On-Chip Enucleation of Bovine Oocytes using Microrobot-Assisted Flow-Speed Control
Abstract
:1. Introduction
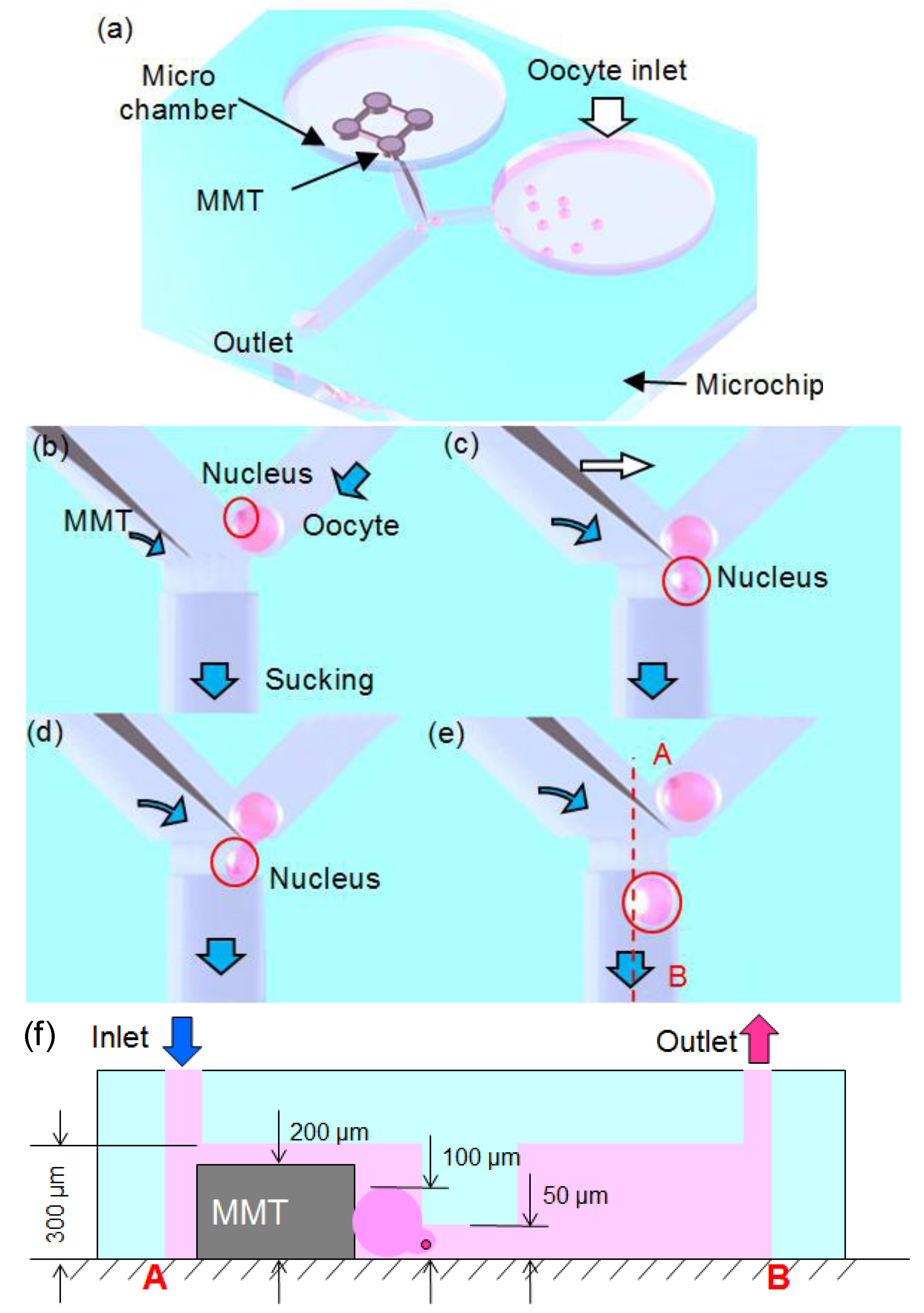
2. Material and Methods
2.1. Fluid Control by MMT
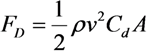
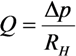
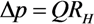
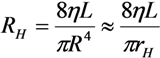
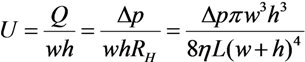

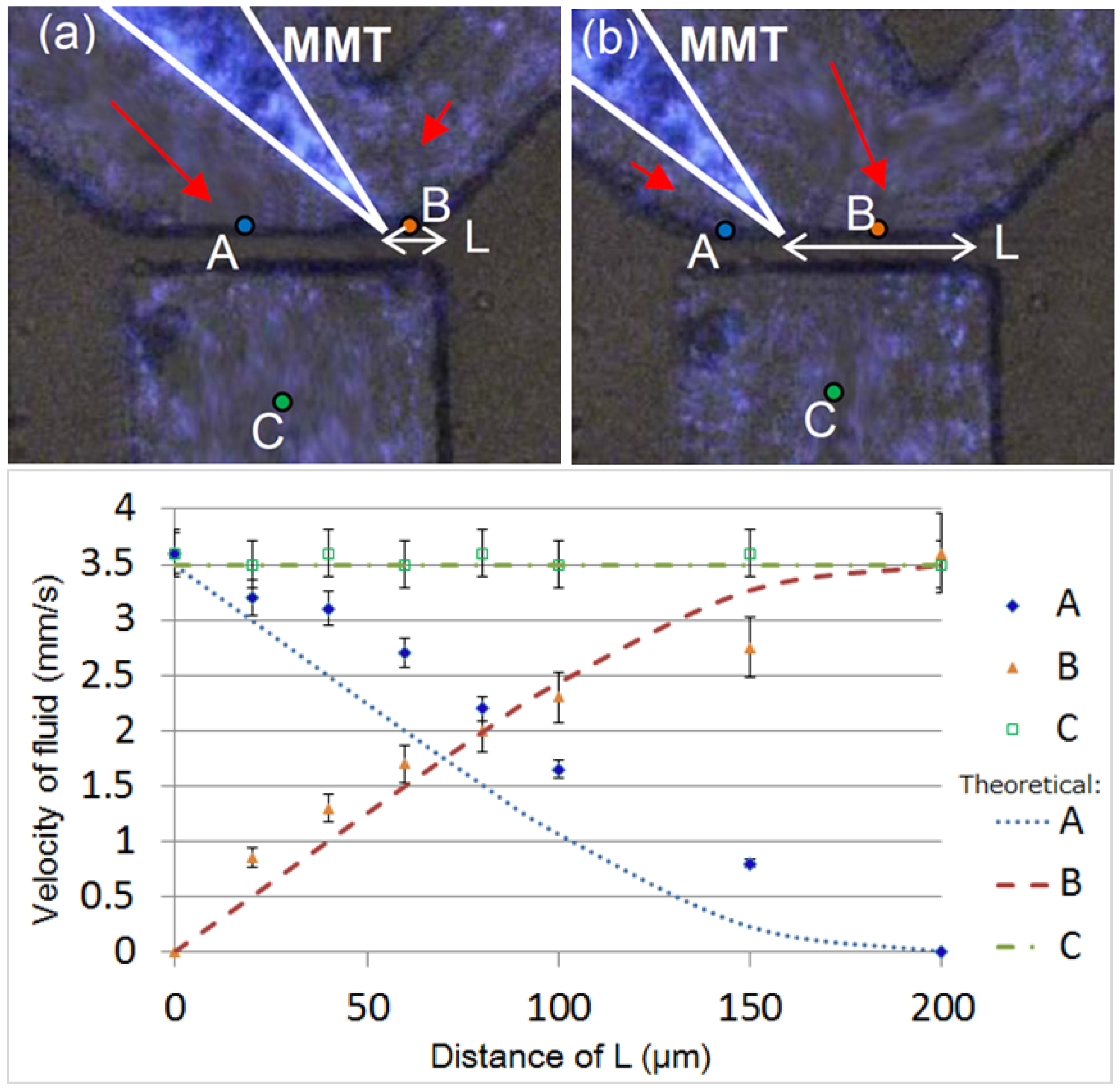
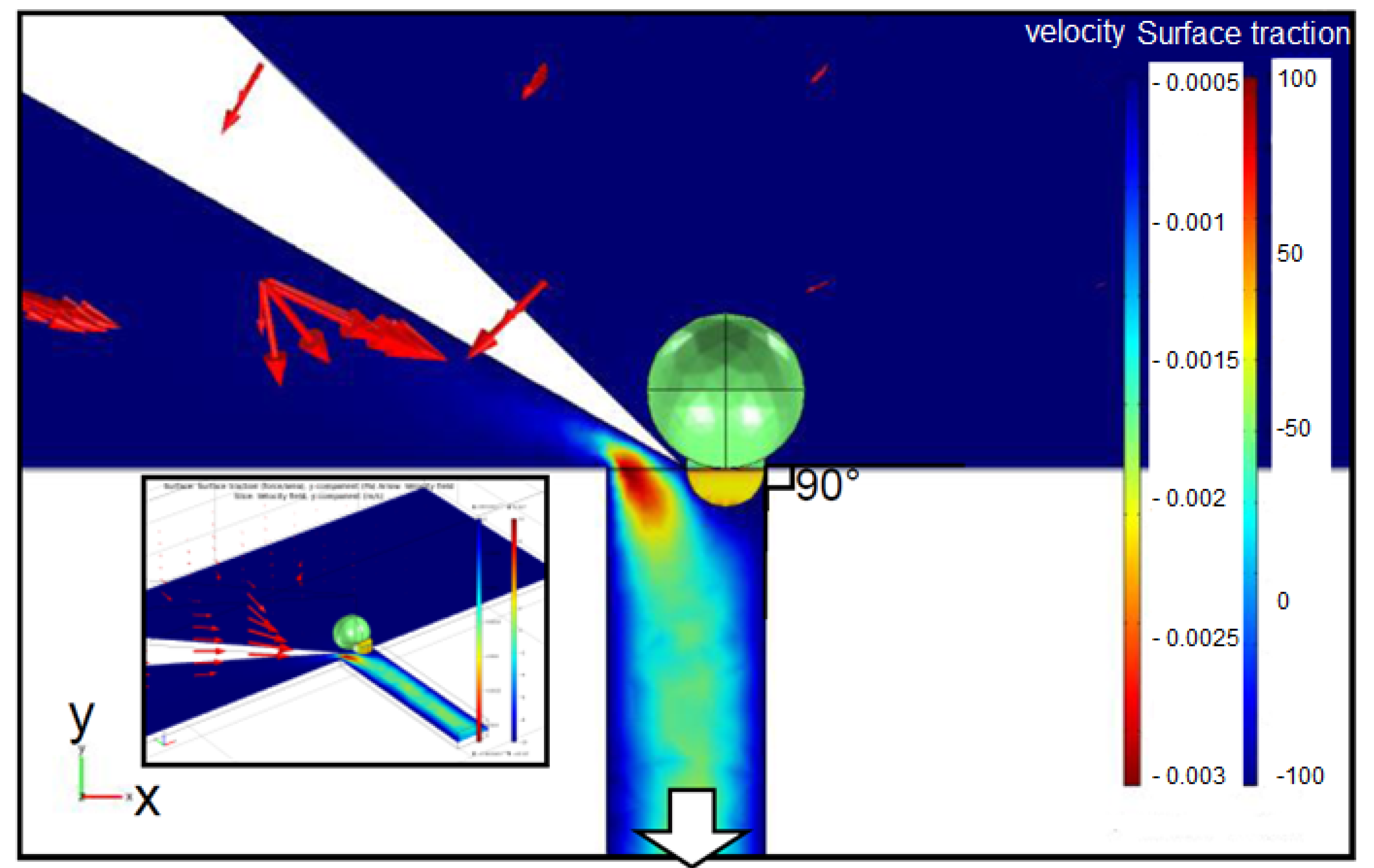
2.2. Cutting Volume Estimation
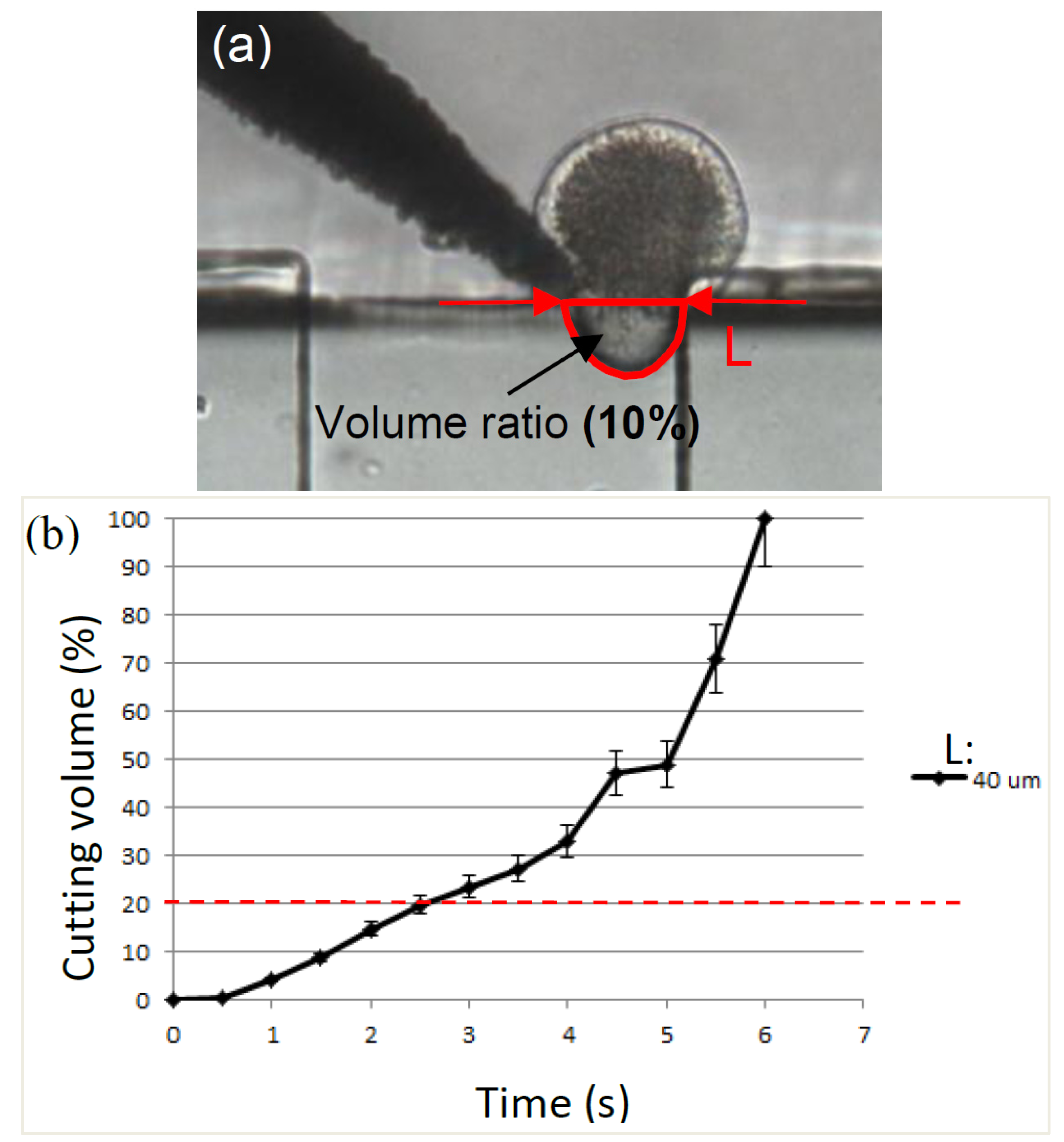
2.3. Separation of Oocyte by Hydraulic Force
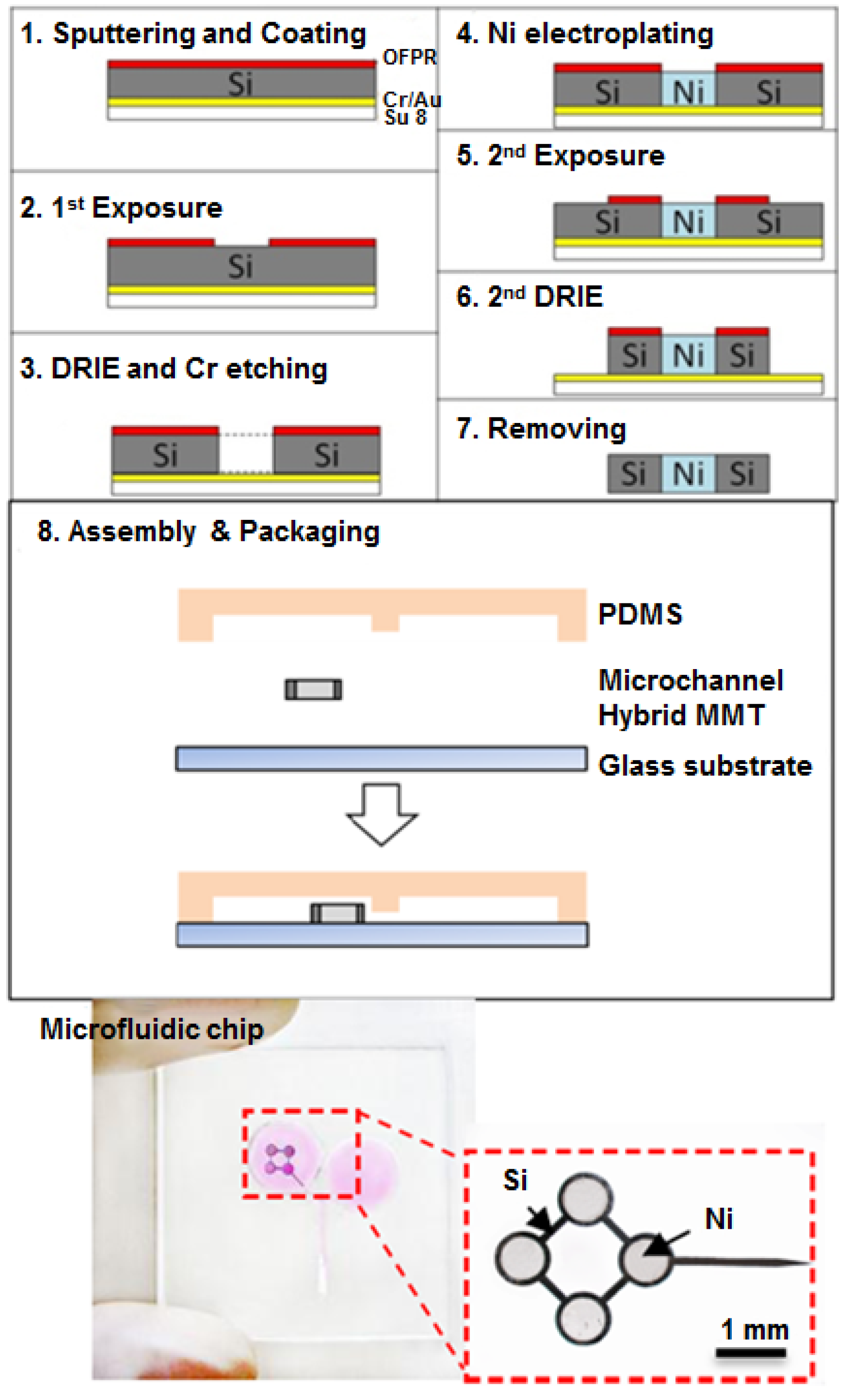
2.4. Fabrication of Hybrid MMT and Microfluidic Chip
3. Oocyte Enucleation Experiments
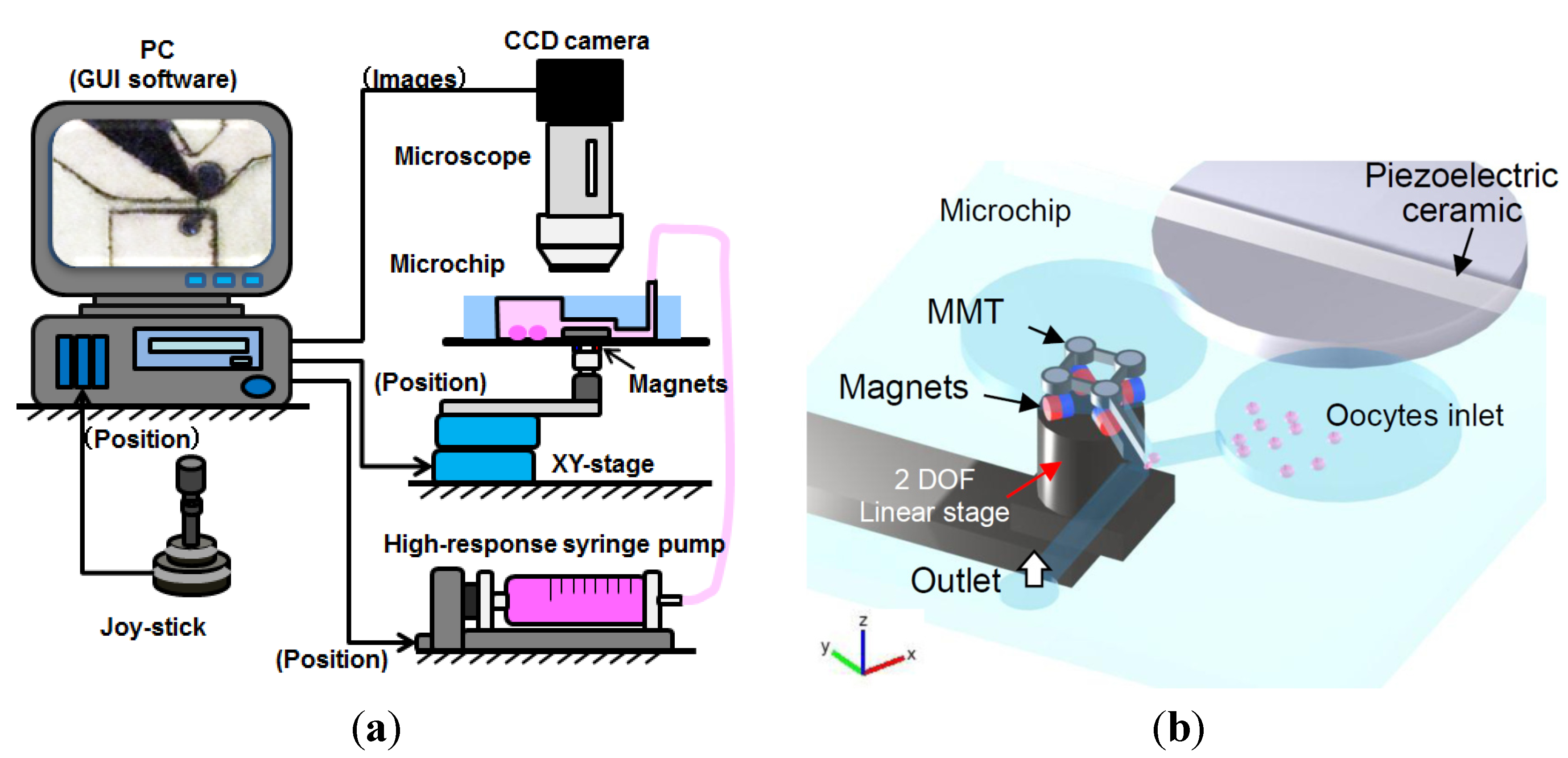
3.1. Experimental Setup
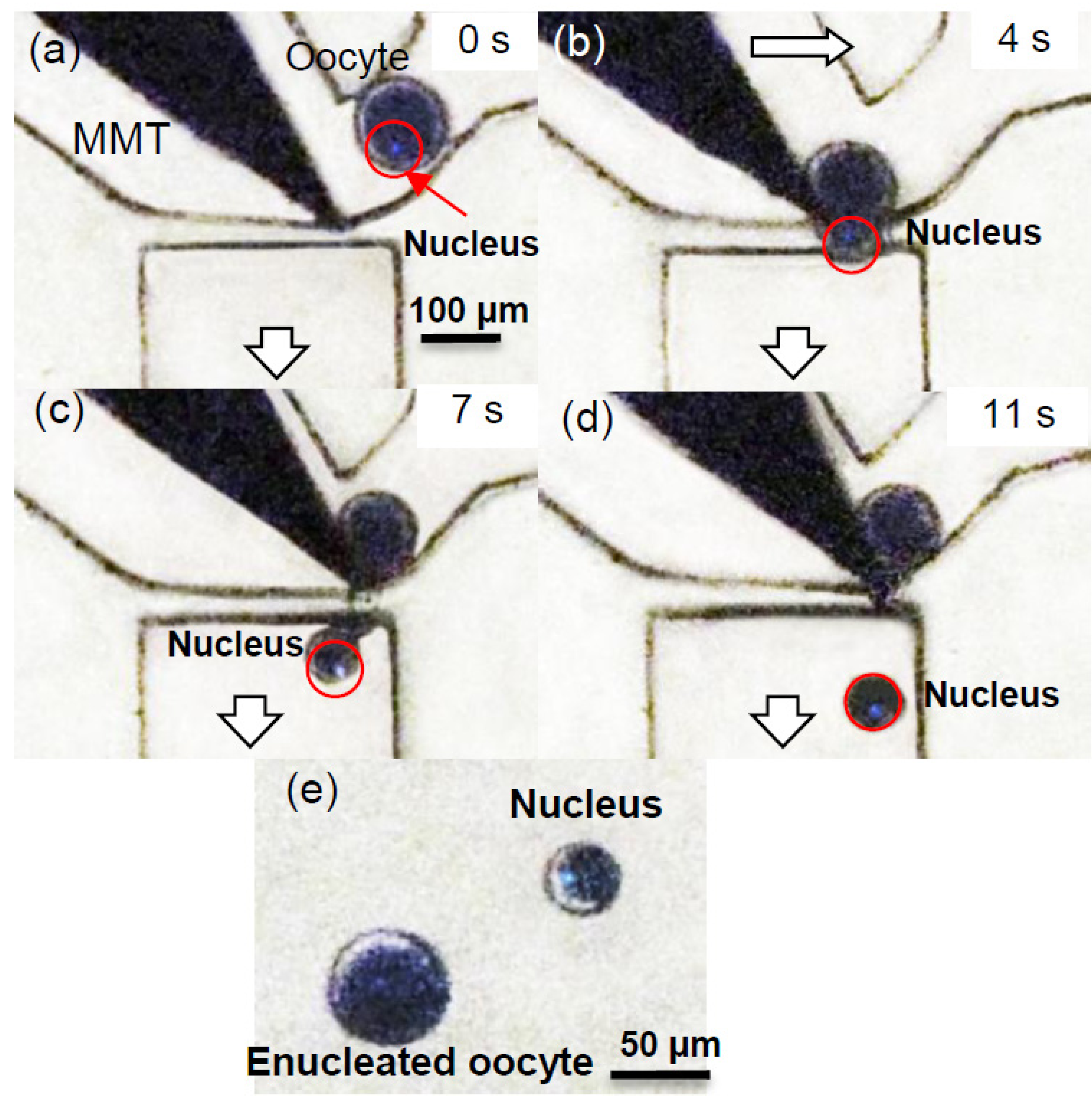
3.2. Experimental Process and Result
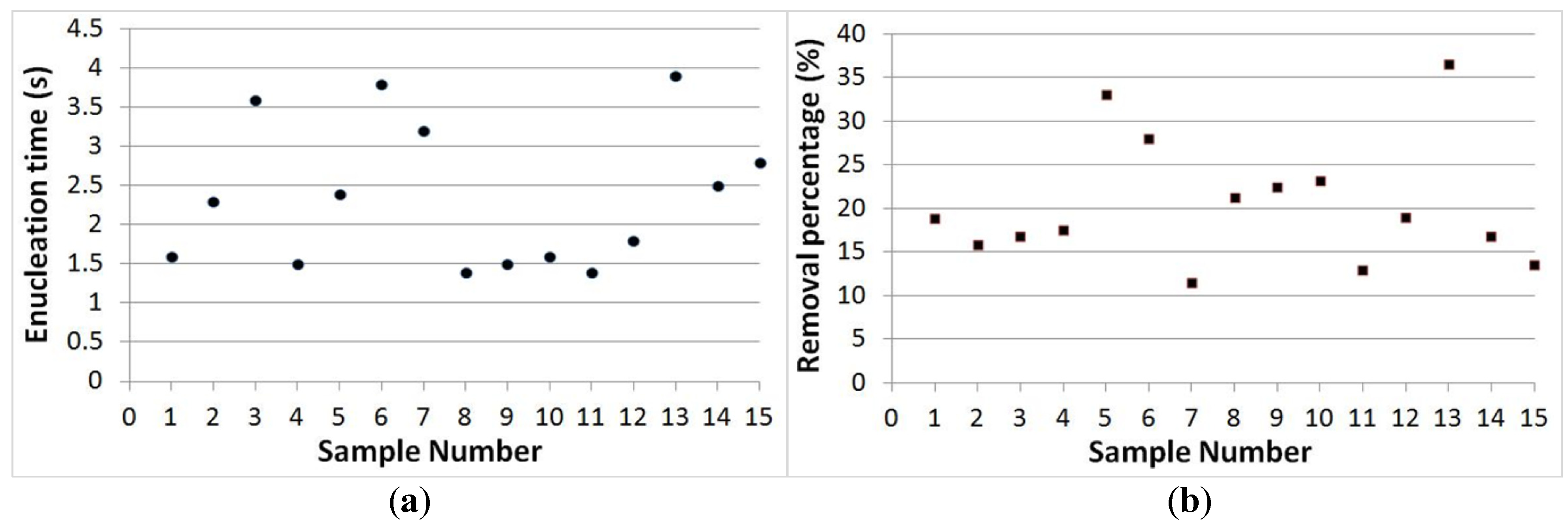
3.3. Separation Time and Removal Proportion Evaluation
4. Conclusions
Acknowledgements
Conflict of Interest
References
- Lassen, J.; Gjerris, M.; Sandøe, P. After Dolly—Ethical limits to the use of biotechnology on farm animals. Theriogenology 2005, 65, 992–1004. [Google Scholar] [CrossRef]
- Edwards, J.L.; Schrick, F.N.; McCracken, M.D.; van Amstel, S.R.; Hopkins, F.M.; Welborn, M.G.; Davies, C.J. Cloning adult farm animals: A review of the possibilities and problems associated with somatic cell nuclear transfer. Am. J. Reprod. Immunol. 2003, 50, 113–123. [Google Scholar] [CrossRef]
- Vanderwall, D.K.; Woods, G.L.; Aston, K.I.; Bunch, T.D.; Li, G.P.; Meerdo, L.N.; White, K.L. 76 cloned horse pregnancies produced using adult cumulus cells. Reprod. Fertil. Dev. 2003, 16, 160–160. [Google Scholar]
- Schramm, R.D.; Paprocki, A.M. Strategies for the production of genetically identical monkeys by embryo splitting. Reprod. Biol. Endocrinol. 2004, 2, 38. [Google Scholar] [CrossRef]
- Peura, T.T.; Lewis, I.M.; Trounson, A.O. The effect of recipient oocyte volume on nuclear transfer in cattle. Mol. Reprod. Dev. 1998, 50, 185–191. [Google Scholar] [CrossRef]
- Wang, H.L.; Chang, Z.L.; Li, K.L.; Lian, H.Y.; Han, D.; Cui, W.; Tan, J.H. Caffeine can be used for oocyte enucleation. Cell. Reprogramming 2011, 13, 225–232. [Google Scholar] [CrossRef]
- Ichikawa, A.; Tanikawa, T.; Akagi, S.; Ohba, K. Automatic cell cutting by high-precision microfluidic control. J. Rob. Mechatron. 2011, 23, 13–18. [Google Scholar]
- Costa-Borges, N.; Paramio, M.T.; Calderón, G.; Santaló, J.; Ibáñez, E. Antimitotic treatments for chemically assisted oocyte enucleation in nuclear transfer procedures. Cloning Stem Cells 2009, 11, 153–166. [Google Scholar] [CrossRef]
- Barbic, M.; Mock, J.J.; Gray, A.P.; Schultz, S. Electromagnetic micromotor for microfluidics applications. Appl. Phys. Lett. 2001, 79, 1399–1401. [Google Scholar] [CrossRef]
- Mensing, G.A.; Pearce, T.M.; Graham, M.D.; Beebe, D.J. An externally driven magnetic microstirrer. Phil. Trans. R. Soc. Lond. A 2004, 362, 1059–1068. [Google Scholar] [CrossRef]
- Atencia, J.; Beebe, D.J. Magnetically driven biomimetic micro pumping using vortices. Lab Chip 2004, 4, 598–602. [Google Scholar] [CrossRef]
- Roper, M.; Dreyfus, R.; Baudry, J.; Fermigier, M.; Bibette, J.; Stone, H.A. On the dynamics of magnetically driven elastic filaments. J. Fluid Mech. 2006, 554, 167–190. [Google Scholar] [CrossRef]
- Gao, L.; Gottron, N.J., III; Virgin, L.N.; Yellen, B.B. The synchronization of superparamagnetic beads driven by a micro-magnetic ratchet. Lab Chip 2010, 10, 2108–2114. [Google Scholar] [CrossRef]
- Zhang, L.; Abbott, J.J.; Dong, L.; Kratochvil, B.E.; Bell, D.; Nelson, B.J. Artificial bacterial flagella: Fabrication and magnetic control. Appl. Phys. Lett. 2009, 94, 064107. [Google Scholar] [CrossRef]
- Park, H.S.; Floyd, S.; Sitti, M. Roll and pitch motion analysis of a biologically inspired water runner robot. Int. J. Rob. Res. 2010, 29, 1281–1297. [Google Scholar] [CrossRef]
- Hagiwara, M.; Kawahara, T.; Yamanishi, Y.; Arai, F. Driving method of microtool by horizontally arranged permanent magnets for single cell manipulation. Appl. Phys. Lett. 2010, 97, 013701. [Google Scholar] [CrossRef]
- Hagiwara, M.; Kawahara, T.; Yamanishi, Y.; Masuda, T.; Feng, L.; Arai, F. On-chip magnetically actuated robot with ultrasonic vibration for single cell manipulations. Lab Chip 2011, 11, 2049–2054. [Google Scholar] [CrossRef]
- Hagiwara, M.; Kawahara, T.; Yamanishi, Y.; Arai, F. Precise control of magnetically driven microtools for enucleation of oocytes in a microfluidic chip. Adv. Rob. 2011, 25, 991–1005. [Google Scholar] [CrossRef]
- Oh, K.W.; Lee, K.; Ahn, B.; Furlani, E.P. Design of pressure-driven microfluidic networks using electric circuit analogy. Lab Chip 2012, 12, 515–545. [Google Scholar] [CrossRef]
- Pfitzner, J. Poiseuille and his law. Anaesthesia 1976, 31, 273–275. [Google Scholar] [CrossRef]
- Cornish, R.J. Flow in a pipe of rectangular cross-section. Proc. R. Soc. Lond. A 1928, 120, 691–700. [Google Scholar] [CrossRef]
- Hua, S.; Zhang, H.; Su, J.M.; Zhang, T.; Quan, F.S.; Liu, L.; Wang, Y.S.; Zhang, Y. Effects of the removal of cytoplasm on the development of early cloned bovine embryos. Anim. Reprod. Sci. 2011, 126, 37–44. [Google Scholar] [CrossRef]
- Alberts, B.; Johnson, A.; Lewis, J.; Raff, M.; Roberts, K.; Walter, P. DNA and Chromosomes. In Molecular Biology of the Cell, 4th ed.; Garland Science: New York, NY, USA, 2002; pp. 191–234. [Google Scholar]
© 2013 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Feng, L.; Hagiwara, M.; Ichikawa, A.; Arai, F. On-Chip Enucleation of Bovine Oocytes using Microrobot-Assisted Flow-Speed Control. Micromachines 2013, 4, 272-285. https://doi.org/10.3390/mi4020272
Feng L, Hagiwara M, Ichikawa A, Arai F. On-Chip Enucleation of Bovine Oocytes using Microrobot-Assisted Flow-Speed Control. Micromachines. 2013; 4(2):272-285. https://doi.org/10.3390/mi4020272
Chicago/Turabian StyleFeng, Lin, Masaya Hagiwara, Akihiko Ichikawa, and Fumihito Arai. 2013. "On-Chip Enucleation of Bovine Oocytes using Microrobot-Assisted Flow-Speed Control" Micromachines 4, no. 2: 272-285. https://doi.org/10.3390/mi4020272




