Impact of Mycotoxins Secreted by Aspergillus Molds on the Inflammatory Response of Human Corneal Epithelial Cells
Abstract
:1. Introduction
2. Results
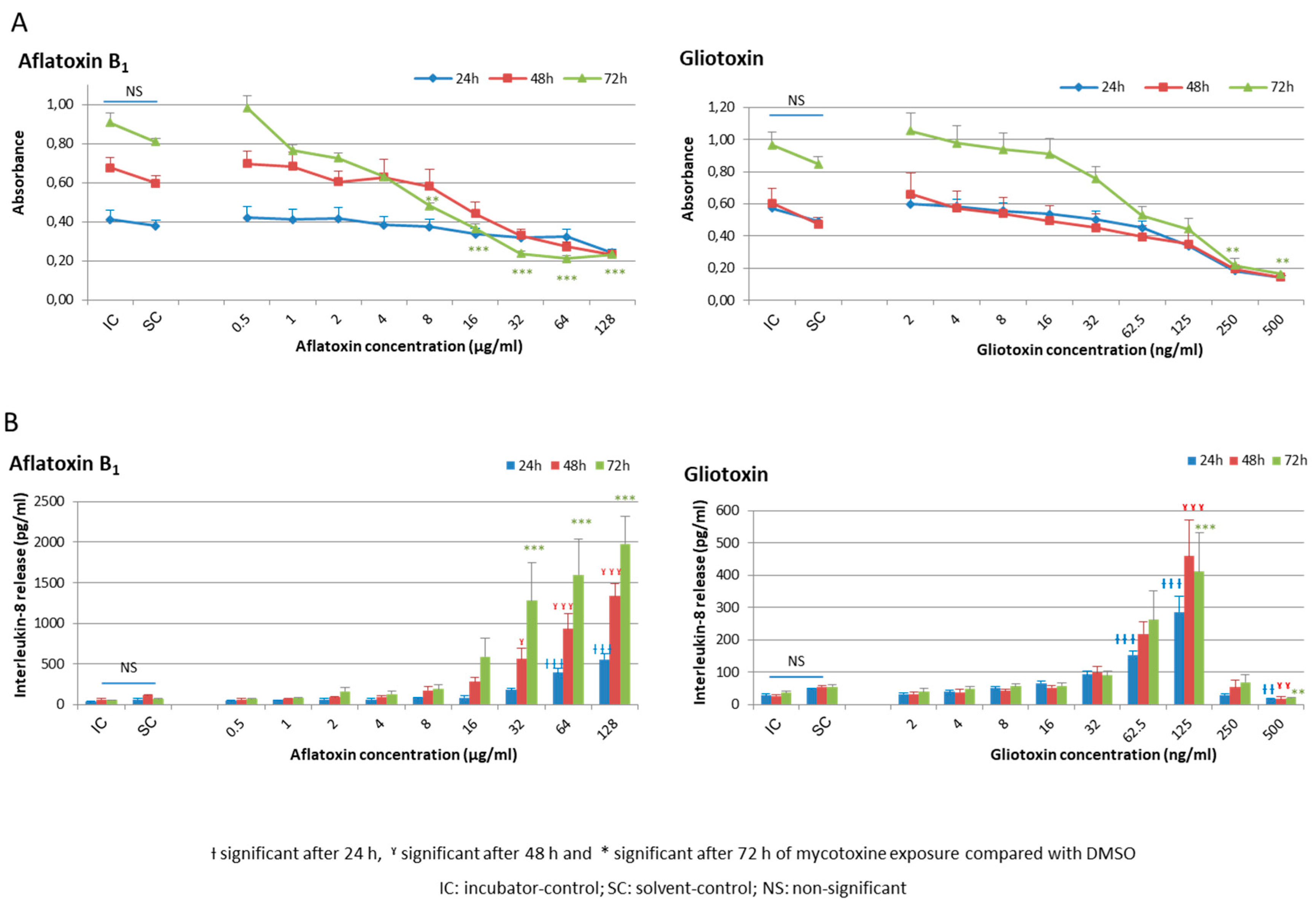
2.1. Cellular Viability and Inflammatory Response of HCE Cells after AFB1 and Gliotoxin Exposures
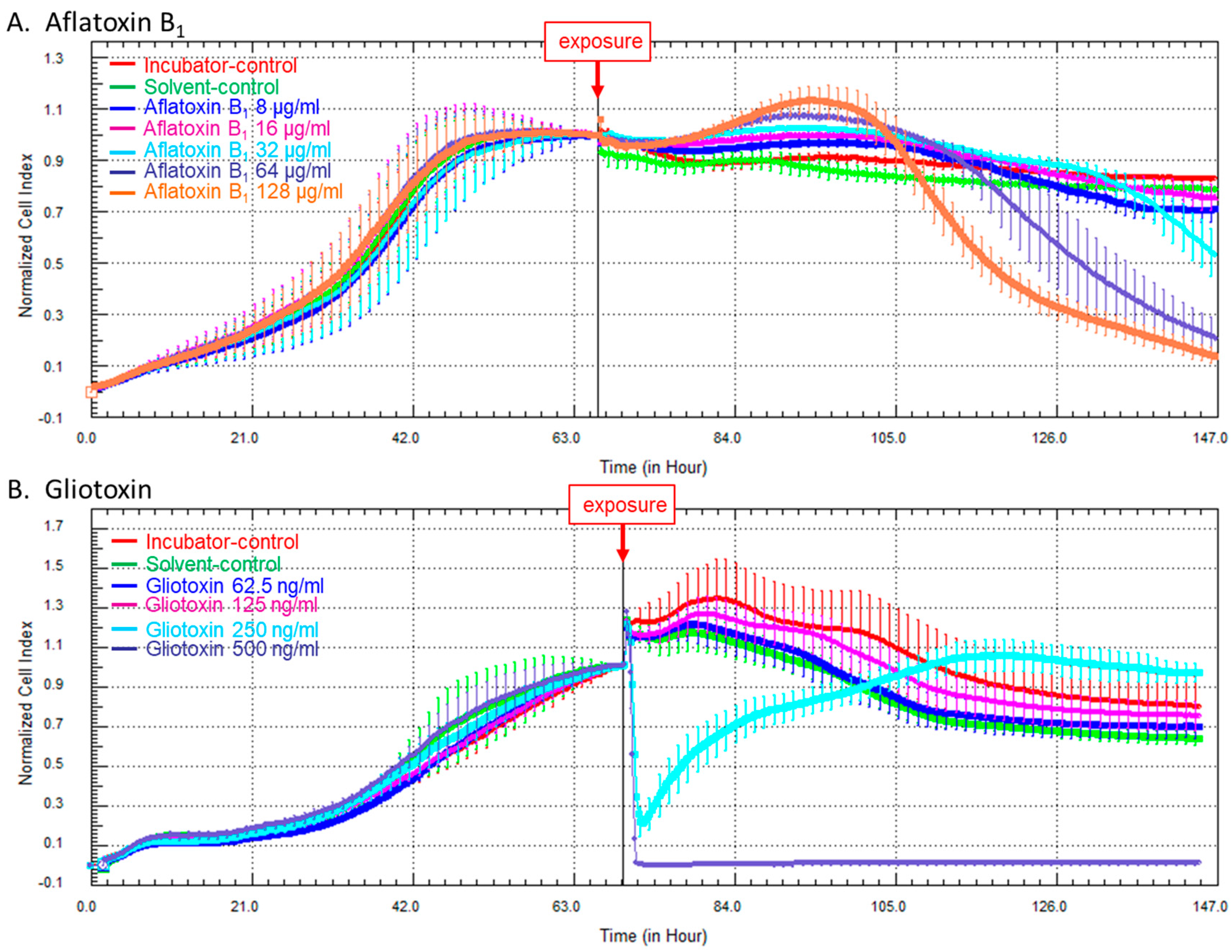
2.2. Kinetics of Cellular Impedance after Exposure of HCE Cells to AFB1 or Gliotoxin
3. Discussion
4. Conclusions
5. Experimental Section
5.1. Chemicals and Reagents
5.2. Cell Line and Culture Conditions
5.3. Cell Exposure to Mycotoxins
5.4. Cell Viability
5.5. Dosage of IL-8 Release
5.6. Quantification of the Gene Expression of Seven Pro-Inflammatory Cytokines
5.7. Real-Time Measurement of Cell Impedance
5.8. Statistical Analyses
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Yiannikouris, A.; Jouany, J.-P. Mycotoxins in feeds and their fate in animals: A review. Anim. Res. 2002, 51, 81–99. [Google Scholar] [CrossRef]
- Bennett, J.W.; Klich, M. Mycotoxins. Clin. Microbiol. Rev. 2003, 16, 497–516. [Google Scholar] [CrossRef] [PubMed]
- Goossens, J.; Pasmans, F.; Verbrugghe, E.; Vandenbroucke, V.; de Baere, S.; Meyer, E.; Haesebrouck, F.; de Backer, P.; Croubels, S. Porcine intestinal epithelial barrier disruption by the Fusarium mycotoxins deoxynivalenol and T-2 toxin promotes transepithelial passage of doxycycline and paromomycin. BMC Vet. Res. 2012, 8, 245. [Google Scholar] [CrossRef] [PubMed]
- Magan, N. Mycotoxin contamination of food in Europe: Early detection and prevention strategies. Mycopathologia 2006, 162, 245–253. [Google Scholar] [CrossRef] [PubMed]
- Richard, J.L. Some major mycotoxins and their mycotoxicoses—An overview. Int. J. Food Microbiol. 2007, 119, 3–10. [Google Scholar] [CrossRef] [PubMed]
- Corcuera, L.A.; Vettorazzi, A.; Arbillaga, L.; Gonzalez Penas, E.; Lopez de Cerain, A. An approach to the toxicity and toxicokinetics of aflatoxin B1 and ochratoxin A after simultaneous oral administration to fasted F344 rats. Food Chem. Toxicol. 2012, 50, 3440–3446. [Google Scholar] [CrossRef] [PubMed]
- Qian, G.; Tang, L.; Guo, X.; Wang, F.; Massey, M.E.; Su, J.; Guo, T.L.; Williams, J.H.; Phillips, T.D.; Wang, J.S. Aflatoxin B1 modulates the expression of phenotypic markers and cytokines by splenic lymphocytes of male F344 rats. J. Appl. Toxicol. 2014, 34, 241–249. [Google Scholar] [CrossRef] [PubMed]
- Marroquin-Cardona, A.G.; Johnson, N.M.; Phillips, T.D.; Hayes, A.W. Mycotoxins in a changing global environment—A review. Food Chem. Toxicol. 2014, 69, 220–230. [Google Scholar] [CrossRef] [PubMed]
- Todd, B.E.; Buchan, R.M. Total dust, respirable dust, and microflora toxin concentrations in Colorado corn storage facilities. Appl. Occup. Environ. Hyg. 2002, 17, 411–415. [Google Scholar] [CrossRef] [PubMed]
- Tsapko, V.G.; Chudnovets, A.J.; Sterenbogen, M.J.; Papach, V.V.; Dutkiewicz, J.; Skorska, C.; Krysinska-Traczyk, E.; Golec, M. Exposure to bioaerosols in the selected agricultural facilities of the Ukraine and Poland—A review. Ann. Agric. Environ. Med. 2011, 18, 19–27. [Google Scholar] [PubMed]
- Lawniczek-Walczyk, A.; Gorny, R.L.; Golofit-Szymczak, M.; Niesler, A.; Wlazlo, A. Occupational exposure to airborne microorganisms, endotoxins and beta-glucans in poultry houses at different stages of the production cycle. Ann. Agric. Environ. Med. 2013, 20, 259–268. [Google Scholar] [PubMed]
- Lanier, C.; Heutte, N.; Richard, E.; Bouchart, V.; Lebailly, P.; Garon, D. Mycoflora and mycotoxin production in oilseed cakes during farm storage. J. Agric. Food Chem. 2009, 57, 1640–1645. [Google Scholar] [CrossRef] [PubMed]
- Reboux, G.; Piarroux, R.; Mauny, F.; Madroszyk, A.; Millon, L.; Bardonnet, K.; Dalphin, J.C. Role of molds in farmer’s lung disease in Eastern France. Am. J. Respir. Crit. Care Med. 2001, 163, 1534–1539. [Google Scholar] [CrossRef] [PubMed]
- Smith, N.L.; Denning, D.W. Underlying conditions in chronic pulmonary aspergillosis including simple aspergilloma. Eur. Respir. J. 2011, 37, 865–872. [Google Scholar] [CrossRef] [PubMed]
- Norback, D.; Hashim, J.H.; Cai, G.H.; Hashim, Z.; Ali, F.; Bloom, E.; Larsson, L. Rhinitis, ocular, throat and dermal symptoms, headache and tiredness among students in schools from johor bahru, Malaysia: Associations with fungal DNA and mycotoxins in classroom dust. PLoS ONE 2016, 11, e0147996. [Google Scholar] [CrossRef] [PubMed]
- Bakand, S.; Hayes, A. Troubleshooting methods for toxicity testing of airborne chemicals in vitro. J. Pharmacol. Toxicol. Methods 2010, 61, 76–85. [Google Scholar] [CrossRef] [PubMed]
- Johannessen, L.; Nilsen, A.; Lovik, M. Mycotoxin-induced depletion of intracellular glutathione and altered cytokine production in the human alveolar epithelial cell line A549. Toxicol. Lett. 2007, 168, 103–112. [Google Scholar] [CrossRef] [PubMed]
- Schutze, N.; Lehmann, I.; Bonisch, U.; Simon, J.C.; Polte, T. Exposure to mycotoxins increases the allergic immune response in a murine asthma model. Am. J. Respir. Crit. Care Med. 2010, 181, 1188–1199. [Google Scholar] [CrossRef] [PubMed]
- Xiang, P.; Liu, R.Y.; Sun, H.J.; Han, Y.H.; He, R.W.; Cui, X.Y.; Ma, L.Q. Molecular mechanisms of dust-induced toxicity in human corneal epithelial cells: Water and organic extract of office and house dust. Environ. Int. 2016, 92–93, 348–356. [Google Scholar] [CrossRef] [PubMed]
- Xiang, P.; He, R.W.; Han, Y.H.; Sun, H.J.; Cui, X.Y.; Ma, L.Q. Mechanisms of housedust-induced toxicity in primary human corneal epithelial cells: Oxidative stress, proinflammatory response and mitochondrial dysfunction. Environ. Int. 2016, 89–90, 30–37. [Google Scholar] [CrossRef] [PubMed]
- Ke, N.; Wang, X.; Xu, X.; Abassi, Y.A. The xCELLigence system for real-time and label-free monitoring of cell viability. Methods Mol. Biol. 2011, 740, 33–43. [Google Scholar] [PubMed]
- Witzel, F.; Fritsche-Guenther, R.; Lehmann, N.; Sieber, A.; Bluthgen, N. Analysis of impedance-based cellular growth assays. Bioinformatics 2015, 31, 2705–2712. [Google Scholar] [CrossRef] [PubMed]
- Saarinen-Savolainen, P.; Jarvinen, T.; Araki-Sasaki, K.; Watanabe, H.; Urtti, A. Evaluation of cytotoxicity of various ophthalmic drugs, eye drop excipients and cyclodextrins in an immortalized human corneal epithelial cell line. Pharm. Res. 1998, 15, 1275–1280. [Google Scholar] [CrossRef] [PubMed]
- Anadon, A.; Martinez, M.A.; Castellano, V.; Martinez-Larranaga, M.R. The role of in vitro methods as alternatives to animals in toxicity testing. Expert Opin. Drug Metab. Toxicol. 2014, 10, 67–79. [Google Scholar] [CrossRef] [PubMed]
- Pahl, H.L.; Krauss, B.; Schulze-Osthoff, K.; Decker, T.; Traenckner, E.B.; Vogt, M.; Myers, C.; Parks, T.; Warring, P.; Muhlbacher, A.; et al. The immunosuppressive fungal metabolite gliotoxin specifically inhibits transcription factor NF-kappaB. J. Exp. Med. 1996, 183, 1829–1840. [Google Scholar] [CrossRef] [PubMed]
- Anselmi, K.; Stolz, D.B.; Nalesnik, M.; Watkins, S.C.; Kamath, R.; Gandhi, C.R. Gliotoxin causes apoptosis and necrosis of rat Kupffer cells in vitro and in vivo in the absence of oxidative stress: Exacerbation by caspase and serine protease inhibition. J. Hepatol. 2007, 47, 103–113. [Google Scholar] [CrossRef] [PubMed]
- Sosne, G.; Albeiruti, A.-R.; Hollis, B.; Siddiqi, A.; Ellenberg, D.; Kurpakus-Wheater, M. Thymosin β4 inhibits benzalkonium chloride-mediated apoptosis in corneal and conjunctival epithelial cells in vitro. Exp. Eye Res. 2006, 83, 502–507. [Google Scholar] [CrossRef] [PubMed]
- Erdinest, N.; Shmueli, O.; Grossman, Y.; Ovadia, H.; Solomon, A. Anti-inflammatory effects of alpha linolenic acid on human corneal epithelial cells. Investig. Ophthalmol. Vis. Sci. 2012, 53, 4396–4406. [Google Scholar] [CrossRef] [PubMed]
- Rozanova, E.; Heilig, P.; Godnić-Cvar, J. The eye-A neglected organ in environmental and occupational medicine: An overview of known environmental and occupational non-traumatic effects on the eyes. Arch. Ind. Hyg. Toxicol. 2009, 60, 205–215. [Google Scholar] [CrossRef] [PubMed]
- Pauly, A.; Roubeix, C.; Liang, H.; Brignole-Baudouin, F.; Baudouin, C. In vitro and in vivo comparative toxicological study of a new preservative-free latanoprost formulation. Investig. Ophthalmol. Vis. Sci. 2012, 53, 8172–8180. [Google Scholar] [CrossRef] [PubMed]
- Pflugfelder, S.C.; Jones, D.; Ji, Z.; Afonso, A.; Monroy, D. Altered cytokine balance in the tear fluid and conjunctiva of patients with Sjogren’s syndrome keratoconjunctivitis sicca. Curr. Eye Res. 1999, 19, 201–211. [Google Scholar] [CrossRef] [PubMed]
- Pedersen, L.; Pilegaard, H.; Hansen, J.; Brandt, C.; Adser, H.; Hidalgo, J.; Olesen, J.; Pedersen, B.K.; Hojman, P. Exercise-induced liver chemokine CXCL-1 expression is linked to muscle-derived interleukin-6 expression. J. Physiol. 2011, 589, 1409–1420. [Google Scholar] [CrossRef] [PubMed]
- Xu, Q.; Zhao, G.; Lin, J.; Wang, Q.; Hu, L.; Jiang, Z. Role of Dectin-1 in the innate immune response of rat corneal epithelial cells to Aspergillus fumigatus. BMC Ophthalmol. 2015, 15, 126. [Google Scholar] [CrossRef] [PubMed]
- Tuomas, P.; Tuomas, R.; Anu, K.; Liisa, M.; Antero, S.; Kaarniranta, K. The preservative polyquaternium-1 increases cytoxicity and NF-kappaB linked inflammation in human corneal epithelial cells. Mol. Vis. 2012, 18, 1189–1196. [Google Scholar]
- Deshmane, S.L.; KremLev, S.; Amini, S.; Sawaya, B.E. Monocyte chemoattractant protein-1 (MCP-1): An overview. J. Interferon Cytokine Res. 2009, 29, 313–326. [Google Scholar] [CrossRef] [PubMed]
- Wills-Karp, M. Interleukin-13 in asthma pathogenesis. Immunol. Rev. 2004, 202, 175–190. [Google Scholar] [CrossRef] [PubMed]
- Di, Y.P.; Tsou, Y.-A.; Lin, C.-D.; Chen, H.-C.; Hsu, H.-Y.; Wu, L.-T.; Chiang-Ni, C.; Chen, C.-J.; Wu, T.-F.; Kao, M.-C. Interleukin-13 inhibits lipopolysaccharide-induced BPIFA1 expression in nasal epithelial cells. PLoS ONE 2015, 10, e0143484. [Google Scholar]
- Braedel, S.; Radsak, M.; Einsele, H.; Latge, J.P.; Michan, A.; Loeffler, J.; Haddad, Z.; Grigoleit, U.; Schild, H.; Hebart, H. Aspergillus fumigatus antigens activate innate immune cells via toll-like receptors 2 and 4. Br. J. Haematol. 2004, 125, 392–399. [Google Scholar] [CrossRef] [PubMed]
- Guo, H.; Wu, X. Innate responses of corneal epithelial cells against Aspergillus fumigatus challenge. FEMS Immunol. Med. Microbiol. 2009, 56, 88–93. [Google Scholar] [CrossRef] [PubMed]
- Larsson, P.; Tjalve, H. Bioactivation of aflatoxin B1 in the nasal and tracheal mucosa in swine. J. Anim. Sci. 1996, 74, 1672–1680. [Google Scholar] [CrossRef] [PubMed]
- Sutton, P.; Waring, P.; Mullbacher, A. Exacerbation of invasive aspergillosis by the immunosuppressive fungal metabolite, gliotoxin. Immunol. Cell. Biol. 1996, 74, 318–322. [Google Scholar] [CrossRef] [PubMed]
- Fauquert, J.L.; Demoly, P. Diagnostic approach to conjunctival hyperreactivity. Rev. Fr. Allergol. 2005, 45, 226–233. [Google Scholar]
- Leonardi, A.; Bogacka, E.; Fauquert, J.L.; Kowalski, M.L.; Groblewska, A.; Jedrzejczak-Czechowicz, M.; Doan, S.; Marmouz, F.; Demoly, P.; Delgado, L. Ocular allergy: Recognizing and diagnosing hypersensitivity disorders of the ocular surface. Allergy 2012, 67, 1327–1337. [Google Scholar] [CrossRef] [PubMed]
- Hom, M.M.; Bielory, L. The anatomical and functional relationship between allergic conjunctivitis and allergic rhinitis. Allergy Rhinol. 2013, 4, 110–119. [Google Scholar] [CrossRef] [PubMed]
- Wichmann, G.; Herbarth, O.; Lehmann, I. The mycotoxins citrinin, gliotoxin, and patulin affect interferon-gamma rather than interleukin-4 production in human blood cells. Environ. Toxicol. 2002, 17, 211–218. [Google Scholar] [CrossRef] [PubMed]
- Johannessen, L.N.; Nilsen, A.M.; Lovik, M. The mycotoxins citrinin and gliotoxin differentially affect production of the pro-inflammatory cytokines tumour necrosis factor-alpha and interleukin-6, and the anti-inflammatory cytokine interleukin-10. Clin. Exp. Allergy 2005, 35, 782–789. [Google Scholar] [CrossRef] [PubMed]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef] [PubMed]

| Aflatoxin B1 (µg/mL) | % DMSO in Culture Medium * | Gliotoxin (ng/mL) | % DMSO in Culture Medium * |
|---|---|---|---|
| 0.5 | 0.005 | 2 | 4 × 10−5 |
| 1 | 0.01 | 4 | 8 × 10−5 |
| 2 | 0.02 | 8 | 1.6 × 10−4 |
| 4 | 0.04 | 16 | 3.2 × 10−4 |
| 8 | 0.08 | 32 | 6.4 × 10−4 |
| 16 | 0.16 | 62.5 | 1.25 × 10−3 |
| 32 | 0.32 | 125 | 2.5 × 10−3 |
| 64 | 0.64 | 250 | 5 × 10−3 |
| 128 | 1.28 | 500 | 0.01 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bossou, Y.M.; Serssar, Y.; Allou, A.; Vitry, S.; Momas, I.; Seta, N.; Menotti, J.; Achard, S. Impact of Mycotoxins Secreted by Aspergillus Molds on the Inflammatory Response of Human Corneal Epithelial Cells. Toxins 2017, 9, 197. https://doi.org/10.3390/toxins9070197
Bossou YM, Serssar Y, Allou A, Vitry S, Momas I, Seta N, Menotti J, Achard S. Impact of Mycotoxins Secreted by Aspergillus Molds on the Inflammatory Response of Human Corneal Epithelial Cells. Toxins. 2017; 9(7):197. https://doi.org/10.3390/toxins9070197
Chicago/Turabian StyleBossou, Yélian Marc, Youssra Serssar, Amel Allou, Sandrine Vitry, Isabelle Momas, Nathalie Seta, Jean Menotti, and Sophie Achard. 2017. "Impact of Mycotoxins Secreted by Aspergillus Molds on the Inflammatory Response of Human Corneal Epithelial Cells" Toxins 9, no. 7: 197. https://doi.org/10.3390/toxins9070197





