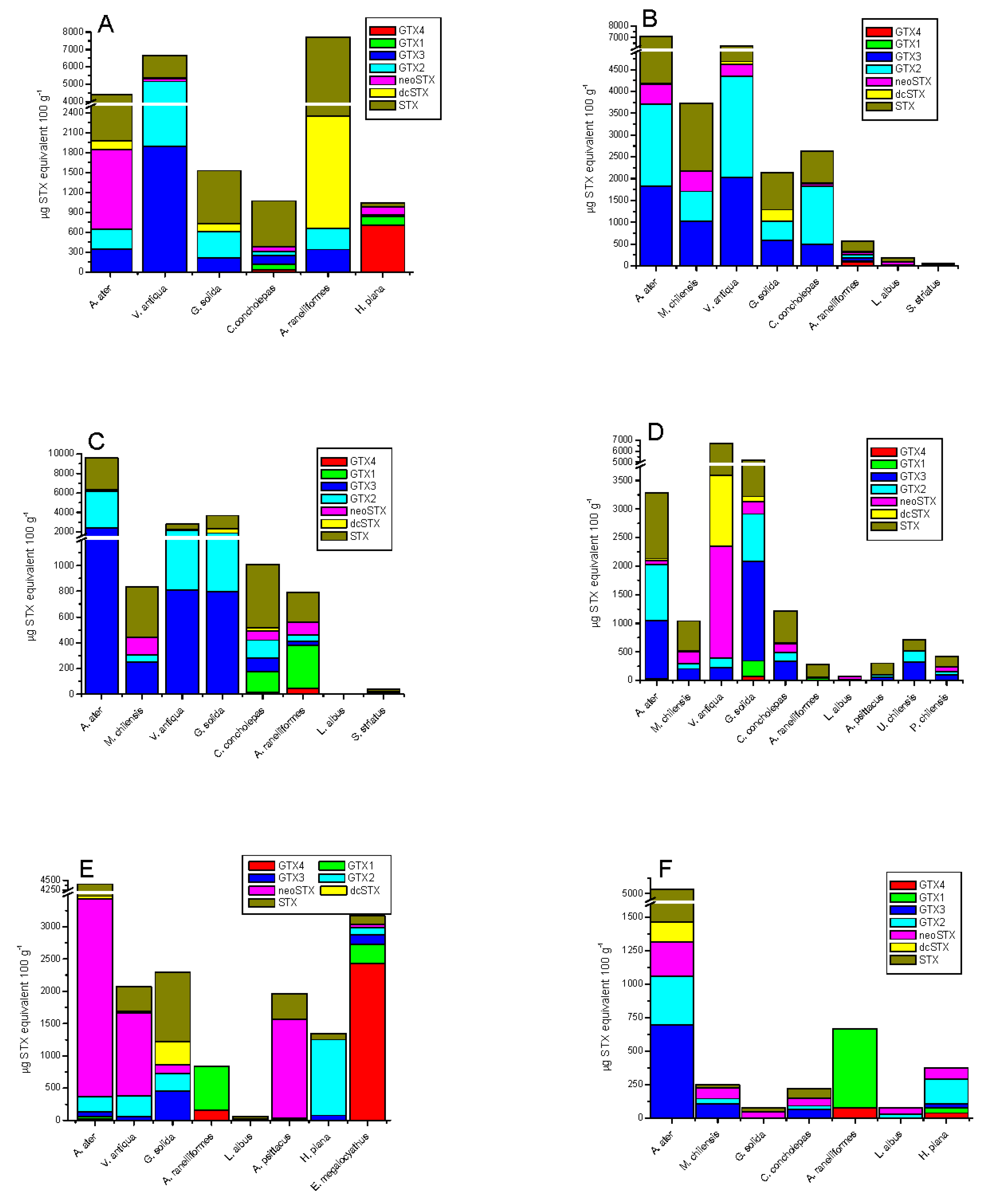
2.2. Prevalence of STX-Group in Fresh Shellfish Samples
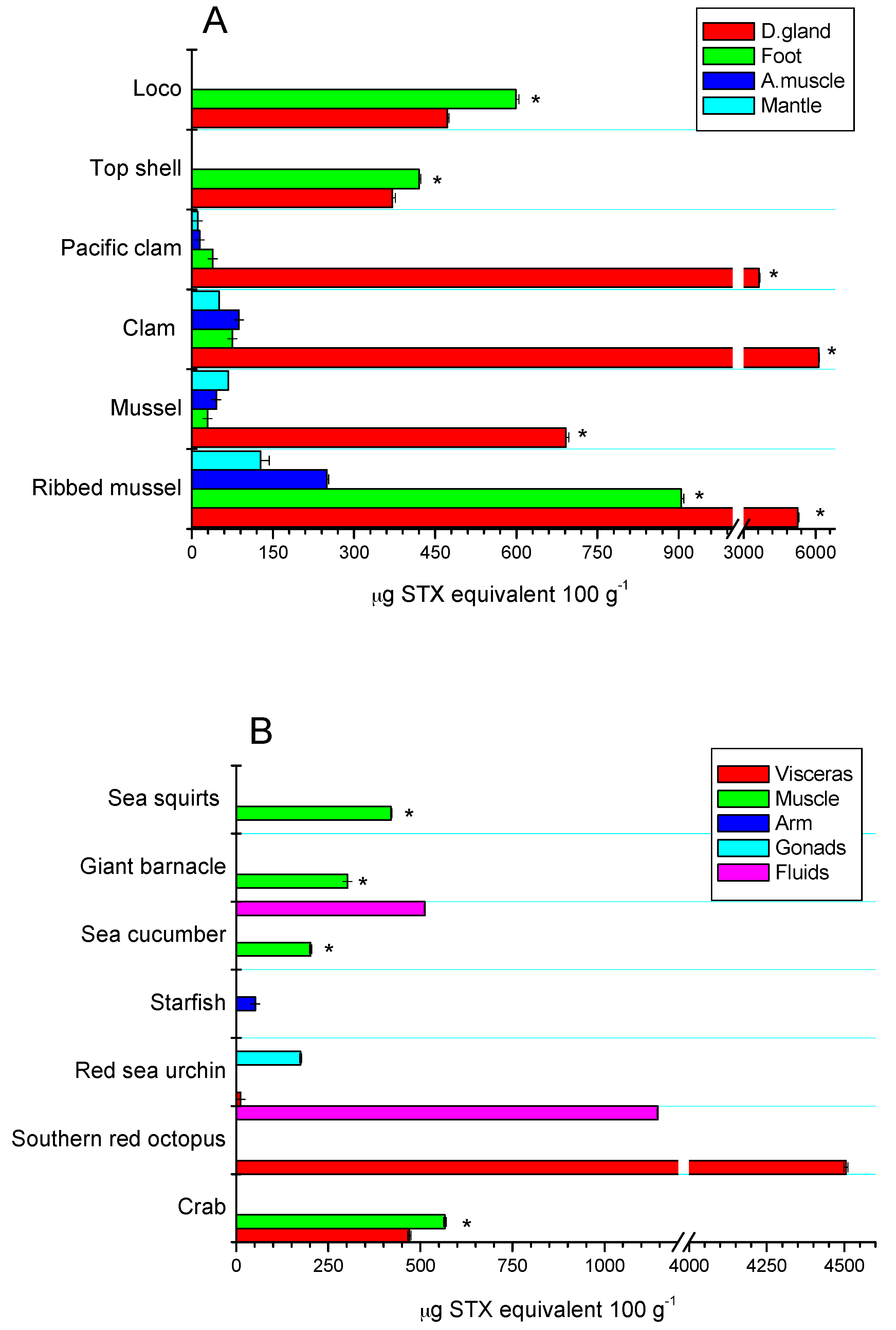
2.3. Tissue Distribution
2.4. Estimation of the Daily Intake
2.5. Food Implications
3. Conclusions
4. Materials and Methods
4.1. Chemical and Reagents
4.2. Standards
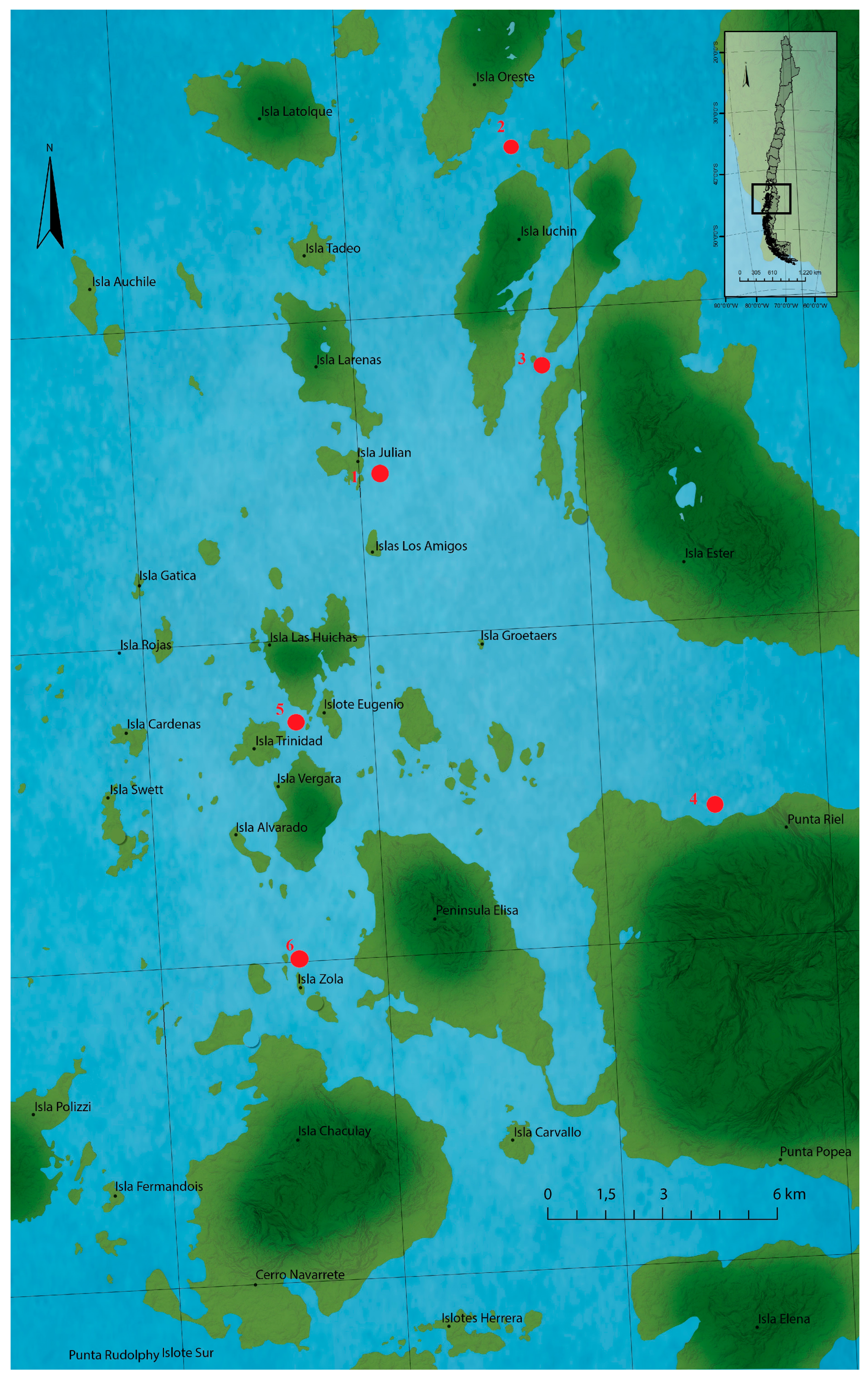
4.3. Sampling
4.4. STX-Group Sample Preparation
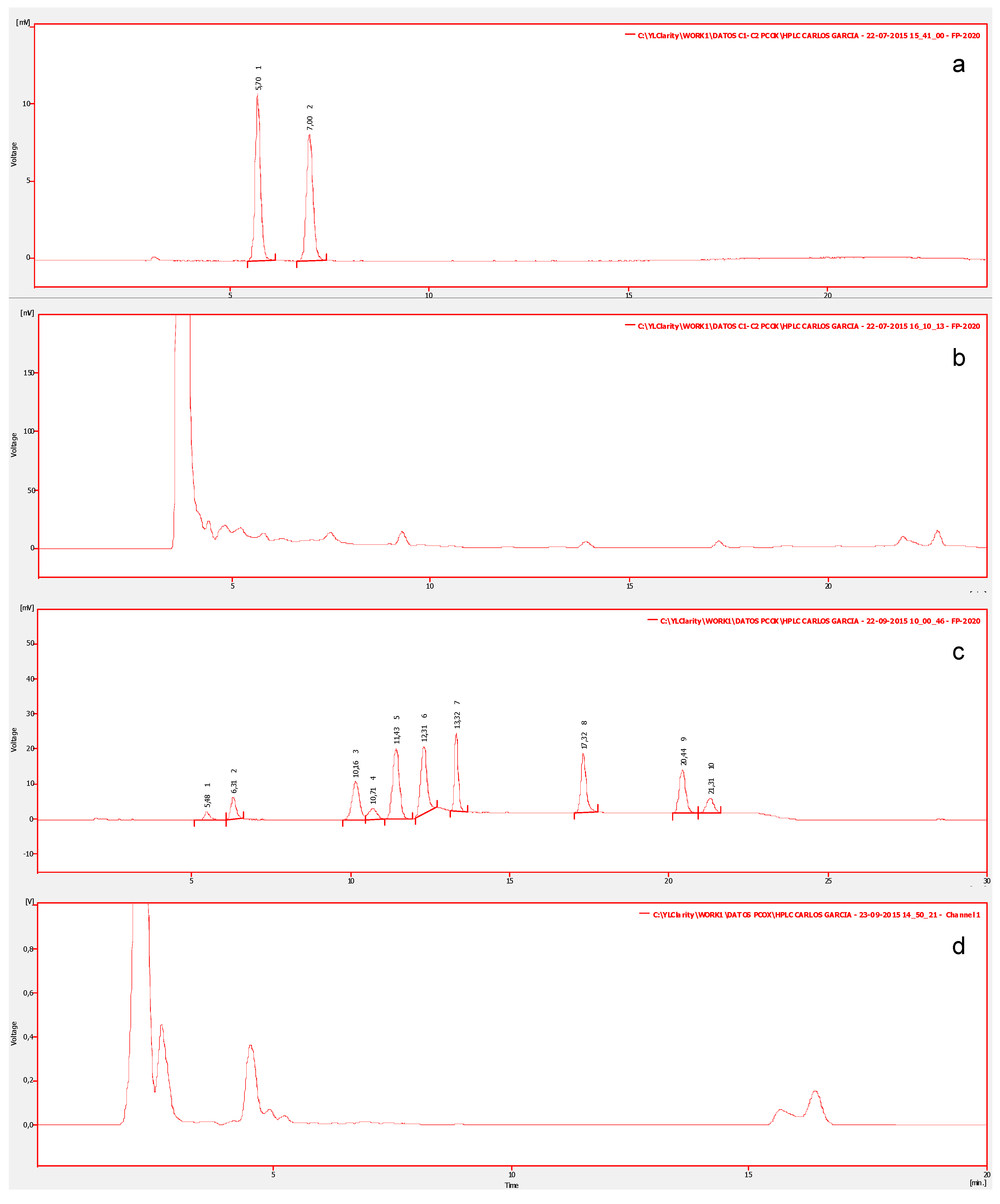
4.5. High Resolution Liquid Chromatography with Fluorescent Detection (LC-PCOX)
4.6. Method Validation
4.7. Statistical Analyses
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Bricelj, V.M.; Connell, L.; Konoki, K.; MacQuarrie, S.P.; Scheuer, T.; Catterall, W.A. Sodium channel mutation leading to saxitoxin resistance in clams increases risk of PSP. Nature 2005, 434, 763–767. [Google Scholar] [CrossRef] [PubMed]
- Llewellyn, L.E. Saxitoxin, a toxic marine natural product that targets a multitude of receptors. Nat. Prod. Rep. 2006, 23, 200–222. [Google Scholar] [CrossRef] [PubMed]
- Wiese, M.; D’Agostino, P.M.; Mihali, T.K.; Moffitt, M.C.; Neilan, B.A. Neurotoxic alkaloids: Saxitoxin and its analogs. Mar. Drugs 2010, 8, 2185–2211. [Google Scholar] [CrossRef] [PubMed]
- Food and Agriculture Organization of the United Nations World Health Organization. Technical Paper on Toxicity Equivalency Factors for Marine Biotoxins Associated with Bivalve Molluscs; FAO/WHO: Rome, Italy, 2016; p. 108. [Google Scholar]
- Botana, L.M.; Hess, P.; Munday, R.; Nathalie, A.; De Grasse, S.L.; Feeley, M.; Suzuki, T.; van der Berg, M.; Fattori, V.; Garrido Gamarra, E.; et al. Derivation of toxicity equivalency factors for marine biotoxins associated with Bivalve Molluscs. Trends Food Sci. Technol. 2017, 50, 15–24. [Google Scholar] [CrossRef]
- EFSA Scientific Opinion of the Panel on Contaminants in the Food Chain. Marine biotoxins in shellfish—Saxitoxin group. EFSA J. 2009, 1019, 1–76. [Google Scholar]
- García, C.; Pérez, F.; Contreras, C.; Figueroa, D.; Barriga, A.; López-Rivera, A.; Araneda, O.F.; Contreras, H.R. Saxitoxins and okadaic acid group: Accumulation and distribution in invertebrate marine vectors from Southern Chile. Food Addit. Contam. 2015, 32, 984–1002. [Google Scholar] [CrossRef] [PubMed]
- Manthey-Karl, M.; Lehmann, I.; Ostermeyer, U.; Rehbein, H.; Schrö, U. Meat Composition and Quality Assessment of King Scallops (Pecten. maximus) and Frozen Atlantic Sea Scallops (Placopecten. magellanicus) on a Retail Level. Foods 2015, 4, 524–546. [Google Scholar] [CrossRef] [PubMed]
- Flores-Moya, A. Effects of adaptation, chance, and history on the evolution of the toxic dinoflagellate Alexandrium. minutum under selection of increased temperature and acidification. Ecol. Evol. 2012, 2, 1251–1259. [Google Scholar] [CrossRef] [PubMed]
- Glibert, P.M.; Allen, J.I.; Artioli, J.; Beusen, A.; Bouwman, L.; Harle, J.; Holmes, R.; Holt, J. Vulnerability of coastal ecosystems to changes in harmful algal bloom distribution in response to climate change: Projections based on model analysis. Glob. Chang. Biol. 2014, 20, 3845–3858. [Google Scholar] [CrossRef] [PubMed]
- Wells, M.L.; Trainer, V.L.; Smayda, T.J.; Karlson, B.S.O.; Trick, C.G.; Kudela, R.M.; Ishikawa, A.; Bernard, S.; Wulff, A.; Anderson, D.M.; et al. Harmful algal blooms and climate change: Learning from the past and present to forecast the future. Harmful Algae 2015, 49, 68–93. [Google Scholar] [CrossRef] [PubMed]
- Banco Central. Foreign Trade Indicators; Banco Central de Chile: Santiago, Chile, 2016; pp. 1–260. [Google Scholar]
- AOAC Official Method 958.08. Paralytic shellfish poison. In Official Methods of Analysis of AOAC International, 18th ed.; Horwitz, W.; Latimer, G.W. (Eds.) AOAC International: Gaithersburg, MD, USA, 2005; pp. 79–82. [Google Scholar]
- AOAC. Method June 2005. Paralytic Shellfish Poisoning Toxins in Shellfish. Prechromatographic Oxidation and Liquid Chromatography with Fluorescence Detection. In Official Methods of Analysis of the Association of Official Analytical Chemists, 1st ed.; AOAC: Gaithersburg, MD, USA, 2005. [Google Scholar]
- AOAC. Official method February 2011. Determination of paralytic shellfish poisoning toxins in mussels, clams, oysters and Scallops. In Post-Column Oxidation Method (PCOX); First Action 2011; AOAC International: Gaithersburg, MD, USA, 2011. [Google Scholar]
- Turner, A.D.; Hatfield, R.G.; Rapkova, M.; Higman, W.; Algoet, M.; Suarez-Isla, B.A.; Cordova, M.; Caceres, C.; van de Riet, J.; Gibbs, R.; et al. Comparison of AOAC June 2005. LC official method with other methodologies for the quantitation of paralytic shellfish poisoning toxins in UK shellfish species. Anal. Bioanal. Chem. 2011, 399, 1257–1270. [Google Scholar] [CrossRef] [PubMed]
- Turner, A.D.; McNabb, P.S.; Harwood, D.T.; Selwood, A.I.; Boundy, M.J. Single-Laboratory Validation of a Multitoxin Ultra-Performance LC-Hydrophilic Interaction LC-MS/MS Method for Quantitation of Paralytic Shellfish Toxins in Bivalve Shellfish. J. AOAC Int. 2015, 98, 609–621. [Google Scholar] [PubMed]
- Minsal. Informe programa de Vigilancia de Floraciones Algales Nocivas (FANs) en Chile; Gobierno de Chile: Santiago, Chile, 2010; pp. 1–28. [Google Scholar]
- Sernapesca. Informe en Actividades de Pesca y Acuicultura; Servicio Nacional de Pesca y Acuicultura: Valparaiso, Chile, 2015; pp. 1–69. [Google Scholar]
- García, C.; Barriga, A.; Díaz, J.C.; Lagos, M.; Lagos, N. Route of metabolization and detoxication of paralytic shellfish toxins in humans. Toxicon 2010, 55, 135–144. [Google Scholar] [CrossRef] [PubMed]
- Visciano, P.; Schirone, M.; Berti, M.; Milandri, A.; Tofalo, R.; Suzzi, G. Marine Biotoxins: Ocurrence, Toxicity, Regulatory Limits and Reference Methods. Front. Microbiol. 2016, 7, 1051. [Google Scholar] [CrossRef] [PubMed]
- Munday, R.; Reeve, J. Risk Assessment of Shellfish Toxins. Toxins 2013, 5, 2109–2137. [Google Scholar] [CrossRef] [PubMed]
- García, C.; Contreras, H.R. Effects of both Paralytic Shellfish Toxins and Diarrhetic Shellfish Toxins in Human Poisoning: Toxicity, Distribution and Biotransformation. In Shellfish Human Consumption, Health Implications and Conservation Concerns; Robert, M.H., Ed.; Nova Science Publishers: New York, NY, USA, 2014; pp. 345–384. [Google Scholar]
- Aune, T. Risk assessment of Marine Toxins. In Seafood and Freshwater Toxins; Botana, L.M., Ed.; CRC Press: Boca Raton, FL, USA, 2008; pp. 3–20. [Google Scholar]
- Etheridge, S.M. Paralytic shellfish poisoning: Seafood safety and human health perspectives. Toxicon 2010, 56, 108–122. [Google Scholar] [CrossRef] [PubMed]
- Navarro, J.M.; Muñoz, M.G.; Contreras, A.M. Temperature as a factor regulating growth and toxin content in the dinoflagellate Alexandrium catenella. Harmful Algae 2006, 5, 762–769. [Google Scholar] [CrossRef]
- Anderson, D.M.; Alpermann, T.J.; Cembella, A.D.; Collos, Y.; Masseret, E.; Montresor, M. The globally distributed genus Alexandrium: Multifaceted roles in marine ecosystems and impacts on human health. Harmful Algae 2012, 14, 10–35. [Google Scholar] [CrossRef] [PubMed]
- García, C.; Seguel, M.; Uribe, J.C. Dynamics of toxic dinoflagellates blooms in the austral pacific region: Distribution, toxicity and impact on aquaculture. In Dinoflagellates: Biology, Geographical Distribution and Economic Importance Tobias RD; Lariree, V.M., Ed.; Nova Science Publishers: New York, NY, USA, 2013; pp. 1–56. [Google Scholar]
- Krock, B.; Seguel, C.G.; Cembella, A.D. Toxin profile of Alexandrium catenella from the Chilean coast as determined by liquid chromatography with fluorescence detection and liquid chromatography coupled with tandem mass spectrometry. Harmful Algae 2007, 6, 734–744. [Google Scholar] [CrossRef]
- Aguilera-Belmonte, A.; Inostroza, I.; Sáez Carrillo, K.; Franco, J.M.; Riobó, P.; Gómez, P.I. The combined effect of salinity and temperature on the growth and toxin content of four Chilean strains of Alexandrium catenella (Whedon and Kofoid) Balech 1985 (Dinophyceae) isolated from outbreak occurring in southern Chile in 2009. Harmful Algae 2013, 23, 55–59. [Google Scholar] [CrossRef]
- Montoya, N.G.; Fulco, V.K.; Carignan, M.O.; Carreto, J.I. Toxin variability in cultured and natural populations of Alexandrium tamarense from southern South America—Evidences of diversity and environmental regulation. Toxicon 2010, 56, 1408–1418. [Google Scholar] [CrossRef] [PubMed]
- Dìaz, P.A.; Molinet, C.; Seguel, M.; Díaz, M.; Labra, G.; Figueroa, R.I. Coupling planktonic and benthic shifts during a bloom of Alexandrium catenella in southern Chile: Implications for bloom dynamics and recurrence. Harmful Algae 2014, 40, 9–22. [Google Scholar] [CrossRef]
- Seguel, M.; Sfeir, A. Distribución de las toxinas marinas y quistes de dinoflagelados tóxicos en los canales occidentales de la Regiòn de Aysén. Cienc. Tecnol. Mar. 2010, 33, 43–55. [Google Scholar]
- Seguel, M.; Sfeir, A.; González, J.; Díaz, P.; Molinet, C.; Labra, G. Quistes de dinoflagelados en sedimentos marinos del sur de Chile con enfásis en Alexandrium catenella y Protoceratium reticulatum. Cienc. Tecnol. Mar. 2011, 34, 5–17. [Google Scholar]
- Mardones, J.I.; Bolch, C.; Guzmán, L.; Paredes, J.; Varela, D.; Hallegraeff, G.M. Role of resting cysts in Chilean Alexandrium catenella dinoflagellate blooms revisited. Harmful Algae 2016, 55, 238–249. [Google Scholar] [CrossRef] [PubMed]
- Smayda, T.J.; Trainer, V. Dinoflagellate blooms in upwelling systems: Seeding, variability, and contrasts with diatom bloom behaviour. Prog. Oceanogr. 2010, 85, 92–107. [Google Scholar] [CrossRef]
- Mardones, J.I.; Mûller, M.N.; Hallegraeff, G.M. Toxic dinoflagellate blooms of Alexandrium catenella in Chilean fjords: A resilient winner from climate change. ICES J. Mar. Sci. 2016. [Google Scholar] [CrossRef]
- Velasco, L.A.; Navarro, J.M. Feeding physiology of infaunal (Mulinia edulis) and epifaunal (Mytilus chilensis) bivalves under a wide range of concentrations and qualities of seston. Mar. Ecol. Prog. Ser. 2002, 240, 143–155. [Google Scholar] [CrossRef]
- Paganini, A.; Kimmerer, W.J.; Stillman, J.H. Metabolic responses to environmental salinity in the invasive clam Corbula amurensis. Aquat. Biol. 2010, 11, 139–147. [Google Scholar] [CrossRef]
- Navarro, J.; Torrijo, R. Physiological energetics of Concholepas concholepas (Bruguiere, 1789) (Gastropoda Muricidae) in Yaldad Bay, South of Chile. Rev. Chil. Hist. Nat. 1995, 68, 61–77. [Google Scholar]
- Cerdal, G.; Wolff, M. Feeding ecology of the crab Cancer polyodon in La Herradura Bay, northern Chile. II. Food spectrum and prey consumption. Mar. Ecol. Prog. Ser. 1993, 100, 119–125. [Google Scholar] [CrossRef]
- Lanas, P.; Riera, M.; Kowal, R.; López, B.A.; López, D.A. Alimentación Natural de Austromegabalanus psittacus (Molina, 1782) (Cirripedia: Balanidae) en el Golfo San Jorge (Chubut, Argentina). BioScriba 2011, 4, 38–43. [Google Scholar]
- Contreras, C.; Luna, N.; Dupré, E. Morfología del aparato reproductor del picoroco Austromegabalanus psittacus (Molina, 1782) (Cirripedia, Balanidae). Lat. Am. J. Aquat. Res. 2015, 43, 607–615. [Google Scholar]
- González, S.J.; Cáceres, C.W.; Ojeda, F.P. Ecología nutricional y alimenticia del erizo comestible Loxechinus albus en el norte de Chile. Rev. Chil. Hist. Nat. 2008, 81, 575–584. [Google Scholar]
- Zhanhui, Q.; Jun, W.; Yuze, M.; Jihong, Z.; Jianguang, F. Prey selection and feeding rate of sea stars Asterias amurensis and Asterina pectinifera on three bivalves. Acta Ecol. Sin. 2013, 33, 4878–4884. [Google Scholar] [CrossRef]
- Aguilera, M.A.; Navarrete, S.A.; Broitman, B.R. Differential effects of grazer species on periphyton of a temperate rocky shore. Mar. Ecol. Prog. Ser. 2013, 484, 63–78. [Google Scholar] [CrossRef]
- Ruiz, J.F.; Ibáñez, C.M.; Cáceres, C.W. Gut morphometry and feeding of the sea cucumber Athyonidium chilensis (Semper, 1868) (Echinodermata: Holothuroidea). Revista de Biología Marina y Oceanografía 2007, 42, 269–274. [Google Scholar] [CrossRef]
- Pérez, M.C.; López, D.A.; Aguila, K.; González, M.A. Feeding and growth in captivity of the octopus Enteroctopus megalocyathus Gould, 1852. Aquac. Res. 2006, 37, 550–555. [Google Scholar] [CrossRef]
- Zamorano, R.; Marín, M.; Cabrera, F.; Figueroa, D.; Contreras, C.; Barriga, A.; Lagos, N.; García, C. Determination of the variability of both hydrophilic and lipophilic toxins in endemic wild bivalves and carnivorous gastropods from the Southern part of Chile. Food Addit. Contam. 2013, 30, 1660–1677. [Google Scholar] [CrossRef] [PubMed]
- Lopes, V.M.; Baptista, M.; Repolho, T.; Rosa, R.; Reis Costa, P. Uptake, transfer and elimination kinetics of paralytic shellfish toxins in common octopus (Octopus vulgaris). Aquat. Toxicol. 2014, 146, 205–211. [Google Scholar] [CrossRef] [PubMed]
- Turner, A.D.; Dhanji-Rapkova, M.; Algoet, M.; Suarez-Isla, B.A.; Cordova, M.; Caceres, C.; van de Riet, J.; Murphy, C.J.; Case, M.; Lees, D.N. Investigations into matrix components affecting the performance of the official bioassay reference method for quantitation of paralytic shellfish poisoning toxins in oysters. Toxicon 2012, 59, 215–230. [Google Scholar] [CrossRef] [PubMed]
- Hansen, H.K.; Gutierrez, C.; Callejas, J.; Cameselle, C. Biosorption of lead from acidic aqueous solutions using Durvillaea Antarctica as adsorbent. Miner. Eng. 2013, 46, 95–99. [Google Scholar] [CrossRef]
- Navarro, J.M.; González, K.; Cisternas, B.; López, J.A.; Chaparro, O.R.; Segura, C.J.; Córdova, M.; Suárez-Isla, B.; Fernández-Reiriz, M.J.; Labarta, U. Contrasting physiological responses of two populations of the razor clam Tagelus dombeii with different histories of exposure to paralytic shellfish poisoning (PSP). PLoS ONE 2014, 9, e105794. [Google Scholar] [CrossRef] [PubMed]
- Shumway, S. Phycotoxin related shellfish poisoning: Bivalve molluscs are not the only vectors. Rev. Fish. Sci. 1995, 3, 1–31. [Google Scholar] [CrossRef]
- Fernández-Reiriz, M.J.; Navarro, J.M.; Contreras, A.M.; Labarta, U. Trophic interactions between the toxic dinoflagellate Alexandrium catenella and Mytilus chilensis: Feeding and digestive behaviour to long-term exposure. Aquat. Toxicol. 2008, 87, 245–251. [Google Scholar] [CrossRef] [PubMed]
- Deeds, J.; Landsberg, J.; Etheridge, S.; Pitcher, G.; Longan, S. Non-traditional vectors for paralytic shellfish poisoning. Mar. Drugs 2008, 6, 308–348. [Google Scholar] [CrossRef] [PubMed]
- Mardones, J.I.; Dorantes-Aranda, J.J.; Nichols, P.D.; Hallegraeff, G.M. Fish gill damage by the dinoflagellate Alexandrium catenella from Chilean fjords: Synergistic action of ROS and PUFA. Harmful Algae 2015, 49, 40–49. [Google Scholar] [CrossRef]
- Chen, C.Y.; Chou, H.N. Ichthyotoxicity studies of milkfish Chanos chanos fingerlings exposed to a harmful dinoflagellate Alexandrium minutum. J. Exp. Mar. Biol. Ecol. 2001, 262, 211–219. [Google Scholar] [CrossRef]
- Kwong, R.W.M.; Wang, W.; Lam, P.K.S.; Yu, P.K.N. The uptake, distribution and elimination of paralytic shellfish toxins in mussels and fish exposed to toxic dinoflagellates. Aquat. Toxicol. 2006, 80, 82–91. [Google Scholar] [CrossRef] [PubMed]
- Gestal-Otero, J.J. Epidemiological of marine toxins. seafood and freshwater toxins: Pharmacology, physiology, and detection. In Seafood and Freshwater Toxins: Pharmacology, Physiology and Detection, 3rd ed.; Botana, L.M., Ed.; CRC Press: New York, NY, USA, 2014; pp. 123–195. [Google Scholar]
- García, C.; Bravo, M.C.; Lagos, M.; Lagos, N. Paralytic shellfish poisoning: Post-mortem analysis of tissue and body fluid samples from human victims in the Patagonia fjords. Toxicon 2004, 43, 149–158. [Google Scholar] [CrossRef] [PubMed]
- Asakawa, M.; Tsuruda, S.; Ishimoto, Y.; Shimomura, M.; Kishimoto, K.; Shida, Y.; Barte-Quilantang, M.; Gomez-Delan, G. Paralytic toxin profiles of xanthid crab Atergatis floridus collected on reefs of Ishigaki Island, Okinawa Prefecture, Japan and Camotes Island, Cebu Province, Philippines. Sci. J. Clin. Med. 2014, 3, 75–81. [Google Scholar] [CrossRef]
- Oikawa, H.; Fujita, T.; Saito, K.; Satomi, M.; Yano, Y. Difference in the level of paralytic shellfish poisoning toxin accumulation between the crabs Telmessus acutidens and Charybdis japonica collected in Onahama, Fukushima Prefecture. Fish. Sci. 2007, 73, 395–403. [Google Scholar] [CrossRef]
- Seguel, M.; Sfeir, A.; Albornoz, V. Floraciones de microalgas tóxicas en la región de Aysén y su relación con larvas de peces. Cienc. Tecnol. Mar. 2010, 33, 31–42. [Google Scholar]
- Campbell, K.; Rawn, D.F.K.; Niedzwiadek, B.; Elliott, C.T. Paralytic shellfish poisoning (PSP) toxin binders for optical biosensor technology: Problems and possibilities for the future: A review. Food Addit. Contam. 2011, 28, 711–725. [Google Scholar] [CrossRef] [PubMed]
- FAO. Contributing to food security and nutrition for all. In The State of World Fisheries and Aquaculture (SOFIA); FAO: Rome, Italy, 2016; p. 200. [Google Scholar]
- Wong, C.W.; Hung, P.; Lee, K.L.H.; Mok, T.; Kam, K.M. Effect of steam cooking on distribution of paralytic shellfish toxins in different tissue compartments of scallops Patinopecten yessoensis. Food Chem. 2009, 114, 72–80. [Google Scholar] [CrossRef]
- Varela, D.; Paredes, J.; Alves-de-Souza, C.; Seguel, M.; Sfeir, A.; Frangópulos, M. Intraregional variation among Alexandrium catenella (Dinophyceae) strains from southern Chile: Morphological, toxicological and genetic Diversity. Harmful Algae 2012, 15, 8–18. [Google Scholar] [CrossRef]
- AOAC. Paralytic shellfish poison: Biological method, First Action, 1959, Final Action, Sec. 49.10.01. In Official Methods of Analysis of AOAC International, 17th ed.; Horwitz, W., Ed.; AOAC International: Gaithersburg, MD, USA, 2000. [Google Scholar]
- Van de Riet, J.; Gibbs, R.S.; Muggah, P.M.; Rourke, W.A.; Macneil, J.D.; Quilliam, M.A. Liquid chromatography post-column oxidation (PCOX) method for the determination of paralytic shellfish toxins in mussels, clams, oysters, and scallops: Collaborative study. J. AOAC Int. 2011, 94, 1154–1176. [Google Scholar] [PubMed]
- Whitmire, M.; Ammerman, J.; de Lisio, P.; Killmer, J.; Kyle, D.; Mainstone, E.; Porter, L.; Zhang, T. LC-MS/MS Bioanalysis Method Development, Validation, and Sample Analysis: Points to Consider When Conducting Nonclinical and Clinical Studies in Accordance with Current Regulatory Guidances. J. Anal. Bioanal. Tech. 2011, S4, 2. [Google Scholar] [CrossRef]

| Species | μg STX eq 100 g−1 | Dose of STX Equiv Ingested by a Person of 60 kg from 400 g of Shellfish (μg STX equiv/kg Body Weight) | ARfD * μg STX equiv/kg Body Weight |
|---|---|---|---|
| Venus antiqua | >1900 ± 7.3 | 126.6 | 0.5 |
| Gari solida | >180 ± 1.2 | 12.0 | 0.5 |
| Aulacomya ater | >4100 ± 5.1 | 273.3 | 0.5 |
| Mytilus chilensis | >1500 ± 2.6 | 100.0 | 0.5 |
| Argobuccinum ranelliformes | >300 ± 1.1 | 20.0 | 0.5 |
| Concholepas concholepas | >129.0 ± 2.1 | 8.6 | 0.5 |
| Homalaspis plana | 2524 ± 4.1 | 168.2 | 0.5 |
| Loxechinus albus | 81.9 ± 0.6 | 5.4 | 0.5 |
| Pyura chilensis | 420.0 ± 2.4 | 28 | 0.5 |
| Austromegabalanus psittacus | >301.1 ± 0.7 | 20.1 | 0.5 |
| Urechis chilensis | 714.2 ± 3.5 | 47.6 | 0.5 |
| Fissurella nigra | 15.0 ± 0.1 | 1.0 | 0.5 |
| Enteroctopus megalocyathus | n.d. | --- | 0.5 |
| Salmo salar | n.d. | --- | 0.5 |
| Macrocystis pyrifera | n.d. | --- | 0.5 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Oyaneder Terrazas, J.; Contreras, H.R.; García, C. Prevalence, Variability and Bioconcentration of Saxitoxin-Group in Different Marine Species Present in the Food Chain. Toxins 2017, 9, 190. https://doi.org/10.3390/toxins9060190
Oyaneder Terrazas J, Contreras HR, García C. Prevalence, Variability and Bioconcentration of Saxitoxin-Group in Different Marine Species Present in the Food Chain. Toxins. 2017; 9(6):190. https://doi.org/10.3390/toxins9060190
Chicago/Turabian StyleOyaneder Terrazas, Javiera, Héctor R. Contreras, and Carlos García. 2017. "Prevalence, Variability and Bioconcentration of Saxitoxin-Group in Different Marine Species Present in the Food Chain" Toxins 9, no. 6: 190. https://doi.org/10.3390/toxins9060190






