Suspended Graphene-Based Gas Sensor with 1-mW Energy Consumption
Abstract
:1. Introduction
2. Device and Methods
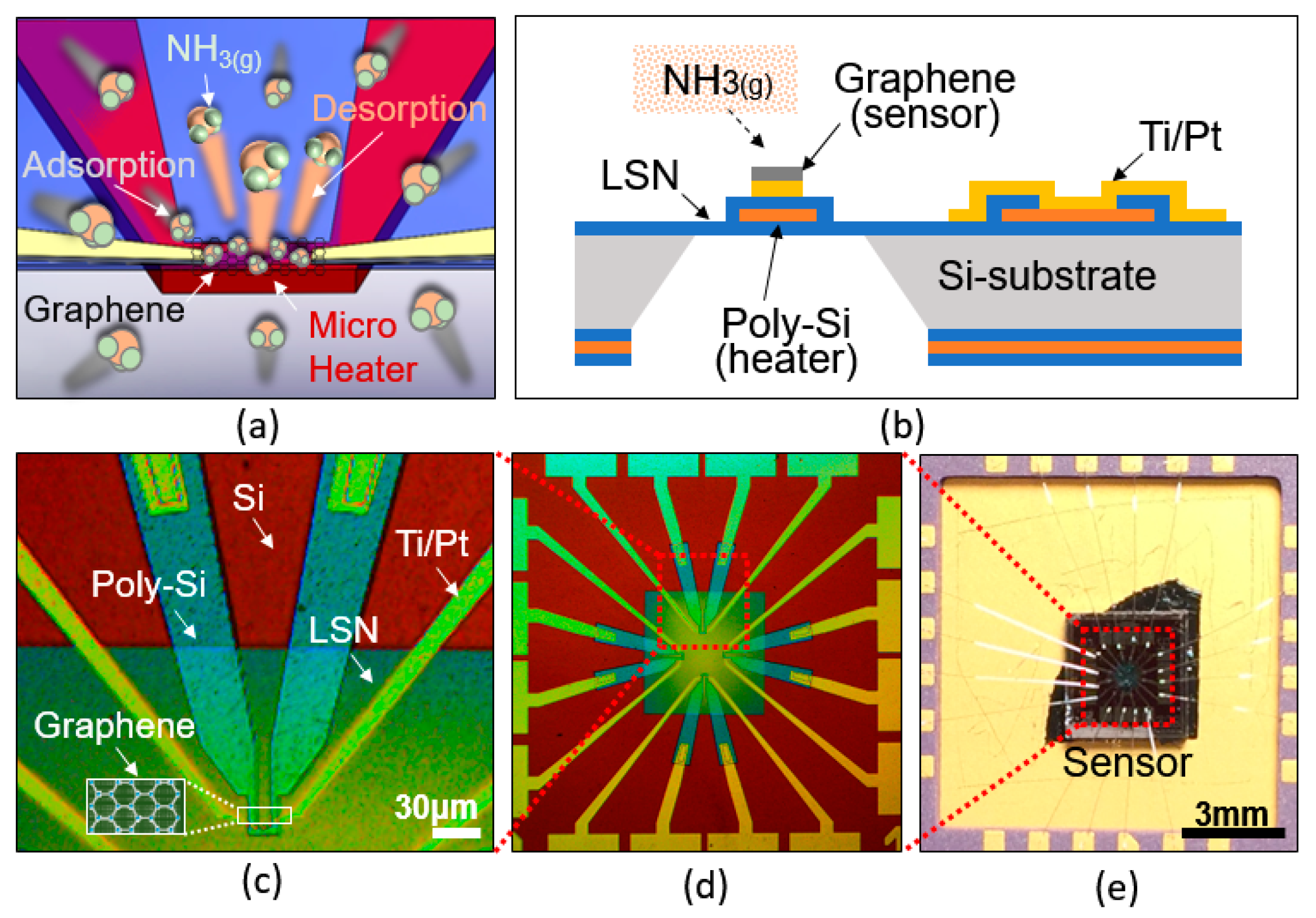
2.1. Structure
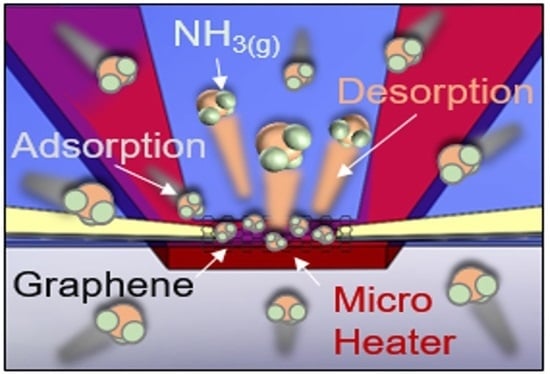
2.2. Working Principle
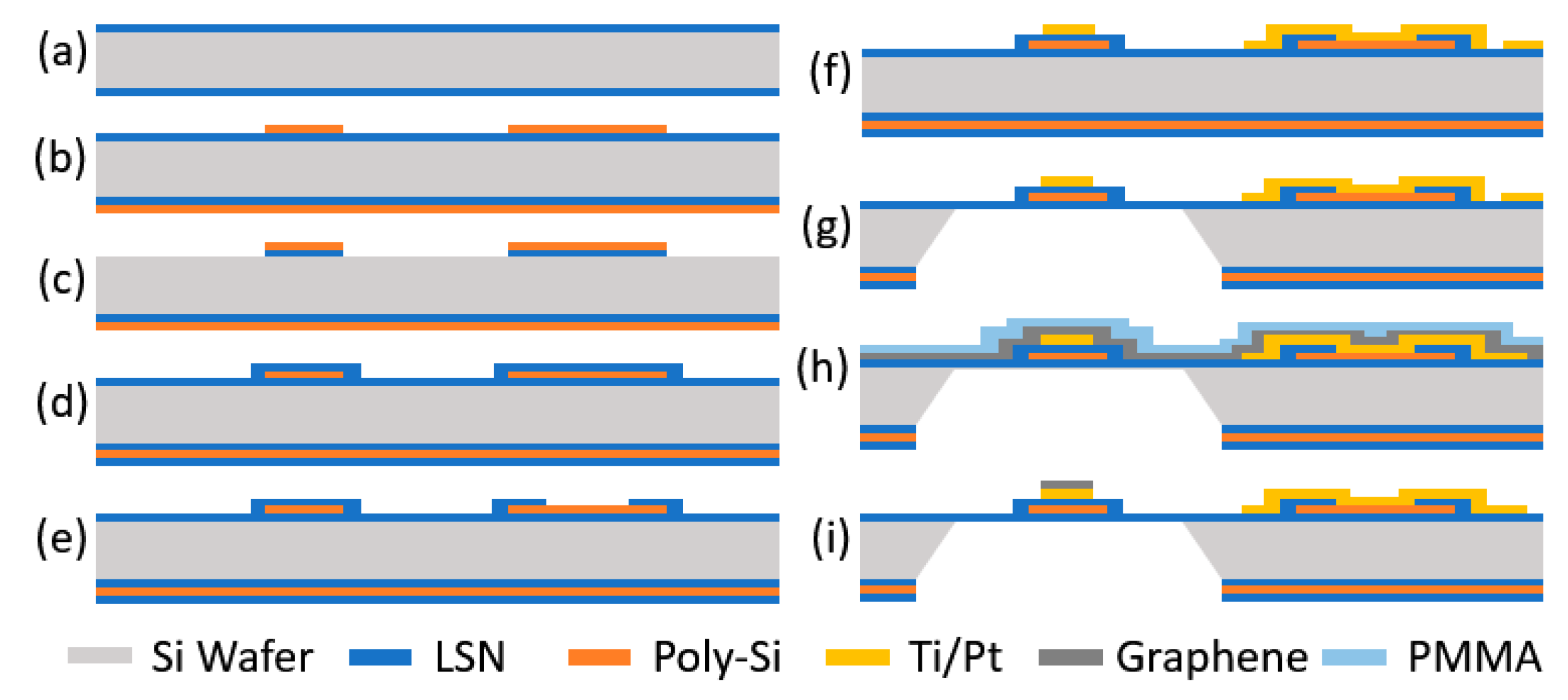
2.3. Device Fabrication
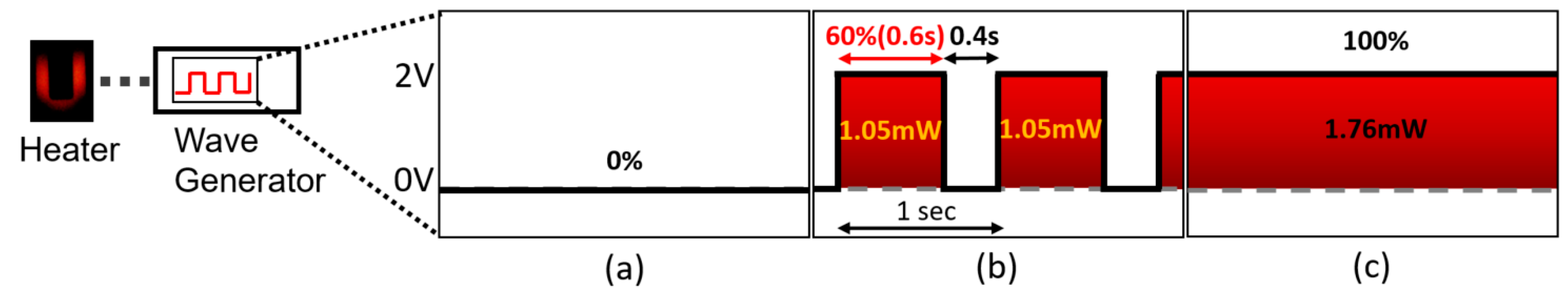
2.4. Measurement
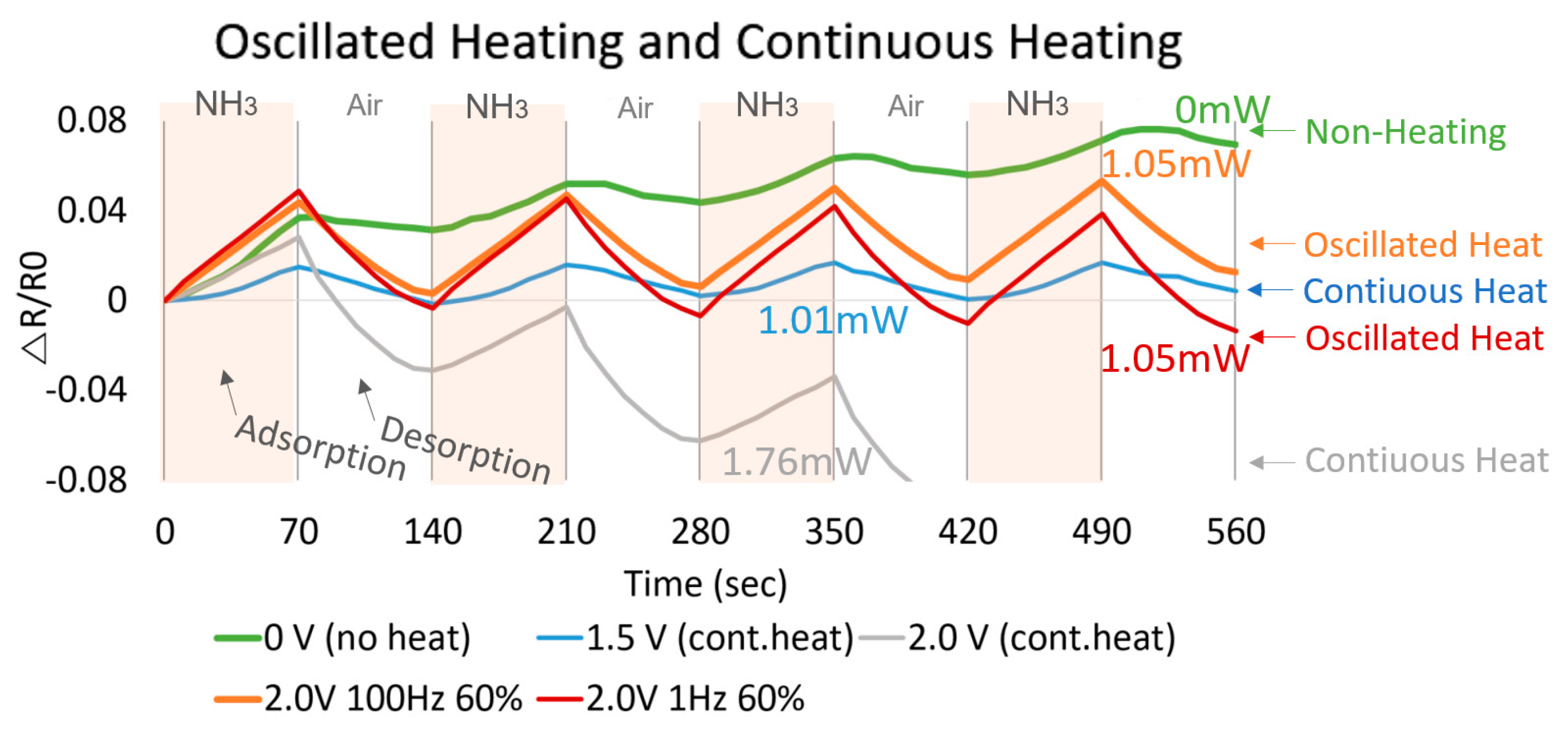
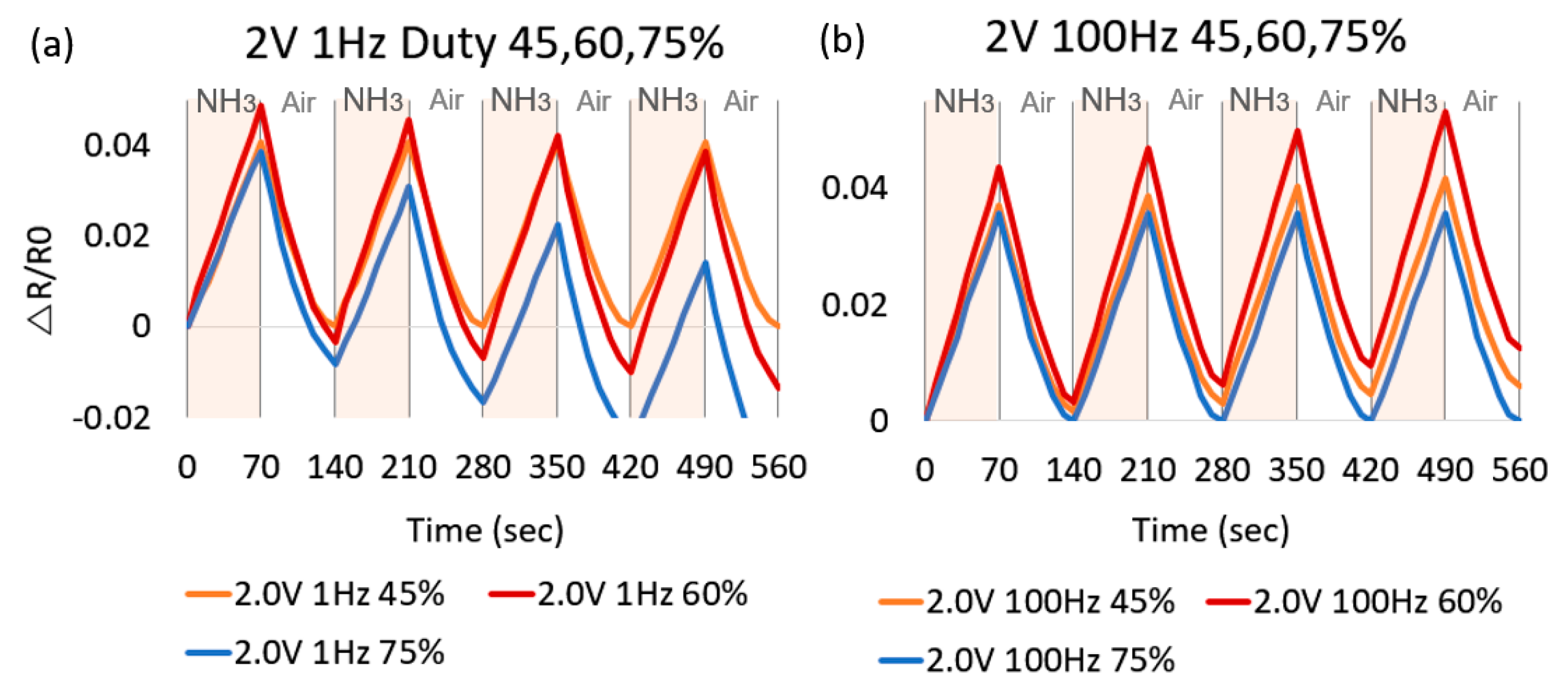
3. Results
4. Discussion
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Cho, B.; Yoon, J.; Hahm, M.G.; Kim, D.H.; Kim, A.R.; Kahng, Y.H.; Park, S.W.; Lee, Y.J.; Park, S.G.; Kwon, J.D.; et al. Graphene-based gas sensor: metal decoration effect and application to a flexible device. J. Mater. Chem. C. 2014, 2, 5280. [Google Scholar] [CrossRef]
- Lee, K.; Scardaci, V.; Kim, H.Y.; Hallam, T.; Nolan, H.; Bolf, B.E.; Maltbie, G.S.; Abbott, J.E.; Duesberg, G.S. Highly sensitive, transparent, and flexible gas sensors based on gold nanoparticle decorated carbon nanotubes. Sens.Actuators B Chem. 2013, 188, 571–575. [Google Scholar] [CrossRef]
- Schedin, F.; Geim, A.K.; Morozov, S.V.; Hill, E.W.; Blake, P.; Katsnelson, M.I.; Novoselov, K.S. Detection of individual gas molecules adsorbed on graphene. Nat. Mater. 2007, 6, 652–655. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sheehan, P.E.; Whitman, L.J. Detection limits for nanoscale biosensors. Nano Lett. 2005, 5, 803–807. [Google Scholar] [CrossRef] [PubMed]
- Leenaerts, O.; Partoens, B.; Peeters, F.M. Adsorption of H2O, NH3, CO, NO2, and NO on graphene: A first-principles study. 2007, 2, 1–6. [Google Scholar]
- Inoue, T.; Ohtsuka, K.; Yoshida, Y.; Matsuura, Y.; Kajiyama, Y. Metal oxide semiconductor NO2 sensor. Sens. Actuators B Chem. 1995, 25, 388–391. [Google Scholar] [CrossRef]
- Mitoma, N.; Nouchi, R.; Tanigaki, K. Enhanced sensing response of oxidized graphene formed by UV irradiation in water. Nanotechnology 2015, 26, 105701. [Google Scholar] [CrossRef] [PubMed]
- Chen, g.; Paronyan, T.M.; Pigos, E.M.; Harutyunyan, A.R. Enhanced gas sensing in pristine carbon nanotubes under continuous ultraviolet light illumination. Sci. Rep. 2012, 2, 343. [Google Scholar] [CrossRef] [PubMed]
- Semancik, S.; Cavicchi, R.E.; Wheeler, M.C.; Tiffany, J.E.; Poirier, G.E.; Walton, R.M.; Suehle, J.S.; Panchapakesan, B.; DeVoe, D.L. Microhotplate platforms for chemical sensor research. Sens. Actuators B Chem. 2001, 77, 579–591. [Google Scholar] [CrossRef]
- Cavicchi, R.E.; Suehle, J.S.; Kreider, K.G.; Shomaker, B.L.; Small, J.A.; Gaitan, M.; Chaparala, P. Growth of SnO2 films on micromachined hotplates. Appl. Phys. Lett. 1995, 66, 812–814. [Google Scholar] [CrossRef]
- Fowler, J.D.; Allen, M.J.; Tung, V.C.; Yang, Y.; Kaner, R.B.; Weiller, B.H. Practical chemical sensors from chemically derived graphene. ACS Nano. 2009, 3, 301–306. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Zhao, L.; Sun, H. Simulation of NH3 temperature-programmed desorption curves using an ab initio force field. J. Phys. Chem. C. 2009, 113, 16051–16057. [Google Scholar] [CrossRef]
- Zhang, Z.; Zhang, X.; Luo, W.; Yang, H.; He, Y.; Liu, Y.; Zhang, X.; Peng, G. Study on adsorption and desorption of ammonia on graphene. Nanoscale Res. Lett. 2015, 10, 359. [Google Scholar] [CrossRef] [PubMed]
- Akinwande, D.; Petrone, N.; Hone, J. Two-dimensional flexible nanoelectronics. Nat. Commun. 2014, 5, 5678. [Google Scholar] [CrossRef] [PubMed]
- Novoselov, K.S.; Geim, A.K.; Morozov, S.V.; Jiang, D.; Zhang, Y.; Dubonos, S.V.; Grigorieva, I.V.; Firsov, A.A. Electric field effect in atomically thin carbon films. Science 2004, 306, 666–669. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Liu, Z.; Lew, W.S.; Wang, Q.J. Temperature dependence of the electrical transport properties in few-layer graphene interconnects. Nanoscale Res. Lett. 2013, 8, 335. [Google Scholar] [CrossRef] [PubMed]
- Silvestrelli, P.L. van der Waals interactions in density functional theory using wannier functions. J. Phys. Chem. A. 2009, 113, 5224–5234. [Google Scholar] [CrossRef] [PubMed]
- Hwang, E.H.; Adam, S.; Das Sarma, S. Transport in chemically doped graphene in the presence of adsorbed molecules. Phys. Rev. B 2007, 76, 195421. [Google Scholar] [CrossRef]
- Lin, X.; Ni, J.; Fang, C. Adsorption capacity of H2O, NH3, CO, and NO2 on the pristine graphene. J. Appl. Phys. 2013, 113, 34306. [Google Scholar] [CrossRef]


© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, J.-H.; Zhou, Q.; Chang, J. Suspended Graphene-Based Gas Sensor with 1-mW Energy Consumption. Micromachines 2017, 8, 44. https://doi.org/10.3390/mi8020044
Kim J-H, Zhou Q, Chang J. Suspended Graphene-Based Gas Sensor with 1-mW Energy Consumption. Micromachines. 2017; 8(2):44. https://doi.org/10.3390/mi8020044
Chicago/Turabian StyleKim, Jong-Hyun, Qin Zhou, and Jiyoung Chang. 2017. "Suspended Graphene-Based Gas Sensor with 1-mW Energy Consumption" Micromachines 8, no. 2: 44. https://doi.org/10.3390/mi8020044