NACC1, as a Target of MicroRNA-331-3p, Regulates Cell Proliferation in Urothelial Carcinoma Cells
Abstract
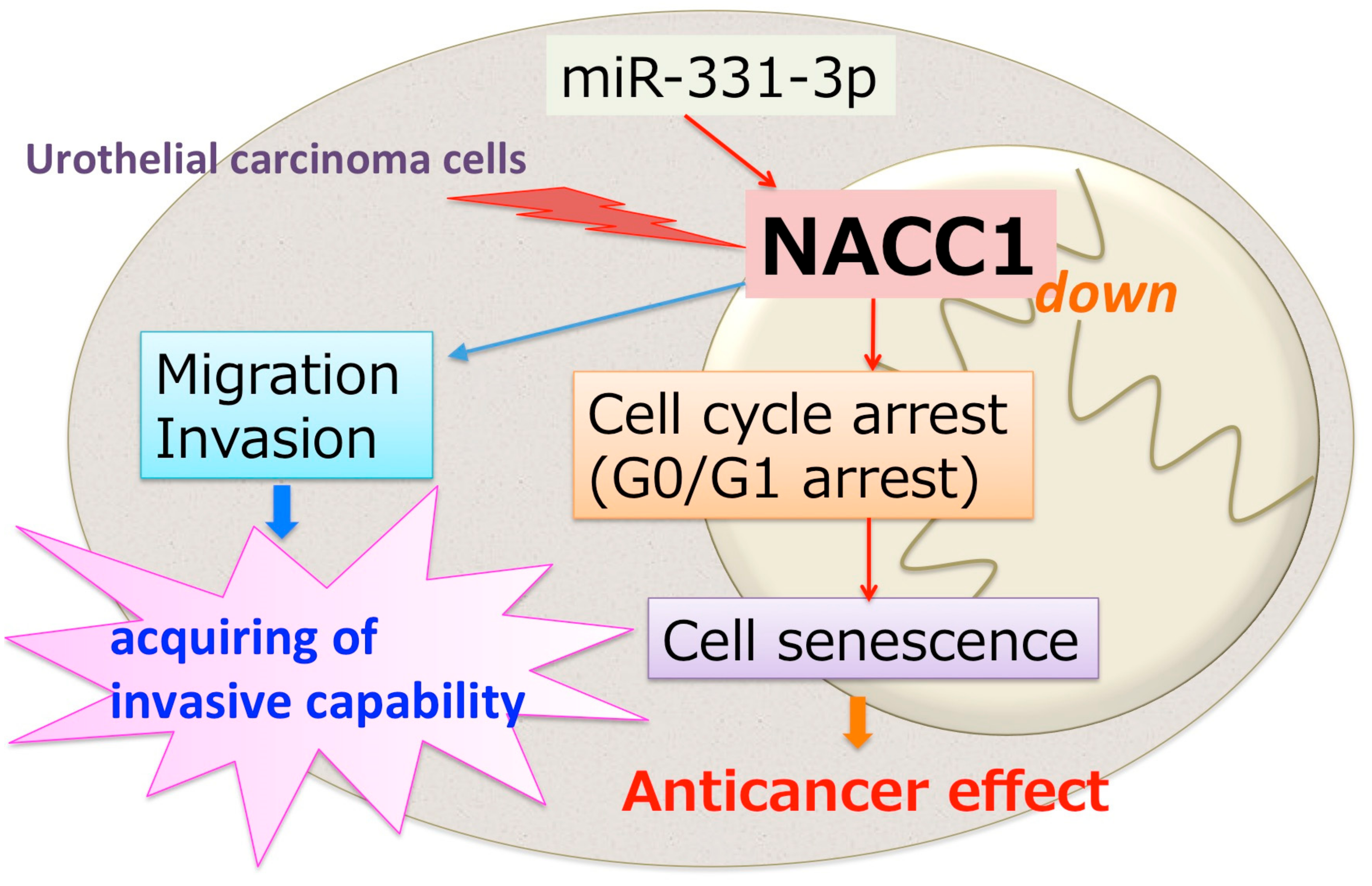
:1. Introduction
2. Results
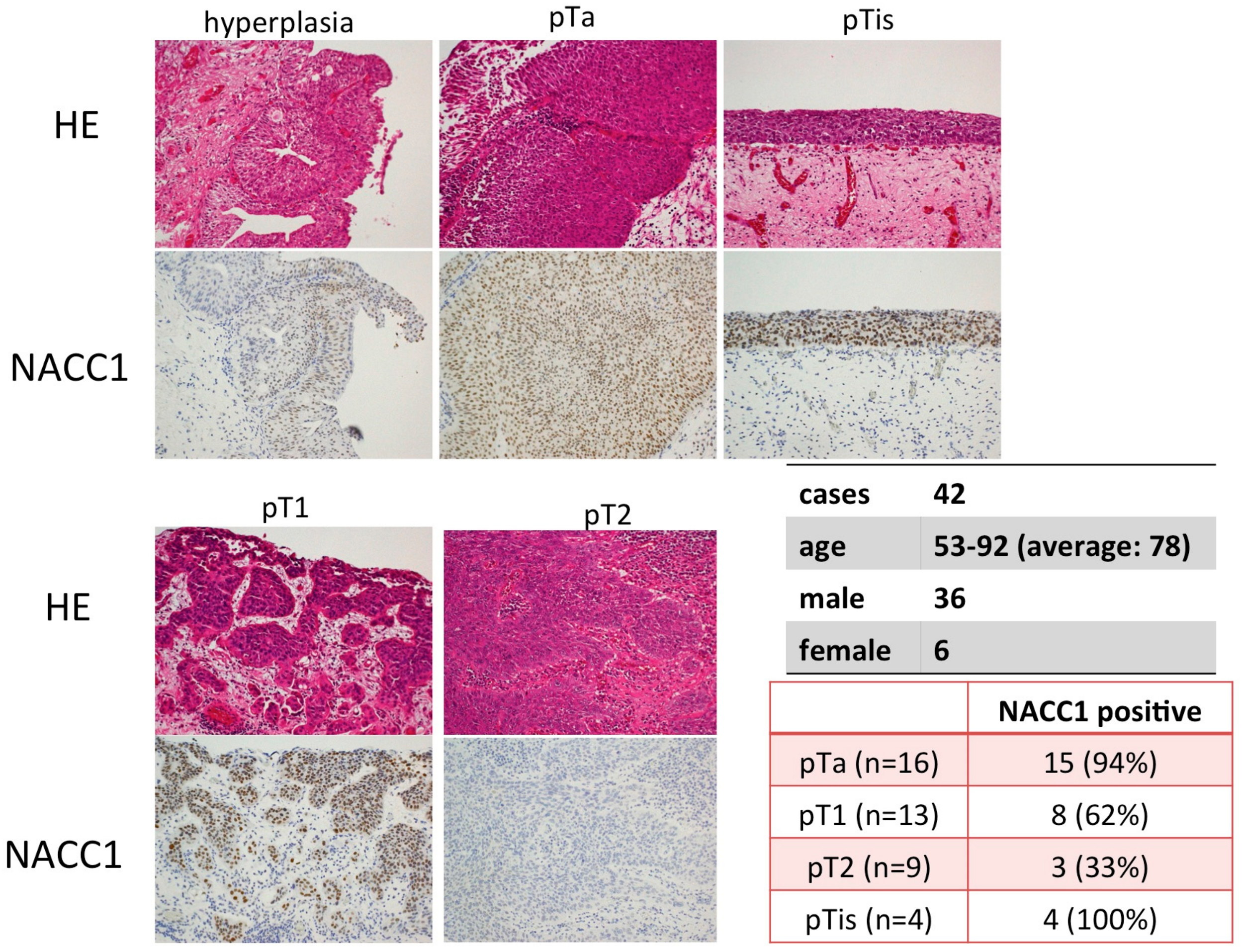
2.1. NACC1 Is Overexpressed in Various Stages of UC in the Urinary Bladder
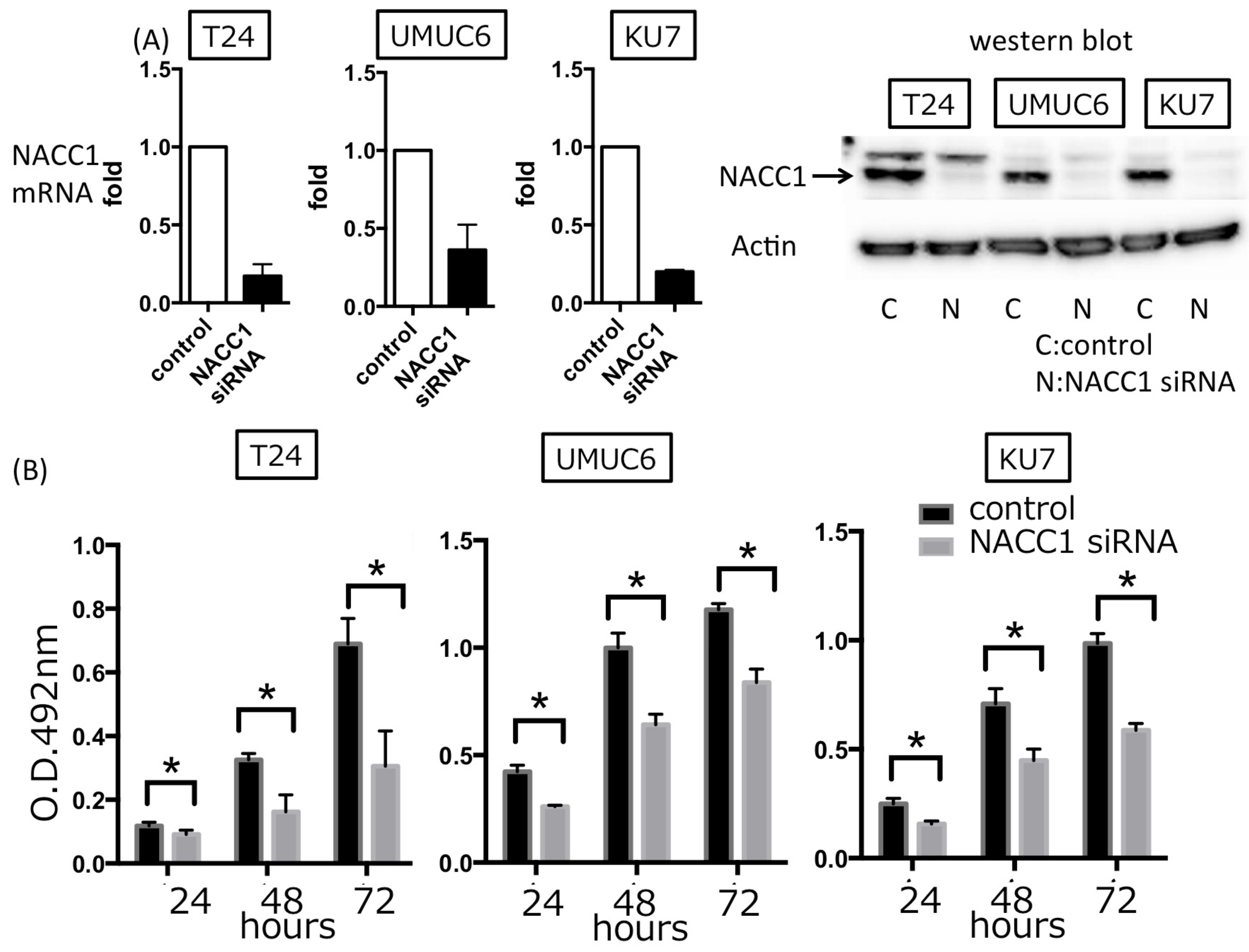
2.2. NACC1 Inhibition Suppresses Proliferation in UC Cells
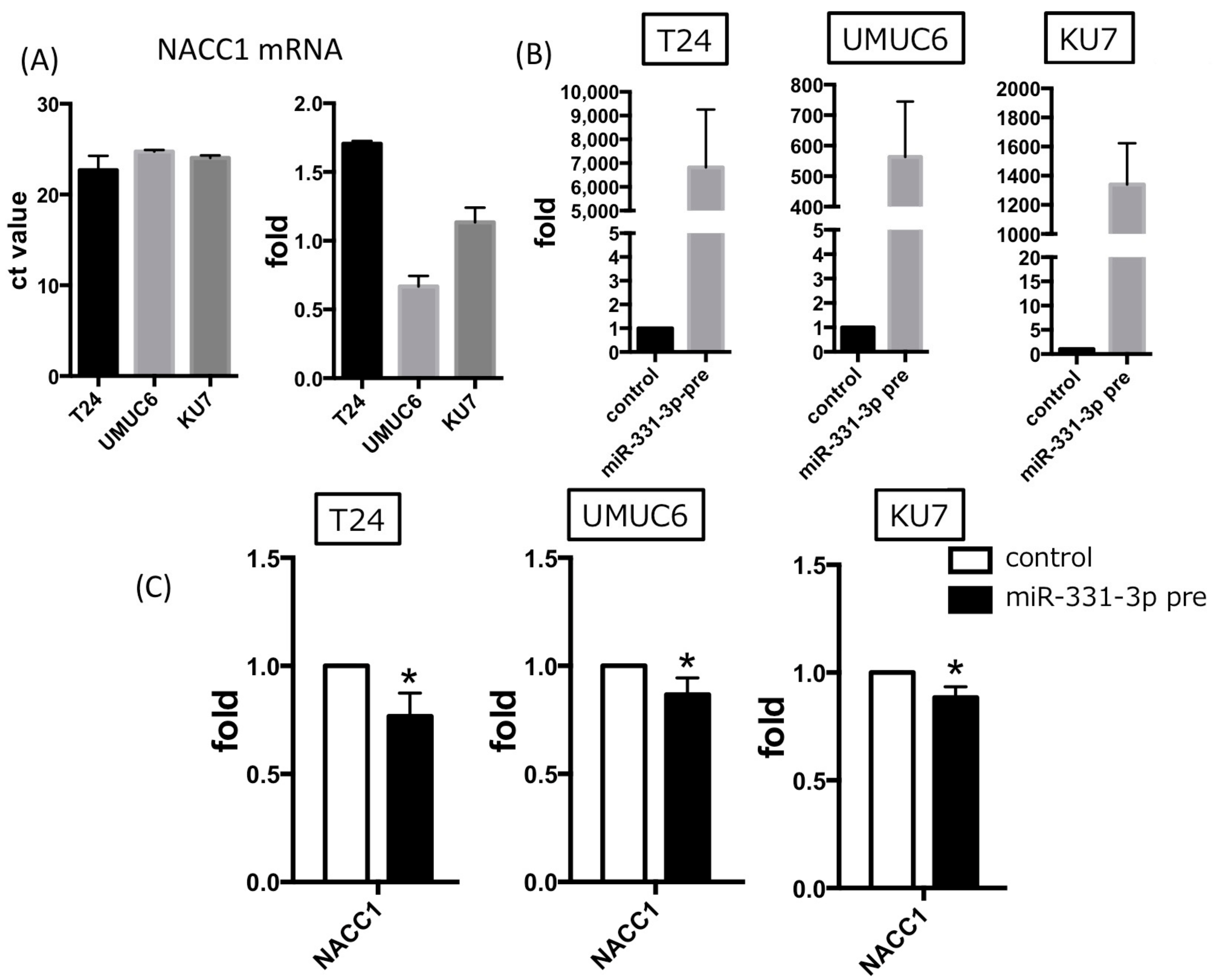
2.3. NACC1 Is One of the Putative Targets of MiR-331-3p
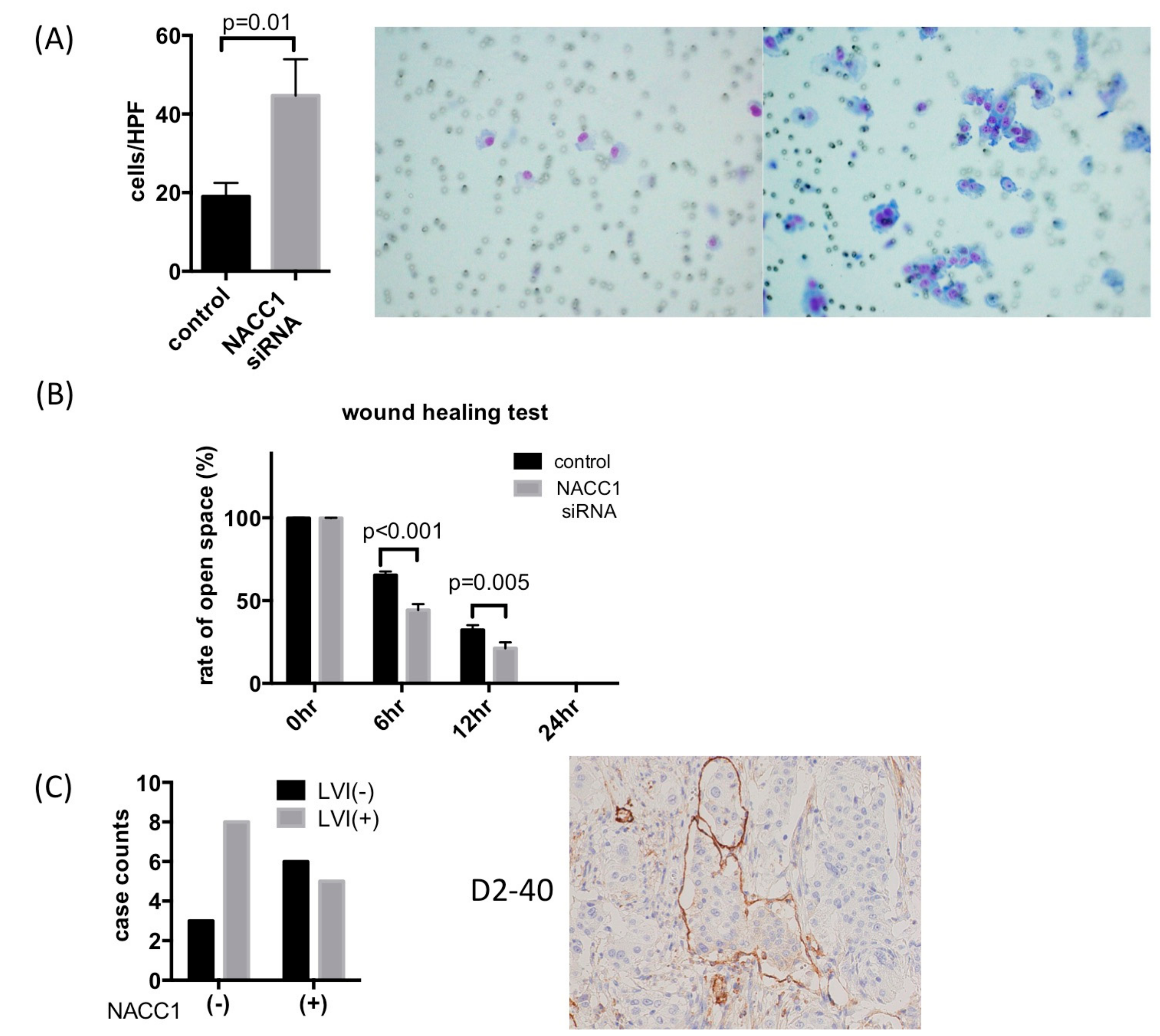
2.4. NACC1 Inhibits UC Cell Invasion and Migration In Vitro
3. Discussion
4. Materials and Methods
4.1. Cell Lines
4.2. siRNA and miRNA Inhibitor/Precursor Transfection in UC Cells
4.3. Quantitative RT-PCR Analysis of miRNA and mRNA
- NACC1 sense 5′-CTCTCCCGGCTGAACTTATCAAC-3′,
- NACC1 antisense 5′-GTACACGTTGGTGCCTGTCAC-3′;
- Actin sense 5′-CTCTTCCAGCCTTCCTTCCT-3′,
- Actin antisense 5′-AGCACTGTGTTGGCGTACAG-3′;
- CCNA1 sense 5′-ACCCCAAGAGTGGAGTTGTG-3′,
- CCNA1 antisense 5′-GGAAGGCATTTTCTGATCCA-3′;
- CCNA2 sense 5′-TTATTGCTGGAGCTGCCTTT-3′,
- CCNA2 antisense 5′-ACTGTTGTGCATGCTGTGGT-3′;
- CCNB1 sense 5′-CGGGAAGTCACTGGAAACAT-3′,
- CCNB1 antisense 5′-AAACATGGCAGTGACACCAA-3′;
- CCNB2 sense 5′-TGGAAAAGTTGGCTCCAAAG-3′,
- CCNB2 antisense 5′-CCTCCAGCTGCCTGAGATAC-3′.
4.4. Cell Proliferation Assay
4.5. Senescence-Associated β-Galactosidase (SA-β-gal) Assay
4.6. Cell Invasion Assay and Wound Healing Assay
4.7. Tissue Samples
4.8. Immunohistochemistry
4.9. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Miller, K.D.; Siegel, R.L.; Lin, C.C.; Mariotto, A.B.; Kramer, J.L.; Rowland, J.H.; Stein, K.D.; Alteri, R.; Jemal, A. Cancer treatment and survivorship statistics, 2016. CA Cancer J. Clin. 2016, 66, 271–289. [Google Scholar] [CrossRef] [PubMed]
- Ro, J.Y.; Staerkel, G.A.; Ayala, A.G. Cytologic and histologic features of superficial bladder cancer. Urol. Clin. N. Am. 1992, 19, 435–453. [Google Scholar] [PubMed]
- Faiola, F.; Yin, N.; Fidalgo, M.; Huang, X.; Saunders, A.; Ding, J.; Guallar, D.; Dang, B.; Wang, J. Nac1 regulates somatic cell reprogramming by controlling zeb1 and e-cadherin expression. Stem Cell Rep. 2017, 9, 913–926. [Google Scholar] [CrossRef] [PubMed]
- Rahman, M.T.; Nakayama, K.; Rahman, M.; Katagiri, H.; Katagiri, A.; Ishibashi, T.; Ishikawa, M.; Iida, K.; Nakayama, N.; Otsuki, Y.; et al. Fatty acid synthase expression associated with nac1 is a potential therapeutic target in ovarian clear cell carcinomas. Br. J. Cancer 2012, 107, 300–307. [Google Scholar] [CrossRef] [PubMed]
- Shan, W.; Li, J.; Bai, Y.; Lu, X. Mir-339-5p inhibits migration and invasion in ovarian cancer cell lines by targeting nacc1 and bcl6. Tumour Biol. 2016, 37, 5203–5211. [Google Scholar] [CrossRef] [PubMed]
- Sekine, J.; Nakatani, E.; Ohira, K.; Hideshima, K.; Kanno, T.; Nariai, Y.; Kagimura, T.; Urano, T. Nucleus accumbens-associated protein 1 expression has potential as a marker for distinguishing oral epithelial dysplasia and squamous cell carcinoma. PLoS ONE 2015, 10, e0131752. [Google Scholar] [CrossRef] [PubMed]
- Ju, T.; Jin, H.; Ying, R.; Xie, Q.; Zhou, C.; Gao, D. Overexpression of nac1 confers drug resistance via hoxa9 in colorectal carcinoma cells. Mol. Med. Rep. 2017, 16, 3194–3200. [Google Scholar] [CrossRef] [PubMed]
- Nakayama, N.; Kato, H.; Sakashita, G.; Nariai, Y.; Nakayama, K.; Kyo, S.; Urano, T. Protein complex formation and intranuclear dynamics of nac1 in cancer cells. Arch. Biochem. Biophys. 2016, 606, 10–15. [Google Scholar] [CrossRef] [PubMed]
- Tatemichi, Y.; Shibazaki, M.; Yasuhira, S.; Kasai, S.; Tada, H.; Oikawa, H.; Suzuki, Y.; Takikawa, Y.; Masuda, T.; Maesawa, C. Nucleus accumbens associated 1 is recruited within the promyelocytic leukemia nuclear body through sumo modification. Cancer Sci. 2015, 106, 848–856. [Google Scholar] [CrossRef] [PubMed]
- Nishi, T.; Maruyama, R.; Urano, T.; Nakayama, N.; Kawabata, Y.; Yano, S.; Yoshida, M.; Nakayama, K.; Miyazaki, K.; Takenaga, K.; et al. Low expression of nucleus accumbens-associated protein 1 predicts poor prognosis for patients with pancreatic ductal adenocarcinoma. Pathol. Int. 2012, 62, 802–810. [Google Scholar] [CrossRef] [PubMed]
- Fujii, T.; Shimada, K.; Asano, A.; Tatsumi, Y.; Yamaguchi, N.; Yamazaki, M.; Konishi, N. Microrna-331-3p suppresses cervical cancer cell proliferation and e6/e7 expression by targeting nrp2. Int. J. Mol. Sci. 2016, 17, 1351. [Google Scholar] [CrossRef] [PubMed]
- Fujii, T.; Shimada, K.; Tatsumi, Y.; Tanaka, N.; Fujimoto, K.; Konishi, N. Syndecan-1 up-regulates microrna-331-3p and mediates epithelial-to-mesenchymal transition in prostate cancer. Mol. Carcinog. 2016, 55, 1378–1386. [Google Scholar] [CrossRef] [PubMed]
- Tsunoda, K.; Oikawa, H.; Tada, H.; Tatemichi, Y.; Muraoka, S.; Miura, S.; Shibazaki, M.; Maeda, F.; Takahashi, K.; Akasaka, T.; et al. Nucleus accumbens-associated 1 contributes to cortactin deacetylation and augments the migration of melanoma cells. J. Investig. Dermatol. 2011, 131, 1710–1719. [Google Scholar] [CrossRef] [PubMed]
- Ishikawa, M.; Nakayama, K.; Yeasmin, S.; Katagiri, A.; Iida, K.; Nakayama, N.; Miyazaki, K. Nac1, a potential stem cell pluripotency factor expression in normal endometrium, endometrial hyperplasia and endometrial carcinoma. Int. J. Oncol. 2010, 36, 1097–1103. [Google Scholar] [PubMed]
- Shih Ie, M.; Davidson, B. Pathogenesis of ovarian cancer: Clues from selected overexpressed genes. Future Oncol. 2009, 5, 1641–1657. [Google Scholar] [CrossRef] [PubMed]
- Andres-Leon, E.; Gonzalez Pena, D.; Gomez-Lopez, G.; Pisano, D.G. Mirgate: A curated database of human, mouse and rat mirna-mrna targets. Database 2015, 2015, bav035. [Google Scholar] [CrossRef] [PubMed]
- Canavese, M.; Santo, L.; Raje, N. Cyclin dependent kinases in cancer: Potential for therapeutic intervention. Cancer Biol. Ther. 2012, 13, 451–457. [Google Scholar] [CrossRef] [PubMed]
- Sperka, T.; Wang, J.; Rudolph, K.L. DNA damage checkpoints in stem cells, ageing and cancer. Nat. Rev. Mol. Cell Biol. 2012, 13, 579–590. [Google Scholar] [CrossRef] [PubMed]
- Van de Putte, E.E.; Mertens, L.S.; Meijer, R.P.; van der Heijden, M.S.; Bex, A.; van der Poel, H.G.; Kerst, J.M.; Bergman, A.M.; Horenblas, S.; van Rhijn, B.W. Neoadjuvant induction dose-dense mvac for muscle invasive bladder cancer: Efficacy and safety compared with classic mvac and gemcitabine/cisplatin. World J. Urol. 2016, 34, 157–162. [Google Scholar] [CrossRef] [PubMed]
- Bognar, Z.; Fekete, K.; Antus, C.; Hocsak, E.; Bognar, R.; Tapodi, A.; Boronkai, A.; Farkas, N.; Gallyas, F., Jr.; Sumegi, B.; et al. Desethylamiodarone-a metabolite of amiodarone-induces apoptosis on t24 human bladder cancer cells via multiple pathways. PLoS ONE 2017, 12, e0189470. [Google Scholar] [CrossRef] [PubMed]
- Fang, C.Y.; Chen, J.S.; Chang, S.K.; Shen, C.H. Reversine induces autophagic cell death through the amp-activated protein kinase pathway in urothelial carcinoma cells. Anticancer Drugs 2018, 29, 29–39. [Google Scholar] [CrossRef] [PubMed]
- Wu, P.; Liu, S.; Su, J.; Chen, J.; Li, L.; Zhang, R.; Chen, T. Apoptosis triggered by isoquercitrin in bladder cancer cells by activating the ampk-activated protein kinase pathway. Food Funct. 2017, 8, 3707–3722. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.C.; Wang, S.T.; Liu, H.T.; Wang, X.Y.; Wu, S.C.; Chen, L.C.; Liu, Y.W. Trichostatin a induces bladder cancer cell death via intrinsic apoptosis at the early phase and sp1survivin downregulation at the late phase of treatment. Oncol. Rep. 2017, 38, 1587–1596. [Google Scholar] [CrossRef] [PubMed]
- Yuan, S.Y.; Shiau, M.Y.; Ou, Y.C.; Huang, Y.C.; Chen, C.C.; Cheng, C.L.; Chiu, K.Y.; Wang, S.S.; Tsai, K.J. Miconazole induces apoptosis via the death receptor 5-dependent and mitochondrial-mediated pathways in human bladder cancer cells. Oncol. Rep. 2017, 37, 3606–3616. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Juengel, E.; Najafi, R.; Rutz, J.; Maxeiner, S.; Makarevic, J.; Roos, F.; Tsaur, I.; Haferkamp, A.; Blaheta, R.A. Hdac inhibition as a treatment concept to combat temsirolimus-resistant bladder cancer cells. Oncotarget 2017, 8, 110016–110028. [Google Scholar] [CrossRef] [PubMed]
- Makarevic, J.; Rutz, J.; Juengel, E.; Kaulfuss, S.; Reiter, M.; Tsaur, I.; Bartsch, G.; Haferkamp, A.; Blaheta, R.A. Amygdalin blocks bladder cancer cell growth in vitro by diminishing cyclin a and cdk2. PLoS ONE 2014, 9, e105590. [Google Scholar] [CrossRef] [PubMed]
- Shen, K.H.; Chang, J.K.; Hsu, Y.L.; Kuo, P.L. Chalcone arrests cell cycle progression and induces apoptosis through induction of mitochondrial pathway and inhibition of nuclear factor kappa b signalling in human bladder cancer cells. Basic Clin. Pharmacol. Toxicol. 2007, 101, 254–261. [Google Scholar] [CrossRef] [PubMed]
- Hussain, S.A.; Palmer, D.H.; Syn, W.K.; Sacco, J.J.; Greensmith, R.M.; Elmetwali, T.; Aachi, V.; Lloyd, B.H.; Jithesh, P.V.; Arrand, J.; et al. Gene expression profiling in bladder cancer identifies potential therapeutic targets. Int. J. Oncol. 2017, 50, 1147–1159. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shih Ie, M.; Nakayama, K.; Wu, G.; Nakayama, N.; Zhang, J.; Wang, T.L. Amplification of the ch19p13.2 nacc1 locus in ovarian high-grade serous carcinoma. Mod. Pathol. 2011, 24, 638–645. [Google Scholar] [CrossRef] [PubMed]
- Davidson, B.; Berner, A.; Trope, C.G.; Wang, T.L.; Shih Ie, M. Expression and clinical role of the bric-a-brac tramtrack broad complex/poxvirus and zinc protein nac-1 in ovarian carcinoma effusions. Hum. Pathol. 2007, 38, 1030–1036. [Google Scholar] [CrossRef] [PubMed]
- Ishibashi, M.; Nakayama, K.; Yeasmin, S.; Katagiri, A.; Iida, K.; Nakayama, N.; Fukumoto, M.; Miyazaki, K. A btb/poz gene, nac-1, a tumor recurrence-associated gene, as a potential target for taxol resistance in ovarian cancer. Clin. Cancer Res. 2008, 14, 3149–3155. [Google Scholar] [CrossRef] [PubMed]
- Jinawath, N.; Vasoontara, C.; Yap, K.L.; Thiaville, M.M.; Nakayama, K.; Wang, T.L.; Shih, I.M. Nac-1, a potential stem cell pluripotency factor, contributes to paclitaxel resistance in ovarian cancer through inactivating gadd45 pathway. Oncogene 2009, 28, 1941–1948. [Google Scholar] [CrossRef] [PubMed]
- Nakayama, K.; Nakayama, N.; Davidson, B.; Sheu, J.J.; Jinawath, N.; Santillan, A.; Salani, R.; Bristow, R.E.; Morin, P.J.; Kurman, R.J.; et al. A btb/poz protein, nac-1, is related to tumor recurrence and is essential for tumor growth and survival. Proc. Natl. Acad. Sci. USA 2006, 103, 18739–18744. [Google Scholar] [CrossRef] [PubMed]
- Nakayama, K.; Nakayama, N.; Wang, T.L.; Shih Ie, M. Nac-1 controls cell growth and survival by repressing transcription of gadd45gip1, a candidate tumor suppressor. Cancer Res. 2007, 67, 8058–8064. [Google Scholar] [CrossRef] [PubMed]
- Nakayama, K.; Rahman, M.T.; Rahman, M.; Yeasmin, S.; Ishikawa, M.; Katagiri, A.; Iida, K.; Nakayama, N.; Miyazaki, K. Biological role and prognostic significance of nac1 in ovarian cancer. Gynecol. Oncol. 2010, 119, 469–478. [Google Scholar] [CrossRef] [PubMed]
- Yeasmin, S.; Nakayama, K.; Ishibashi, M.; Katagiri, A.; Iida, K.; Purwana, I.N.; Nakayama, N.; Miyazaki, K. Expression of the bric-a-brac tramtrack broad complex protein nac-1 in cervical carcinomas seems to correlate with poorer prognosis. Clin. Cancer Res. 2008, 14, 1686–1691. [Google Scholar] [CrossRef] [PubMed]
- Yeasmin, S.; Nakayama, K.; Rahman, M.T.; Rahman, M.; Ishikawa, M.; Katagiri, A.; Iida, K.; Nakayama, N.; Otuski, Y.; Kobayashi, H.; et al. Biological and clinical significance of nac1 expression in cervical carcinomas: A comparative study between squamous cell carcinomas and adenocarcinomas/adenosquamous carcinomas. Hum. Pathol. 2012, 43, 506–519. [Google Scholar] [CrossRef] [PubMed]
- Yap, K.L.; Fraley, S.I.; Thiaville, M.M.; Jinawath, N.; Nakayama, K.; Wang, J.; Wang, T.L.; Wirtz, D.; Shih Ie, M. Nac1 is an actin-binding protein that is essential for effective cytokinesis in cancer cells. Cancer Res. 2012, 72, 4085–4096. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Cheng, Y.; Ren, X.; Hori, T.; Huber-Keener, K.J.; Zhang, L.; Yap, K.L.; Liu, D.; Shantz, L.; Qin, Z.H.; et al. Dysfunction of nucleus accumbens-1 activates cellular senescence and inhibits tumor cell proliferation and oncogenesis. Cancer Res. 2012, 72, 4262–4275. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Cheng, Y.; Ren, X.; Zhang, L.; Yap, K.L.; Wu, H.; Patel, R.; Liu, D.; Qin, Z.H.; Shih, I.M.; et al. Nac1 modulates sensitivity of ovarian cancer cells to cisplatin by altering the hmgb1-mediated autophagic response. Oncogene 2012, 31, 1055–1064. [Google Scholar] [CrossRef] [PubMed]
- Okazaki, K.; Nakayama, N.; Nariai, Y.; Nakayama, K.; Miyazaki, K.; Maruyama, R.; Kato, H.; Kosugi, S.; Urano, T.; Sakashita, G. Nuclear localization signal in a cancer-related transcriptional regulator protein nac1. Carcinogenesis 2012, 33, 1854–1862. [Google Scholar] [CrossRef] [PubMed]
- Gao, M.; Wu, R.C.; Herlinger, A.L.; Yap, K.; Kim, J.W.; Wang, T.L.; Shih Ie, M. Identification of the nac1-regulated genes in ovarian cancer. Am. J. Pathol. 2014, 184, 133–140. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Yang, J.W.; Ren, X.; Yang, J.M. Nac1 and hmgb1 enter a partnership for manipulating autophagy. Autophagy 2011, 7, 1557–1558. [Google Scholar] [CrossRef] [PubMed]
- Gottardo, F.; Liu, C.G.; Ferracin, M.; Calin, G.A.; Fassan, M.; Bassi, P.; Sevignani, C.; Byrne, D.; Negrini, M.; Pagano, F.; et al. Micro-rna profiling in kidney and bladder cancers. Urol. Oncol. 2007, 25, 387–392. [Google Scholar] [CrossRef] [PubMed]
- Nagata, M.; Muto, S.; Horie, S. Molecular biomarkers in bladder cancer: Novel potential indicators of prognosis and treatment outcomes. Dis. Markers 2016, 2016, 8205836. [Google Scholar] [CrossRef] [PubMed]
- Fujii, T.; Shimada, K.; Tatsumi, Y.; Hatakeyama, K.; Obayashi, C.; Fujimoto, K.; Konishi, N. Microrna-145 promotes differentiation in human urothelial carcinoma through down-regulation of syndecan-1. BMC Cancer 2015, 15, 818. [Google Scholar] [CrossRef] [PubMed]
- Tazaki, H.; Tachibana, M. Studies on ku-1 and ku-7 cells as an in vitro model of human transitional cell carcinoma of urinary bladder. Hum. Cell 1988, 1, 78–83. [Google Scholar] [PubMed]
- Shimada, K.; Nakamura, M.; De Velasco, M.A.; Tanaka, M.; Ouji, Y.; Konishi, N. Syndecan-1, a new target molecule involved in progression of androgen-independent prostate cancer. Cancer Sci. 2009, 100, 1248–1254. [Google Scholar] [CrossRef] [PubMed] [Green Version]


© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Morita, K.; Fujii, T.; Itami, H.; Uchiyama, T.; Nakai, T.; Hatakeyama, K.; Sugimoto, A.; Miyake, M.; Nakai, Y.; Tanaka, N.; et al. NACC1, as a Target of MicroRNA-331-3p, Regulates Cell Proliferation in Urothelial Carcinoma Cells. Cancers 2018, 10, 347. https://doi.org/10.3390/cancers10100347
Morita K, Fujii T, Itami H, Uchiyama T, Nakai T, Hatakeyama K, Sugimoto A, Miyake M, Nakai Y, Tanaka N, et al. NACC1, as a Target of MicroRNA-331-3p, Regulates Cell Proliferation in Urothelial Carcinoma Cells. Cancers. 2018; 10(10):347. https://doi.org/10.3390/cancers10100347
Chicago/Turabian StyleMorita, Kohei, Tomomi Fujii, Hiroe Itami, Tomoko Uchiyama, Tokiko Nakai, Kinta Hatakeyama, Aya Sugimoto, Makito Miyake, Yasushi Nakai, Nobumichi Tanaka, and et al. 2018. "NACC1, as a Target of MicroRNA-331-3p, Regulates Cell Proliferation in Urothelial Carcinoma Cells" Cancers 10, no. 10: 347. https://doi.org/10.3390/cancers10100347




