Modeling and Analysis of an Opto-Fluidic Sensor for Lab-on-a-Chip Applications
Abstract
:1. Introduction
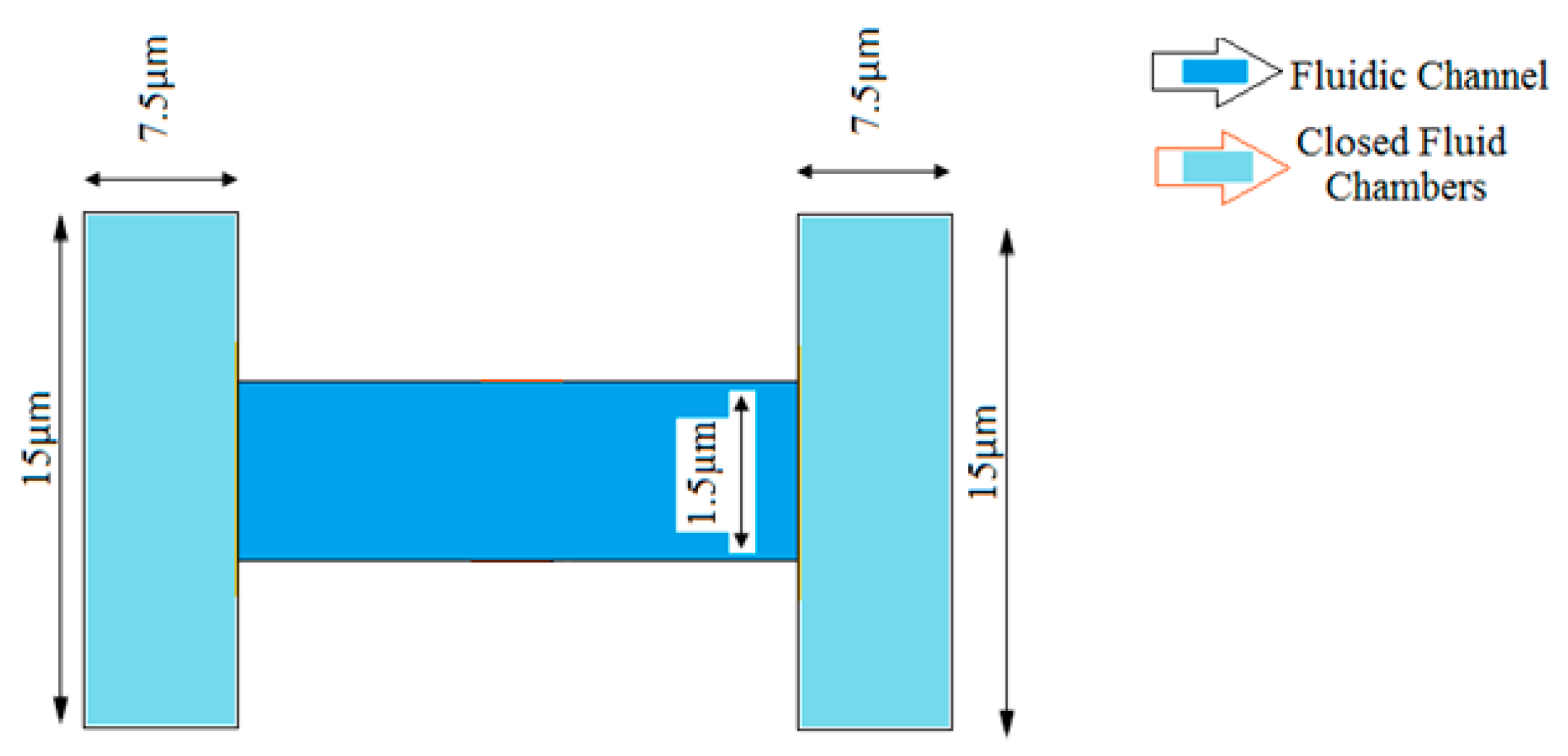
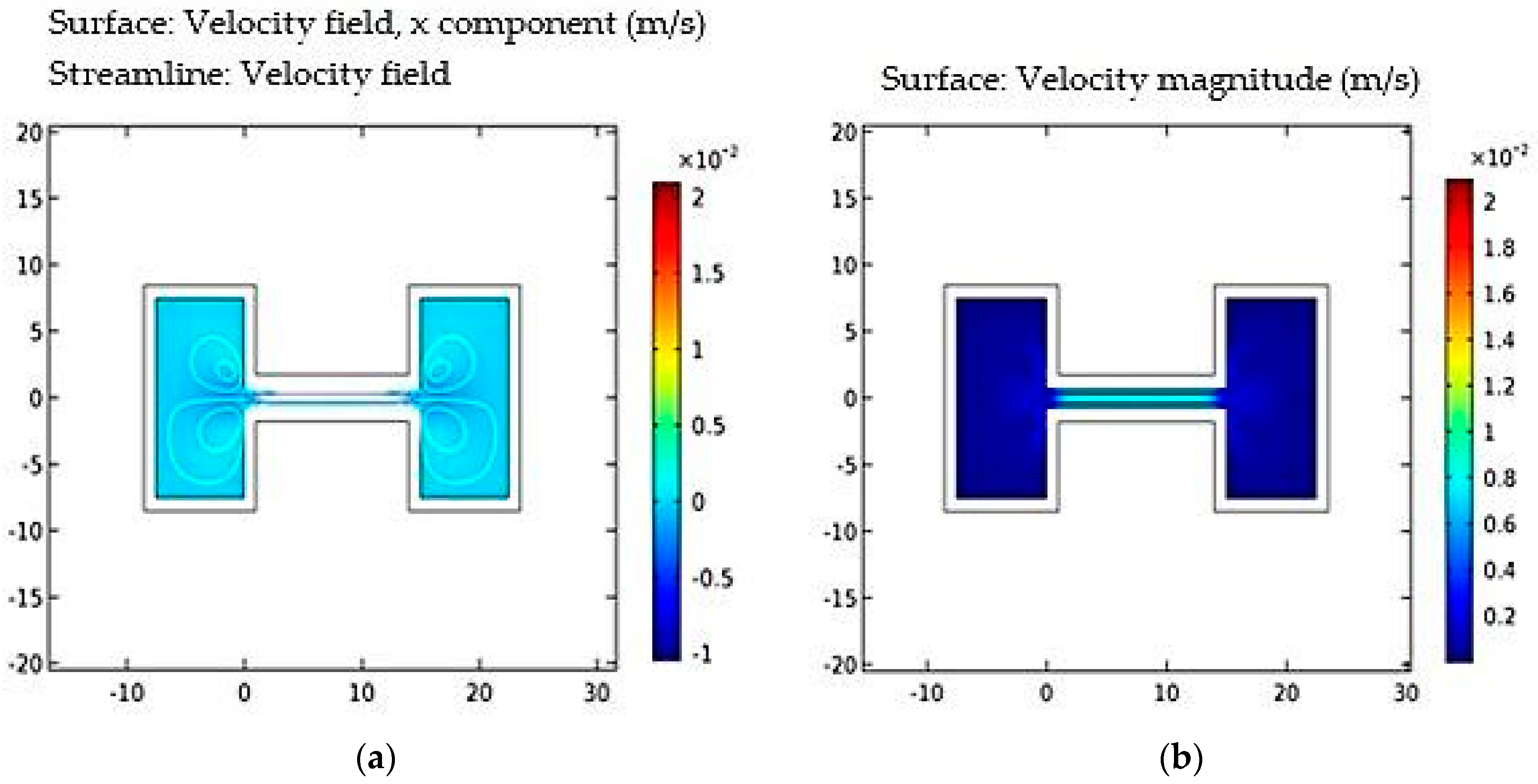
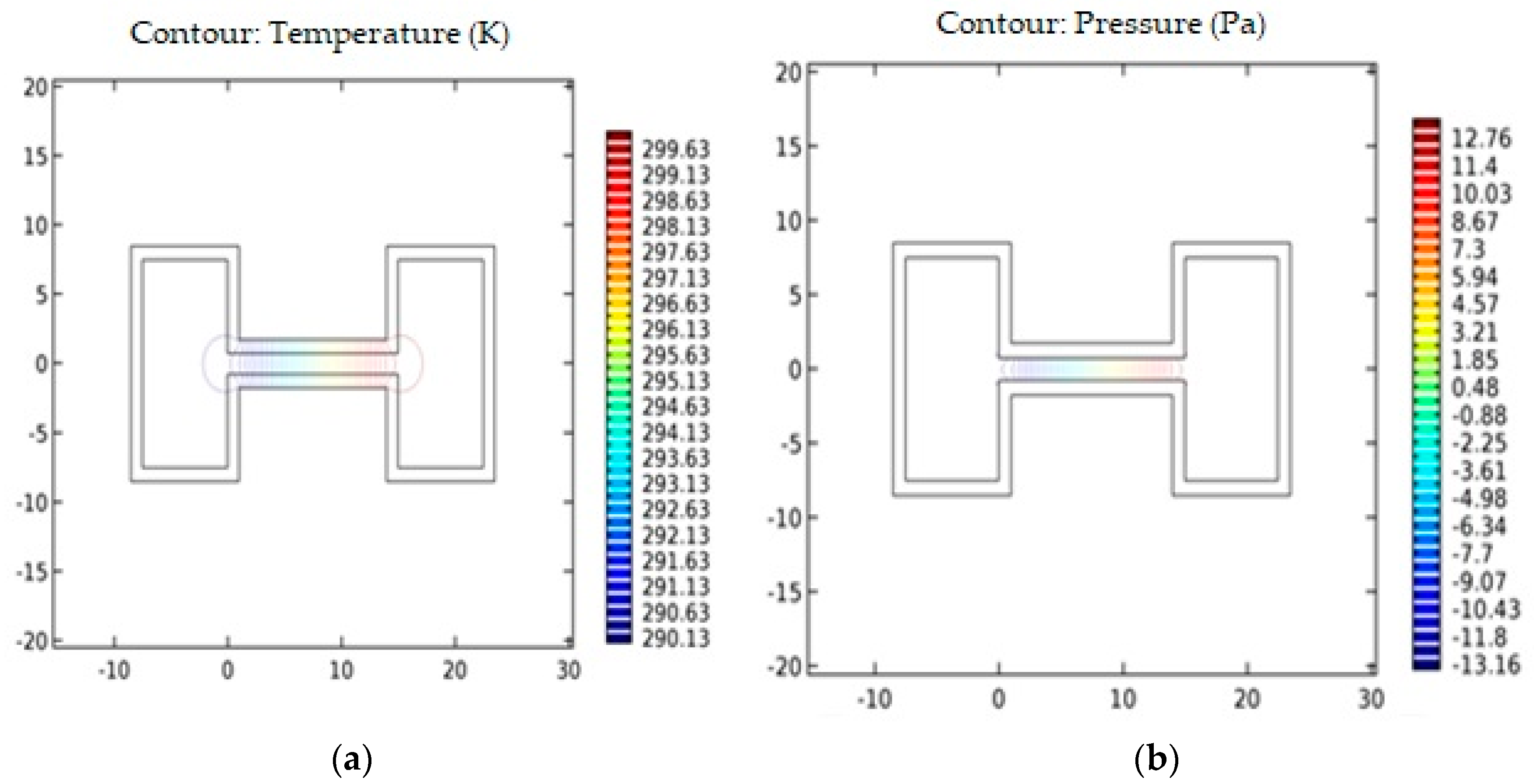
2. Modeling of the Microfluidic Channel
3. Modeling of the Silicon on Insulator (SOI) Waveguide
- SOI wafer definition with top device layer (silicon) having a thickness 250 nm and SiO2 thickness of 1000 µm.
- Deposition of PR-AZ5214 spin (001) (Microchemicals GmbH, Ulm, Germany) having a thickness of 300 nm. PR-AZ5214 is used as positive mask.
- Lithography ultraviolet (UV) contact: in this step mask is exposed to UV light.
- Etch Si Reactive Ion Etching (RIE) (Cl2_CF4), in this step the UV exposed region of 250 nm thickness, top Si is removed by using reactive ion etching.
- Etch PR-AZ5214 wet (lift-off), in this step PR-AZ5214 is removed by using the lift-off technique.
4. Results and Discussion
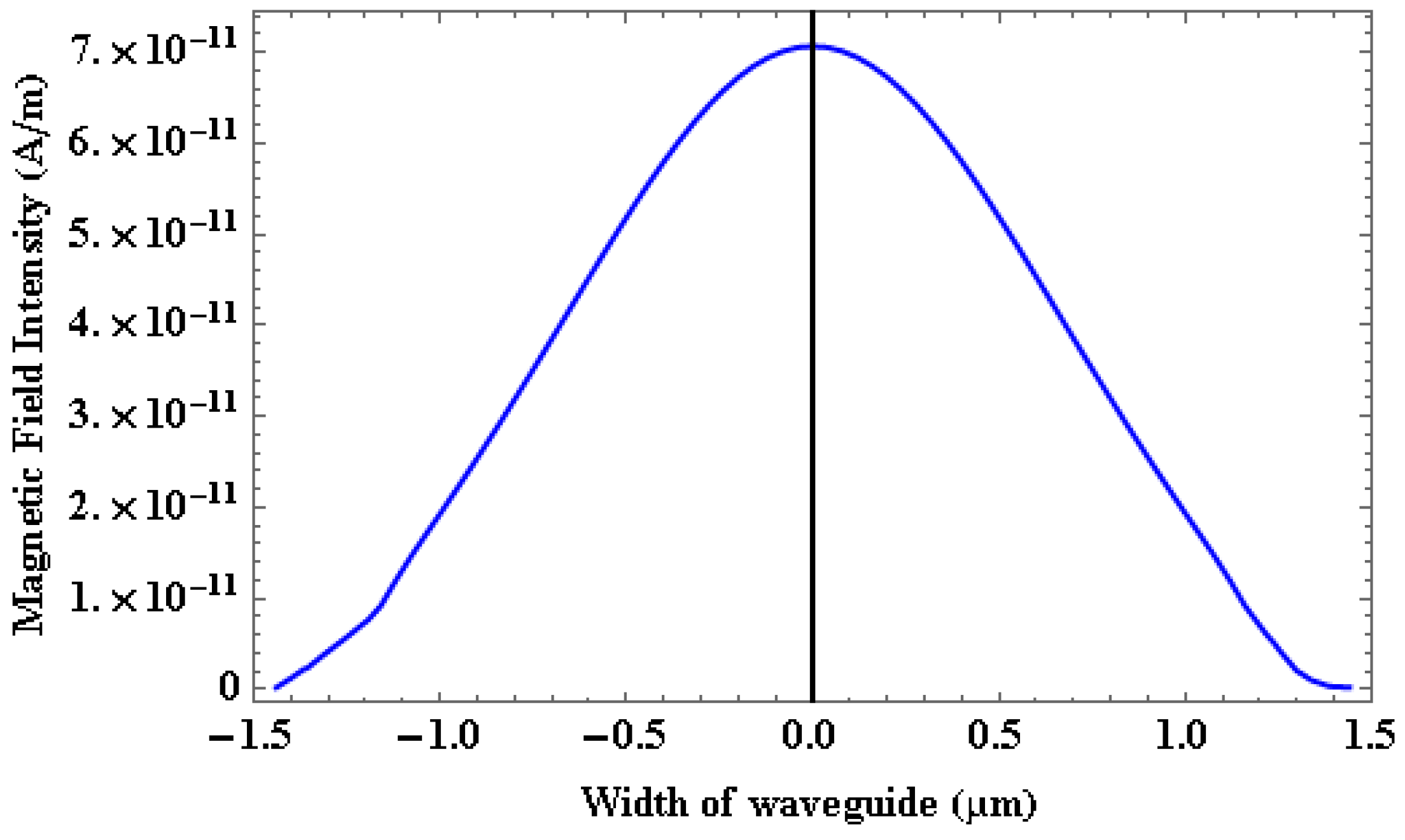
4.1. Power Coupling Analysis
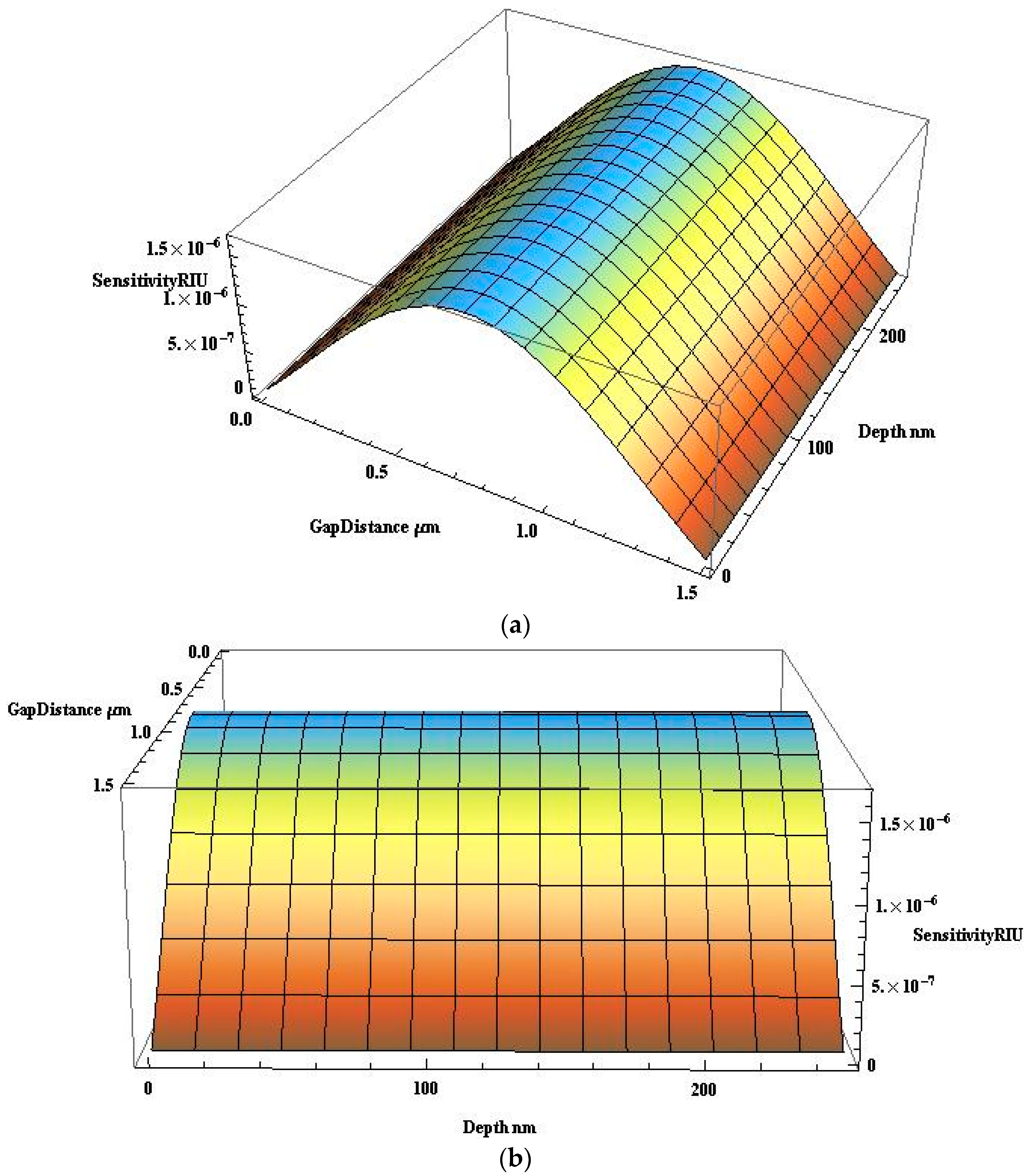
4.2. Power Coupling and Sensitivity Analysis
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Volpatti, L.R.; Yestisen, A.K. Commercialization of microfluidic devices. Trends Biotechnol. 2014, 327, 347–350. [Google Scholar] [CrossRef] [PubMed]
- Saggiomo, V.; Velders, A.H. Sample 3D printed scaffold removal method for the fabrication of intricate microfluidic devices. Adv. Sci. 2015, 2. [Google Scholar] [CrossRef]
- Ghallab, Y.; Badway, W. Sensing methods for di-electrophoresis phenomenon from bulky instruments to lab on a chip. IEEE Circuits Syst. Mag. 2004, 4, 5–15. [Google Scholar] [CrossRef]
- Pawell, R.S.; Inglis, D.W.; Barber, T.J.; Taylor, R.A. Manufacturing and wetting low cost microfluidic cell separation devices. Microfluidics 2013, 7, 056501. [Google Scholar] [CrossRef] [PubMed]
- Tabeling, P. Introduction to Microfluidics; Oxford University Press: Oxford, UK, 2015. [Google Scholar]
- Chokkalingam, V.; Weidonhoff, B.; Kraener, M.; Maier, W.F.; Herminghaus, S.; Seemann, R. Optimized drop let-based microfluidics scheme for sol-gel reaction. Lab Chip 2010, 10, 1700–1705. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Su, L.; Manuzzi, D.; de los Monteros, H.V.E.; Jia, W.; Huo, D.; Hou, C.; Lei, Y. Ultrasensitive and selective non-enzymatic glucose detection using copper nanowires. Biosens. Bioelectron. 2012, 31, 426–432. [Google Scholar] [CrossRef] [PubMed]
- Alejandra, F.-C.; Riera, A.; Herrero, P.; Moreno, F. Glucose levels regulate the nucleo-mitochondrial distribution of Mig2. Mitochondrion 2012, 12, 370–380. [Google Scholar]
- Noiphung, J.; Songjaroen, T.; Dungchai, W.; Henry, C.S.; Chailapakul, O.; Laiwattanapaisal, W. Electrochemical detection of glucose from whole blood using paper-based microfluidic devices. Anal. Chim. Acta 2013, 788, 39–45. [Google Scholar] [CrossRef] [PubMed]
- Park, H.; Yoon, H.S.; Patil, U.; Anoop, R.; Lee, J.; Lim, J.; Lee, W.; Jun, S.C. Radio frequency based label-free detection of glucose. Biosens. Bioelectron. 2014, 54, 141–145. [Google Scholar] [CrossRef] [PubMed]
- Luo, Y.; Chen, X.; Xu, M.; Chen, Z.; Fan, X. Optofluidic glucose detection by capillary-based ring resonators. Opt. Laser Technol. 2014, 56, 12–14. [Google Scholar] [CrossRef]
- Guerrero-Romero, F.; Simental-Mendía, L.E.; Rodríguez-Morán, M. Association of C-reactive protein levels with fasting and postload glucose levels according to glucose tolerance status. Arch. Med. Res. 2014, 45, 70–75. [Google Scholar] [CrossRef] [PubMed]
- Galant, A.L.; Kaufman, R.C.; Wilson, J.D. Glucose: Detection and analysis. Food Chem. 2015, 188, 149–160. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.; Sheng, Y.; Sun, Y.; Feng, J.; Wang, S.; Zhang, J.; Xu, J.; Jiang, D. A glucose oxidase-coupled DNAzyme sensor for glucose detection in tears and saliva. Biosens. Bioelectron. 2015, 70, 455–461. [Google Scholar] [CrossRef] [PubMed]
- Kim, N.Y.; Dhakal, R.; Adhikari, K.K.; Kim, E.S.; Wang, C. A reusable robust radio frequency biosensor using microwave resonator by integrated passive device technology for quantitative detection of glucose level. Biosens. Bioelectron. 2015, 67, 687–693. [Google Scholar] [CrossRef] [PubMed]
- Ma, G.; Yang, M.; Li, C.; Tan, H.; Deng, L.; Xie, S.; Xu, F.; Wang, L.; Song, Y. Preparation of spinel nickel-cobalt oxide nanowrinkles/reduced graphene oxide hybrid for nonenzymatic glucose detection at physiological level. Electrochim. Acta 2016, 220, 545–553. [Google Scholar] [CrossRef]
- Shen, W.; Sun, J.; Seah, J.Y.H.; Shi, L.; Tang, S.; Lee, H.K. Needle-based sampling coupled with colorimetric reaction catalyzed by layered double hydroxide peroxidase mimic for rapid detection of the change of d-glucose levels with time in bananas. Anal. Chim. Acta 2018, 1001, 32–39. [Google Scholar] [CrossRef] [PubMed]
- Narayan, K.T.; Srinivas, G.; Mohan, R.; Varma, M.M. Analysis of integrated optical lab-on-a-chip sensor based on refractive index and absorbance sensing. IEEE Sens. J. 2013, 13, 1730–1741. [Google Scholar]
- Kennard, E.H. Kinetic Theory of Gases; McGraw-Hill: New York, NY, USA, 1938. [Google Scholar]
- Maxwell, J.C. On stresses in rarefied fluides arising from inequalities of temperature. Phil. Trans. R. Soc. Lond. 1879, 170, 231–256. [Google Scholar] [CrossRef]
- Pollock, C.R. Fundamentals of Optoelectronics; Irwin: Hunt Sverre, NC, USA, 1995; ISBN 978-0-256-10104-1. [Google Scholar]
- Berenger, J. A perfectly matched layer for the absorption of electromagnetic waves. J. Comput. Phys. 1994, 114, 185–200. [Google Scholar] [CrossRef]
- Amerov, A.K.; Chen, J.; Arnold, M.A. Molar absorptivities of glucose and other biological molecules in aqueous solutions over the first overtone and combination regions of the near-infrared spectrum. Appl. Spectrosc. 2004, 58, 1195–1204. [Google Scholar] [CrossRef] [PubMed]
- Domachuk, P.; Littler, I.; Cronin-Golomb, M.; Eggleton, B. Compact resonant integrated microfluidic refractometer. Appl. Phys. Lett. 2006, 88, 093513. [Google Scholar] [CrossRef]
- Mandal, S.; Erickson, D. Nanoscale optofluidic sensor arrays. Opt. Express 2008, 16, 1623–1631. [Google Scholar] [CrossRef] [PubMed]
- Cole, C.F.; Hill, G.M.; Adams, A.J. Automated refractive index measurement of catalyst-laden edible oils undergoing partial hydrogenation. J. Am. Oil Chem. Soc. 1994, 71, 1339–1342. [Google Scholar] [CrossRef]
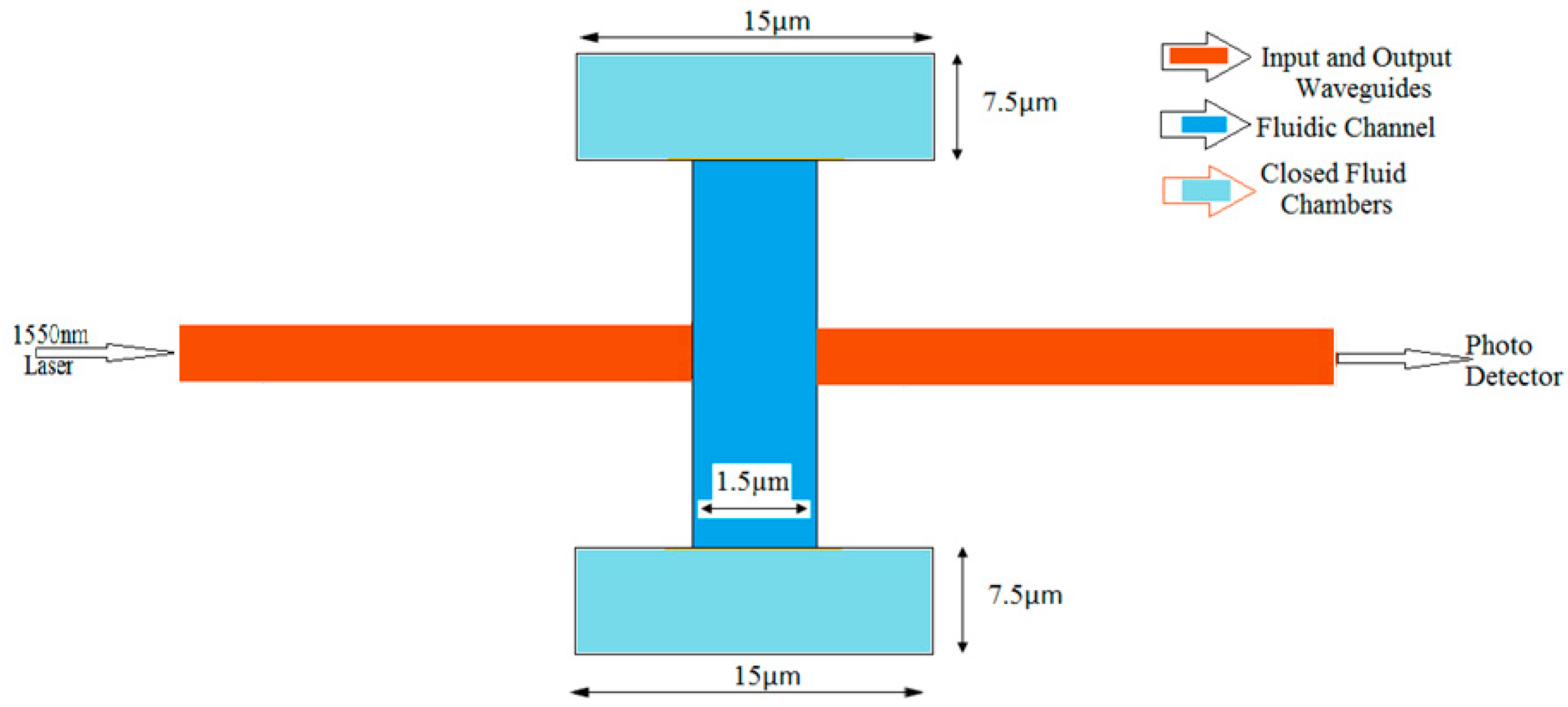




| Material | Width (nm) | Height (nm) | Refractive Index at 1550 nm |
|---|---|---|---|
| Si (Core) | 500 | 250 | 3.4714 |
| SiO2 (Substrate) | 4000 | 1000 | 1.55 |
| Parameter | Value |
|---|---|
| Mesh Resolution | 50 × 50 |
| Wavelength | 1550 nm |
| Boundary conditions | Perfectly matched layers (PML) |
| Background index | 1 (Air) |
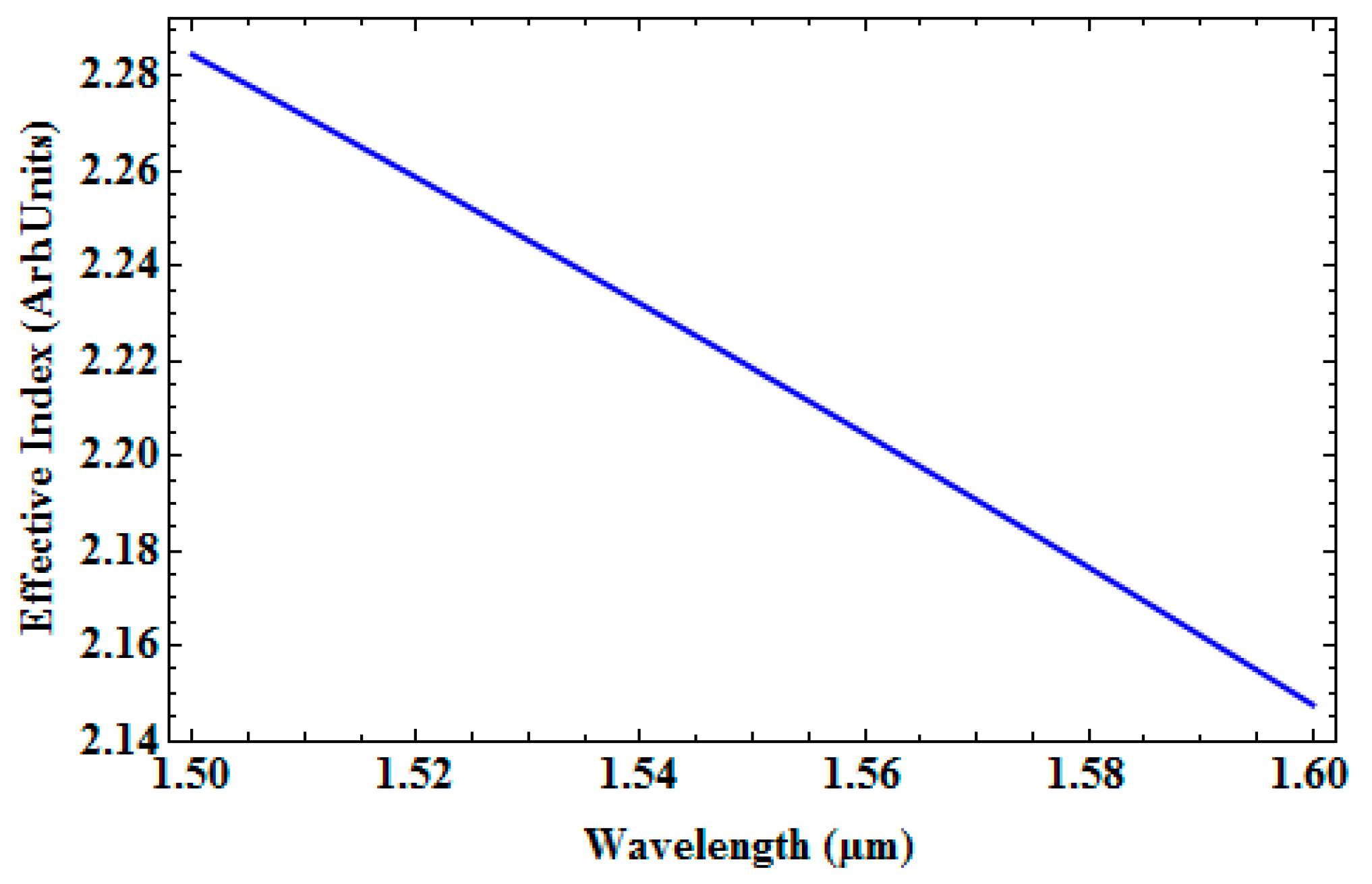
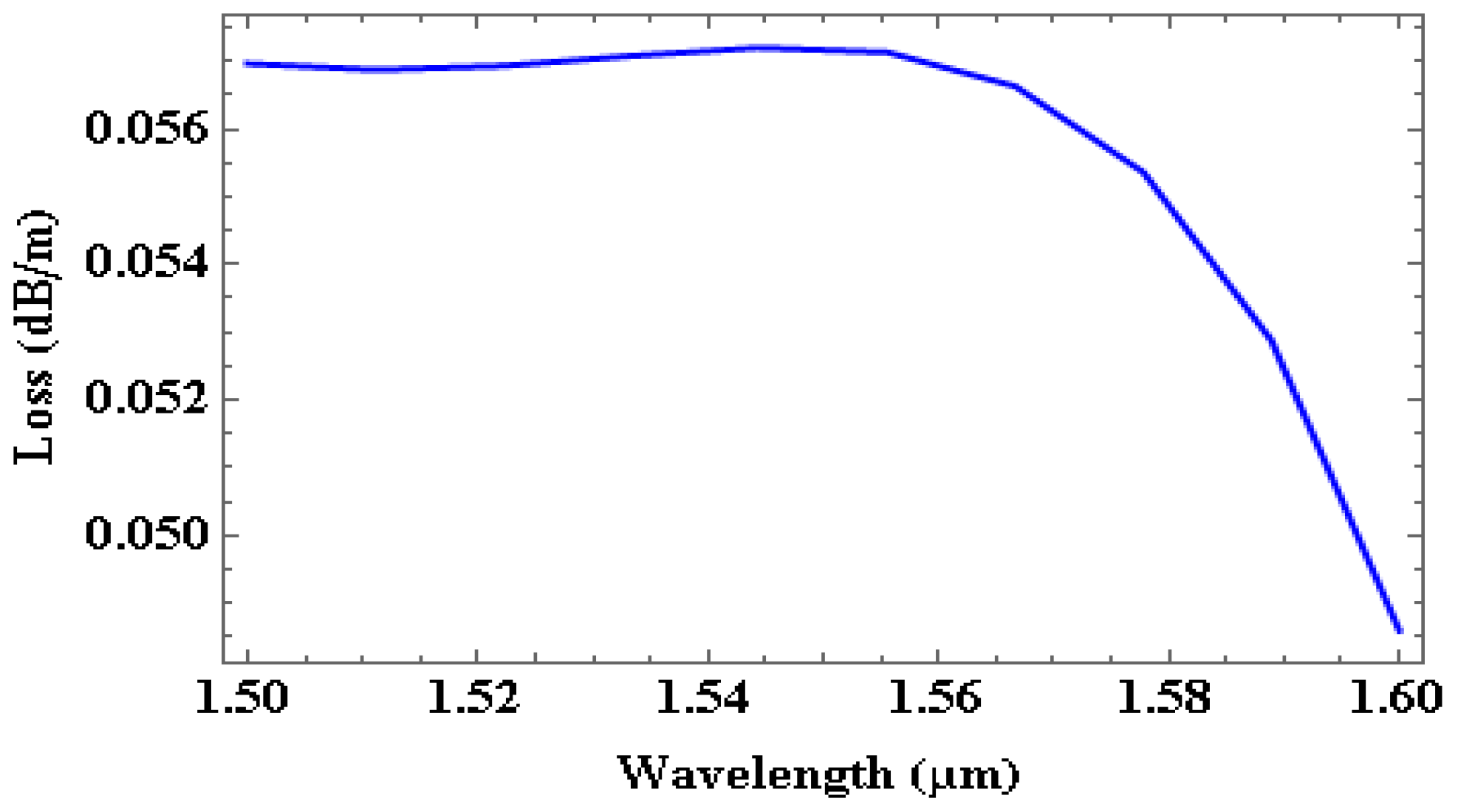
| Wavelength (nm) | Effective Index | Loss in dB/cm | % (TE/TM) Fraction |
|---|---|---|---|
| 1500 | 2.3619 | 0.00057 | 73.96/81.6 |
| 1530 | 2.322 | 0.00057 | 72.97/81.43 |
| 1550 | 2.2963 | 0.00057 | 72.32/81.33 |
| Parameter | Value |
|---|---|
| Wavelength | 1550 nm |
| Height of input/output waveguide | 250 nm |
| Width of input/output waveguide | 500 nm |
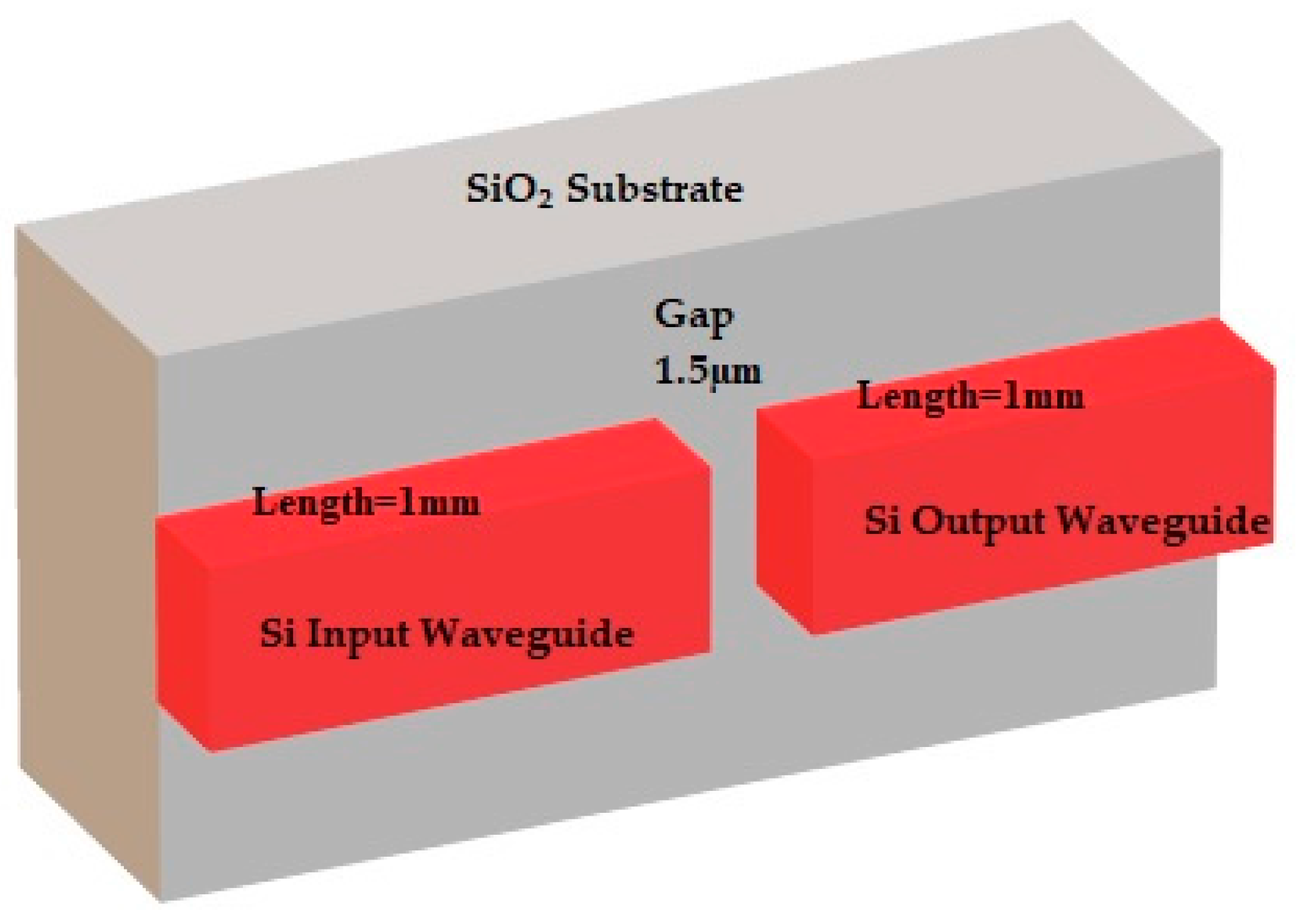
| Width of fluidic channel | 1.5 µm |
| Length of input/output waveguide | 1 mm |
| Cross section of fluidic chambers | 7.5 µm × 15 µm |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Muniswamy, V.; Bangalore Muniraju, C.; Kumar Pattnaik, P.; Krishnaswamy, N. Modeling and Analysis of an Opto-Fluidic Sensor for Lab-on-a-Chip Applications. Micromachines 2018, 9, 134. https://doi.org/10.3390/mi9030134
Muniswamy V, Bangalore Muniraju C, Kumar Pattnaik P, Krishnaswamy N. Modeling and Analysis of an Opto-Fluidic Sensor for Lab-on-a-Chip Applications. Micromachines. 2018; 9(3):134. https://doi.org/10.3390/mi9030134
Chicago/Turabian StyleMuniswamy, Venkatesha, Chaya Bangalore Muniraju, Prasant Kumar Pattnaik, and Narayan Krishnaswamy. 2018. "Modeling and Analysis of an Opto-Fluidic Sensor for Lab-on-a-Chip Applications" Micromachines 9, no. 3: 134. https://doi.org/10.3390/mi9030134








