The Role of MicroRNAs in the Metastatic Process of High-Risk HPV-Induced Cancers
Abstract
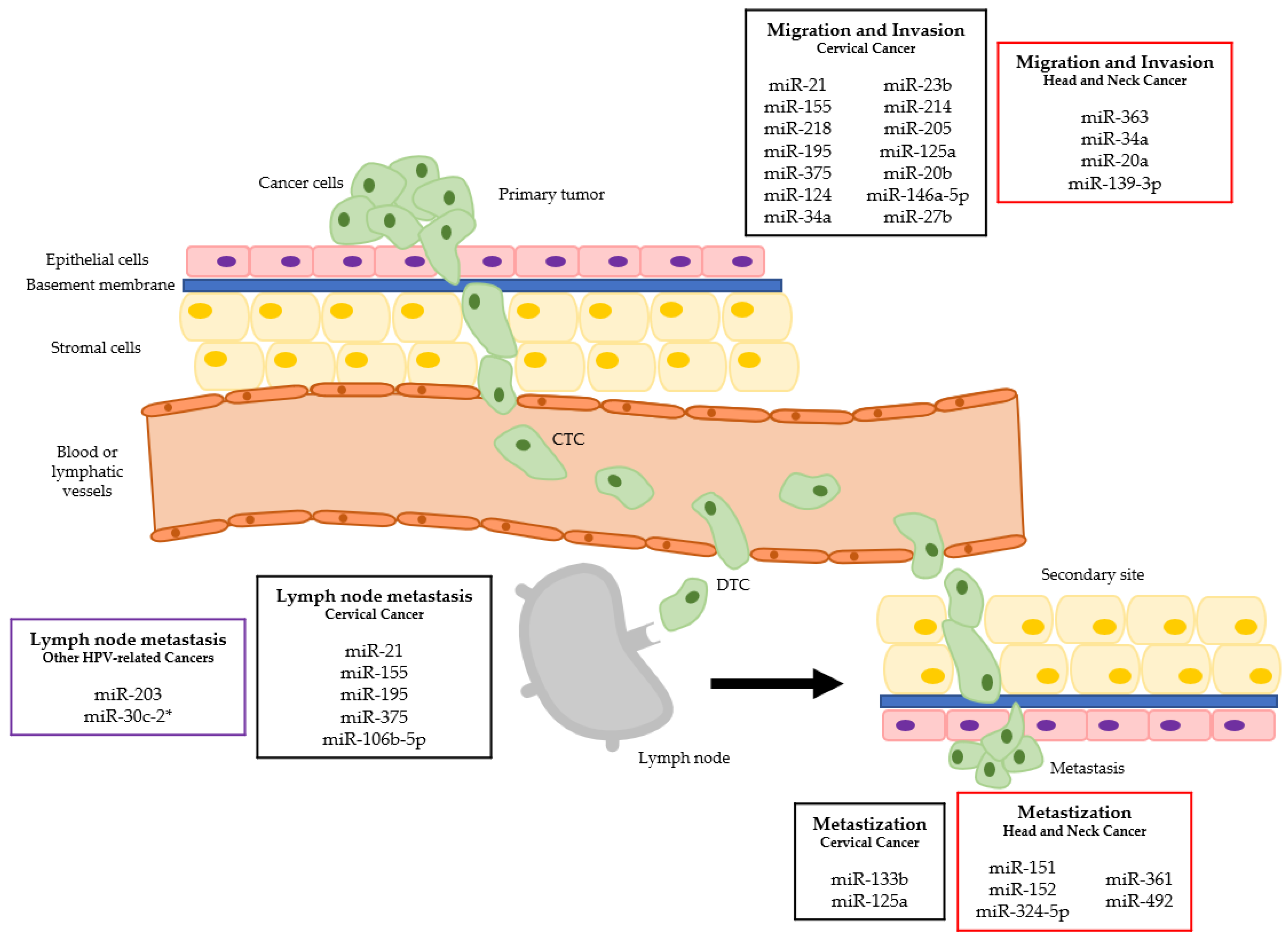
:1. Introduction
2. Cervical Cancer
3. Other Anogenital Cancers
4. Head and Neck Cancers
5. Other Cancers Potentially Related to High-Risk HPV
5.1. Esophageal Cancer
5.2. Lung Cancer
6. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Roden, R.B.S.; Stern, P.L. Opportunities and challenges for human papillomavirus vaccination in cancer. Nat. Rev. Cancer 2018, 18, 240–254. [Google Scholar] [CrossRef] [PubMed]
- Muller-Coan, B.G.; Caetano, B.F.R.; Pagano, J.S.; Elgui de Oliveira, D. Cancer Progression Goes Viral: The Role of Oncoviruses in Aggressiveness of Malignancies. Trends Cancer 2018, 4, 485–498. [Google Scholar] [CrossRef] [PubMed]
- Boulenouar, S.; Weyn, C.; Van Noppen, M.; Moussa Ali, M.; Favre, M.; Delvenne, P.O.; Bex, F.; Noel, A.; Englert, Y.; Fontaine, V. Effects of HPV-16 E5, E6 and E7 proteins on survival, adhesion, migration and invasion of trophoblastic cells. Carcinogenesis 2010, 31, 473–480. [Google Scholar] [CrossRef] [PubMed]
- Hu, D.; Zhou, J.; Wang, F.; Shi, H.; Li, Y.; Li, B. HPV-16 E6/E7 promotes cell migration and invasion in cervical cancer via regulating cadherin switch in vitro and in vivo. Arch Gynecol. Obstet. 2015, 292, 1345–1354. [Google Scholar] [CrossRef] [PubMed]
- Cheung, K.J.; Ewald, A.J. A collective route to metastasis: Seeding by tumor cell clusters. Science 2016, 352, 167–169. [Google Scholar] [CrossRef] [PubMed]
- Jiang, W.G.; Sanders, A.J.; Katoh, M.; Ungefroren, H.; Gieseler, F.; Prince, M.; Thompson, S.K.; Zollo, M.; Spano, D.; Dhawan, P.; et al. Tissue invasion and metastasis: Molecular, biological and clinical perspectives. Semin. Cancer Biol. 2015, 35, S244–S275. [Google Scholar] [CrossRef]
- Turajlic, S.; Swanton, C. Metastasis as an evolutionary process. Science 2016, 352, 169–175. [Google Scholar] [CrossRef]
- Dasgupta, A.; Lim, A.R.; Ghajar, C.M. Circulating and disseminated tumor cells: Harbingers or initiators of metastasis? Mol. Oncol. 2017, 11, 40–61. [Google Scholar] [CrossRef]
- Luo, X.; Zhao, X.; Cheng, C.; Li, N.; Liu, Y.; Cao, Y. The implications of signaling lipids in cancer metastasis. Exp. Mol. Med. 2018, 50, 127. [Google Scholar] [CrossRef]
- Chitty, J.L.; Filipe, E.C.; Lucas, M.C.; Herrmann, D.; Cox, T.R.; Timpson, P. Recent advances in understanding the complexities of metastasis. F1000Research 2018, 7. [Google Scholar] [CrossRef]
- Cortesina, G.; Martone, T. Molecular metastases markers in head and neck squamous cell carcinoma: Review of the literature. Acta Otorhinolaryngol. Ital. 2006, 26, 317–325. [Google Scholar] [PubMed]
- Bielenberg, D.R.; Zetter, B.R. The Contribution of Angiogenesis to the Process of Metastasis. Cancer J. 2015, 21, 267–273. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Healy, J.C. Detection of peritoneal metastases. Cancer Imaging 2001, 1, 4–12. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, D.X.; Bos, P.D.; Massague, J. Metastasis: From dissemination to organ-specific colonization. Nat. Rev. Cancer 2009, 9, 274–284. [Google Scholar] [CrossRef] [PubMed]
- Malanchi, I.; Santamaria-Martinez, A.; Susanto, E.; Peng, H.; Lehr, H.A.; Delaloye, J.F.; Huelsken, J. Interactions between cancer stem cells and their niche govern metastatic colonization. Nature 2011, 481, 85–89. [Google Scholar] [CrossRef] [Green Version]
- Fehm, T.; Mueller, V.; Marches, R.; Klein, G.; Gueckel, B.; Neubauer, H.; Solomayer, E.; Becker, S. Tumor cell dormancy: Implications for the biology and treatment of breast cancer. APMIS 2008, 116, 742–753. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.; Shen, X.J.; Zou, Q.; Wang, S.P.; Tang, S.M.; Zhang, G.Z. Biological functions of microRNAs: A review. J. Physiol. Biochem. 2011, 67, 129–139. [Google Scholar] [CrossRef] [PubMed]
- Esquela-Kerscher, A.; Slack, F.J. Oncomirs-microRNAs with a role in cancer. Nat. Rev. Cancer 2006, 6, 259–269. [Google Scholar] [CrossRef] [PubMed]
- Santos, J.M.O.; Gil da Costa, R.M.; Medeiros, R. Dysregulation of cellular microRNAs by human oncogenic viruses-Implications for tumorigenesis. Biochim. Biophys. Acta 2018, 1861, 95–105. [Google Scholar] [CrossRef] [PubMed]
- Garzon, R.; Calin, G.A.; Croce, C.M. MicroRNAs in Cancer. Annu. Rev. Med. 2009, 60, 167–179. [Google Scholar] [CrossRef]
- Paiva, I.; Gil da Costa, R.M.; Ribeiro, J.; Sousa, H.; Bastos, M.M.; Faustino-Rocha, A.; Lopes, C.; Oliveira, P.A.; Medeiros, R. MicroRNA-21 expression and susceptibility to HPV-induced carcinogenesis-role of microenvironment in K14-HPV16 mice model. Life Sci. 2015, 128, 8–14. [Google Scholar] [CrossRef] [PubMed]
- Araujo, R.; Santos, J.M.O.; Fernandes, M.; Dias, F.; Sousa, H.; Ribeiro, J.; Bastos, M.; Oliveira, P.A.; Carmo, D.; Casaca, F.; et al. Expression profile of microRNA-146a along HPV-induced multistep carcinogenesis: A study in HPV16 transgenic mice. J. Cancer Res. Clin. Oncol. 2018, 144, 241–248. [Google Scholar] [CrossRef] [PubMed]
- Paiva, I.; Gil da Costa, R.M.; Ribeiro, J.; Sousa, H.; Bastos, M.; Faustino-Rocha, A.; Lopes, C.; Oliveira, P.A.; Medeiros, R. A role for microRNA-155 expression in microenvironment associated to HPV-induced carcinogenesis in K14-HPV16 transgenic mice. PLoS ONE 2015, 10, e0116868. [Google Scholar] [CrossRef] [PubMed]
- Santos, J.M.O.; Fernandes, M.; Araujo, R.; Sousa, H.; Ribeiro, J.; Bastos, M.; Oliveira, P.A.; Carmo, D.; Casaca, F.; Silva, S.; et al. Dysregulated expression of microRNA-150 in human papillomavirus-induced lesions of K14-HPV16 transgenic mice. Life Sci. 2017, 175, 31–36. [Google Scholar] [CrossRef] [PubMed]
- Vojtechova, Z.; Tachezy, R. The Role of miRNAs in Virus-Mediated Oncogenesis. Int. J. Mol. Sci. 2018, 19. [Google Scholar] [CrossRef] [PubMed]
- Gutierrez, D.A.; Varela-Ramirez, A.; Rodriguez-Esquivel, M.; Mendoza-Rodriguez, M.G.; Ayala-Sumuano, J.T.; Pineda, D.; Garrido-Guerrero, E.; Jimenez-Vega, F.; Aguilar, S.; Quinones, M.; et al. Predicting Human miRNA-like Sequences within Human Papillomavirus Genomes. Arch Med. Res. 2018. [Google Scholar] [CrossRef]
- White, N.M.; Fatoohi, E.; Metias, M.; Jung, K.; Stephan, C.; Yousef, G.M. Metastamirs: A stepping stone towards improved cancer management. Nat. Rev. Clin. Oncol. 2011, 8, 75–84. [Google Scholar] [CrossRef] [PubMed]
- Melo, S.A.; Esteller, M. Dysregulation of microRNAs in cancer: Playing with fire. FEBS Lett. 2011, 585, 2087–2099. [Google Scholar] [CrossRef]
- Ferlay, J.; Soerjomataram, I.; Dikshit, R.; Eser, S.; Mathers, C.; Rebelo, M.; Parkin, D.M.; Forman, D.; Bray, F. Cancer incidence and mortality worldwide: Sources, methods and major patterns in GLOBOCAN 2012. Int. J. Cancer 2015, 136, E359–E386. [Google Scholar] [CrossRef]
- Hasanzadeh, M.; Movahedi, M.; Rejali, M.; Maleki, F.; Moetamani-Ahmadi, M.; Seifi, S.; Hosseini, Z.; Khazaei, M.; Amerizadeh, F.; Ferns, G.A.; et al. The potential prognostic and therapeutic application of tissue and circulating microRNAs in cervical cancer. J. Cell. Physiol. 2018. [Google Scholar] [CrossRef]
- Vu, M.; Yu, J.; Awolude, O.A.; Chuang, L. Cervical cancer worldwide. Curr. Probl. Cancer 2018. [Google Scholar] [CrossRef] [PubMed]
- Bosch, F.X.; Broker, T.R.; Forman, D.; Moscicki, A.B.; Gillison, M.L.; Doorbar, J.; Stern, P.L.; Stanley, M.; Arbyn, M.; Poljak, M.; et al. Comprehensive control of human papillomavirus infections and related diseases. Vaccine 2013, 31, H1–H31. [Google Scholar] [CrossRef] [PubMed]
- Walboomers, J.M.; Jacobs, M.V.; Manos, M.M.; Bosch, F.X.; Kummer, J.A.; Shah, K.V.; Snijders, P.J.; Peto, J.; Meijer, C.J.; Munoz, N. Human papillomavirus is a necessary cause of invasive cervical cancer worldwide. J. Pathol. 1999, 189, 12–19. [Google Scholar] [CrossRef]
- Czerniak, B.; Olszewska-Slonina, D. Biomarkers could facilitate prediction of worse clinical outcome of cancer with special insight to cervical cancer. Contemp. Oncol. (Pozn) 2018, 22, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Kong, Q.; Wang, W.; Li, P. Regulator role of HPV E7 protein on miR-21 expression in cervical carcinoma cells and its functional implication. Int. J. Clin. Exp. Pathol. 2015, 8, 15808–15813. [Google Scholar] [PubMed]
- Deftereos, G.; Corrie, S.R.; Feng, Q.; Morihara, J.; Stern, J.; Hawes, S.E.; Kiviat, N.B. Expression of mir-21 and mir-143 in cervical specimens ranging from histologically normal through to invasive cervical cancer. PLoS ONE 2011, 6, e28423. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Zhan, X.; Yan, D.; Wang, Z. Circulating MicroRNA-21 Is Involved in Lymph Node Metastasis in Cervical Cancer by Targeting RASA1. Int. J. Gynecol. Cancer 2016, 26, 810–816. [Google Scholar] [CrossRef] [PubMed]
- Fang, H.; Shuang, D.; Yi, Z.; Sheng, H.; Liu, Y. Up-regulated microRNA-155 expression is associated with poor prognosis in cervical cancer patients. Biomed. Pharmacother. 2016, 83, 64–69. [Google Scholar] [CrossRef] [PubMed]
- Lei, C.; Wang, Y.; Huang, Y.; Yu, H.; Huang, Y.; Wu, L.; Huang, L. Up-regulated miR155 reverses the epithelial-mesenchymal transition induced by EGF and increases chemo-sensitivity to cisplatin in human Caski cervical cancer cells. PLoS ONE 2012, 7, e52310. [Google Scholar] [CrossRef] [PubMed]
- Yamamoto, N.; Kinoshita, T.; Nohata, N.; Itesako, T.; Yoshino, H.; Enokida, H.; Nakagawa, M.; Shozu, M.; Seki, N. Tumor suppressive microRNA-218 inhibits cancer cell migration and invasion by targeting focal adhesion pathways in cervical squamous cell carcinoma. Int. J. Oncol. 2013, 42, 1523–1532. [Google Scholar] [CrossRef]
- Jiang, Z.; Song, Q.; Zeng, R.; Li, J.; Li, J.; Lin, X.; Chen, X.; Zhang, J.; Zheng, Y. MicroRNA-218 inhibits EMT, migration and invasion by targeting SFMBT1 and DCUN1D1 in cervical cancer. Oncotarget 2016, 7, 45622–45636. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhong, J.; Yuan, H.; Xu, X.; Kong, S. MicroRNA195 inhibits cell proliferation, migration and invasion by targeting defective in cullin neddylation 1 domain containing 1 in cervical cancer. Int. J. Mol. Med. 2018, 42, 779–788. [Google Scholar] [CrossRef]
- Wang, F.; Li, Y.; Zhou, J.; Xu, J.; Peng, C.; Ye, F.; Shen, Y.; Lu, W.; Wan, X.; Xie, X. miR-375 is down-regulated in squamous cervical cancer and inhibits cell migration and invasion via targeting transcription factor SP1. Am. J. Pathol. 2011, 179, 2580–2588. [Google Scholar] [CrossRef]
- Liu, S.; Song, L.; Yao, H.; Zhang, L.; Xu, D.; Gao, F.; Li, Q. MiR-375 Is Epigenetically Downregulated by HPV-16 E6 Mediated DNMT1 Upregulation and Modulates EMT of Cervical Cancer Cells by Suppressing lncRNA MALAT1. PLoS ONE 2016, 11, e0163460. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Song, L.; Zeng, S.; Zhang, L. MALAT1-miR-124-RBG2 axis is involved in growth and invasion of HR-HPV-positive cervical cancer cells. Tumour Biol. 2016, 37, 633–640. [Google Scholar] [CrossRef] [PubMed]
- Pang, R.T.; Leung, C.O.; Ye, T.M.; Liu, W.; Chiu, P.C.; Lam, K.K.; Lee, K.F.; Yeung, W.S. MicroRNA-34a suppresses invasion through downregulation of Notch1 and Jagged1 in cervical carcinoma and choriocarcinoma cells. Carcinogenesis 2010, 31, 1037–1044. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Au Yeung, C.L.; Tsang, T.Y.; Yau, P.L.; Kwok, T.T. Human papillomavirus type 16 E6 induces cervical cancer cell migration through the p53/microRNA-23b/urokinase-type plasminogen activator pathway. Oncogene 2011, 30, 2401–2410. [Google Scholar] [CrossRef] [Green Version]
- Qiang, R.; Wang, F.; Shi, L.Y.; Liu, M.; Chen, S.; Wan, H.Y.; Li, Y.X.; Li, X.; Gao, S.Y.; Sun, B.C.; et al. Plexin-B1 is a target of miR-214 in cervical cancer and promotes the growth and invasion of HeLa cells. Int. J. Biochem. Cell Biol. 2011, 43, 632–641. [Google Scholar] [CrossRef]
- Peng, R.Q.; Wan, H.Y.; Li, H.F.; Liu, M.; Li, X.; Tang, H. MicroRNA-214 suppresses growth and invasiveness of cervical cancer cells by targeting UDP-N-acetyl-alpha-D-galactosamine:polypeptide N-acetylgalactosaminyltransferase 7. J. Biol. Chem. 2012, 287, 14301–14309. [Google Scholar] [CrossRef]
- Xie, H.; Zhao, Y.; Caramuta, S.; Larsson, C.; Lui, W.O. miR-205 expression promotes cell proliferation and migration of human cervical cancer cells. PLoS ONE 2012, 7, e46990. [Google Scholar] [CrossRef]
- Qin, W.; Dong, P.; Ma, C.; Mitchelson, K.; Deng, T.; Zhang, L.; Sun, Y.; Feng, X.; Ding, Y.; Lu, X.; et al. MicroRNA-133b is a key promoter of cervical carcinoma development through the activation of the ERK and AKT1 pathways. Oncogene 2012, 31, 4067–4075. [Google Scholar] [CrossRef] [PubMed]
- Fan, Z.; Cui, H.; Xu, X.; Lin, Z.; Zhang, X.; Kang, L.; Han, B.; Meng, J.; Yan, Z.; Yan, X.; et al. MiR-125a suppresses tumor growth, invasion and metastasis in cervical cancer by targeting STAT3. Oncotarget 2015, 6, 25266–25280. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cheng, Y.; Geng, L.; Zhao, L.; Zuo, P.; Wang, J. Human papillomavirus E6-regulated microRNA-20b promotes invasion in cervical cancer by targeting tissue inhibitor of metalloproteinase 2. Mol. Med. Rep. 2017, 16, 5464–5470. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Peta, E.; Sinigaglia, A.; Masi, G.; Di Camillo, B.; Grassi, A.; Trevisan, M.; Messa, L.; Loregian, A.; Manfrin, E.; Brunelli, M.; et al. HPV16 E6 and E7 upregulate the histone lysine demethylase KDM2B through the c-MYC/miR-146a-5p axys. Oncogene 2018, 37, 1654–1668. [Google Scholar] [CrossRef] [PubMed]
- Liu, F.; Zhang, S.; Zhao, Z.; Mao, X.; Huang, J.; Wu, Z.; Zheng, L.; Wang, Q. MicroRNA-27b up-regulated by human papillomavirus 16 E7 promotes proliferation and suppresses apoptosis by targeting polo-like kinase2 in cervical cancer. Oncotarget 2016, 7, 19666–19679. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, S.; Liu, F.; Mao, X.; Huang, J.; Yang, J.; Yin, X.; Wu, L.; Zheng, L.; Wang, Q. Elevation of miR-27b by HPV16 E7 inhibits PPARgamma expression and promotes proliferation and invasion in cervical carcinoma cells. Int. J. Oncol. 2015, 47, 1759–1766. [Google Scholar] [CrossRef]
- Yi, Y.; Liu, Y.; Wu, W.; Wu, K.; Zhang, W. The role of miR-106p-5p in cervical cancer: From expression to molecular mechanism. Cell Death Discov. 2018, 5, 36. [Google Scholar] [CrossRef]
- Zur Hausen, H. The role of papillomaviruses in anogenital cancer. Scand. J. Infect. Dis. Suppl. 1990, 69, 107–111. [Google Scholar]
- Pogoda, C.S.; Roden, R.B.; Garcea, R.L. Immunizing against Anogenital Cancer: HPV Vaccines. PLoS Pathog. 2016, 12, e1005587. [Google Scholar] [CrossRef]
- de Sanjose, S.; Bruni, L.; Alemany, L. HPV in genital cancers (at the exception of cervical cancer) and anal cancers. Presse Med. 2014, 43, e423–e428. [Google Scholar] [CrossRef]
- Gao, G.; Smith, D.I. Role of the Common Fragile Sites in Cancers with a Human Papillomavirus Etiology. Cytogenet. Genome. Res. 2016, 150, 217–226. [Google Scholar] [CrossRef] [PubMed]
- Trottier, H.; Franco, E.L. The epidemiology of genital human papillomavirus infection. Vaccine 2006, 24, S1/4–S1/15. [Google Scholar] [CrossRef] [PubMed]
- de Sanjose, S.; Alemany, L.; Ordi, J.; Tous, S.; Alejo, M.; Bigby, S.M.; Joura, E.A.; Maldonado, P.; Laco, J.; Bravo, I.G.; et al. Worldwide human papillomavirus genotype attribution in over 2000 cases of intraepithelial and invasive lesions of the vulva. Eur. J. Cancer 2013, 49, 3450–3461. [Google Scholar] [CrossRef] [PubMed]
- Rakislova, N.; Saco, A.; Sierra, A.; Del Pino, M.; Ordi, J. Role of Human Papillomavirus in Vulvar Cancer. Adv. Anat. Pathol. 2017, 24, 201–214. [Google Scholar] [CrossRef] [PubMed]
- Joura, E.A.; Losch, A.; Haider-Angeler, M.G.; Breitenecker, G.; Leodolter, S. Trends in vulvar neoplasia. Increasing incidence of vulvar intraepithelial neoplasia and squamous cell carcinoma of the vulva in young women. J. Reprod. Med. 2000, 45, 613–615. [Google Scholar] [PubMed]
- Zhang, J.; Zhang, Y.; Zhang, Z. Prevalence of human papillomavirus and its prognostic value in vulvar cancer: A systematic review and meta-analysis. PLoS ONE 2018, 13. [Google Scholar] [CrossRef] [PubMed]
- de Melo Maia, B.; Lavorato-Rocha, A.M.; Rodrigues, L.S.; Coutinho-Camillo, C.M.; Baiocchi, G.; Stiepcich, M.M.; Puga, R.; de, A.L.L.; Soares, F.A.; Rocha, R.M. microRNA portraits in human vulvar carcinoma. Cancer Prev. Res. (Phila.) 2013, 6, 1231–1241. [Google Scholar] [CrossRef] [PubMed]
- de Melo Maia, B.; Ling, H.; Monroig, P.; Ciccone, M.; Soares, F.A.; Calin, G.A.; Rocha, R.M. Design of a miRNA sponge for the miR-17 miRNA family as a therapeutic strategy against vulvar carcinoma. Mol. Cell. Probes 2015, 29, 420–426. [Google Scholar] [CrossRef] [PubMed]
- de Melo Maia, B.; Rodrigues, I.S.; Akagi, E.M.; Soares do Amaral, N.; Ling, H.; Monroig, P.; Soares, F.A.; Calin, G.A.; Rocha, R.M. MiR-223-5p works as an oncomiR in vulvar carcinoma by TP63 suppression. Oncotarget 2016, 7, 49217–49231. [Google Scholar] [CrossRef]
- Yang, X.H.; Guo, F. miR3147 serves as an oncomiR in vulvar squamous cell cancer via Smad4 suppression. Mol. Med. Rep. 2018, 17, 6397–6404. [Google Scholar] [CrossRef]
- Agostini, A.; Brunetti, M.; Davidson, B.; Trope, C.G.; Heim, S.; Panagopoulos, I.; Micci, F. Expressions of miR-30c and let-7a are inversely correlated with HMGA2 expression in squamous cell carcinoma of the vulva. Oncotarget 2016, 7, 85058–85062. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; Wu, X. miRNA expression profile of vulvar squamous cell carcinoma and identification of the oncogenic role of miR-590-5p. Oncol. Rep. 2016, 35, 398–408. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.M.; Wang, X.B.; Chen, M.M.; Liu, T.; Li, Y.X.; Jia, W.H.; Liu, M.; Li, X.; Tang, H. MicroRNA-19a and -19b regulate cervical carcinoma cell proliferation and invasion by targeting CUL5. Cancer Lett. 2012, 322, 148–158. [Google Scholar] [CrossRef] [PubMed]
- Tang, R.; Cui, Z.M.; Lou, Y.H. MicroRNA-16 regulates the proliferation, invasion and apoptosis of ovarian epithelial carcinoma cells in vitro. Zhonghua Fu Chan Ke Za Zhi 2012, 47, 846–850. [Google Scholar] [PubMed]
- Nyitray, A.G.; Iannacone, M.R. The epidemiology of human papillomaviruses. Curr. Probl. Dermatol. 2014, 45, 75–91. [Google Scholar] [CrossRef] [PubMed]
- Daling, J.R.; Madeleine, M.M.; Schwartz, S.M.; Shera, K.A.; Carter, J.J.; McKnight, B.; Porter, P.L.; Galloway, D.A.; McDougall, J.K.; Tamimi, H. A population-based study of squamous cell vaginal cancer: HPV and cofactors. Gynecol. Oncol. 2002, 84, 263–270. [Google Scholar] [CrossRef] [PubMed]
- Ostrow, R.S.; Manias, D.A.; Clark, B.A.; Fukushima, M.; Okagaki, T.; Twiggs, L.B.; Faras, A.J. The analysis of carcinomas of the vagina for human papillomavirus DNA. Int. J. Gynecol. Pathol. 1988, 7, 308–314. [Google Scholar] [CrossRef]
- Brianti, P.; De Flammineis, E.; Mercuri, S.R. Review of HPV-related diseases and cancers. New Microbiol. 2017, 40, 80–85. [Google Scholar]
- Castor, M.; da Silva, H.J.; Gondim Martins, D.B.; de Mello, R.J. HPV and precancerous lesions of anal canal in women: Systematic review. Int. J. Colorectal. Dis. 2012, 27, 271–276. [Google Scholar] [CrossRef]
- Krzowska-Firych, J.; Lucas, G.; Lucas, C.; Lucas, N.; Pietrzyk, L. An overview of Human Papillomavirus (HPV) as an etiological factor of the anal cancer. J. Infect. Public Health 2018. [Google Scholar] [CrossRef]
- Islami, F.; Ferlay, J.; Lortet-Tieulent, J.; Bray, F.; Jemal, A. International trends in anal cancer incidence rates. Int. J. Epidemiol. 2017, 46, 924–938. [Google Scholar] [CrossRef] [PubMed]
- Maniar, K.P.; Nayar, R. HPV-related squamous neoplasia of the lower anogenital tract: An update and review of recent guidelines. Adv. Anat. Pathol. 2014, 21, 341–358. [Google Scholar] [CrossRef] [PubMed]
- Myklebust, M.P.; Bruland, O.; Fluge, O.; Skarstein, A.; Balteskard, L.; Dahl, O. MicroRNA-15b is induced with E2F-controlled genes in HPV-related cancer. Br. J. Cancer 2011, 105, 1719–1725. [Google Scholar] [CrossRef]
- Schlenker, B.; Schneede, P. The Role of Human Papilloma Virus in Penile Cancer Prevention and New Therapeutic Agents. Eur. Urol. Focus 2018. [Google Scholar] [CrossRef] [PubMed]
- Giuliano, A.R.; Anic, G.; Nyitray, A.G. Epidemiology and pathology of HPV disease in males. Gynecol. Oncol. 2010, 117, S15–S19. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bleeker, M.C.; Heideman, D.A.; Snijders, P.J.; Horenblas, S.; Dillner, J.; Meijer, C.J. Penile cancer: Epidemiology, pathogenesis and prevention. World J. Urol. 2009, 27, 141–150. [Google Scholar] [CrossRef] [PubMed]
- Wakeham, K.; Kavanagh, K. The burden of HPV-associated anogenital cancers. Curr. Oncol. Rep. 2014, 16, 402. [Google Scholar] [CrossRef] [Green Version]
- Kutlubay, Z.; Engin, B.; Zara, T.; Tuzun, Y. Anogenital malignancies and premalignancies: Facts and controversies. Clin. Dermatol. 2013, 31, 362–373. [Google Scholar] [CrossRef]
- Backes, D.M.; Kurman, R.J.; Pimenta, J.M.; Smith, J.S. Systematic review of human papillomavirus prevalence in invasive penile cancer. Cancer Causes Control 2009, 20, 449–457. [Google Scholar] [CrossRef]
- Anic, G.M.; Giuliano, A.R. Genital HPV infection and related lesions in men. Prev. Med. 2011, 53, S36–S41. [Google Scholar] [CrossRef] [Green Version]
- Alemany, L.; Cubilla, A.; Halec, G.; Kasamatsu, E.; Quiros, B.; Masferrer, E.; Tous, S.; Lloveras, B.; Hernandez-Suarez, G.; Lonsdale, R.; et al. Role of Human Papillomavirus in Penile Carcinomas Worldwide. Eur. Urol. 2016, 69, 953–961. [Google Scholar] [CrossRef] [PubMed]
- Stankiewicz, E.; Kudahetti, S.C.; Prowse, D.M.; Ktori, E.; Cuzick, J.; Ambroisine, L.; Zhang, X.; Watkin, N.; Corbishley, C.; Berney, D.M. HPV infection and immunochemical detection of cell-cycle markers in verrucous carcinoma of the penis. Mod. Pathol. 2009, 22, 1160–1168. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, L.; Wei, P.; Shen, X.; Zhang, Y.; Xu, B.; Zhou, J.; Fan, S.; Hao, Z.; Shi, H.; Zhang, X.; et al. MicroRNA Expression Profile in Penile Cancer Revealed by Next-Generation Small RNA Sequencing. PLoS ONE 2015, 10. [Google Scholar] [CrossRef] [PubMed]
- Barzon, L.; Cappellesso, R.; Peta, E.; Militello, V.; Sinigaglia, A.; Fassan, M.; Simonato, F.; Guzzardo, V.; Ventura, L.; Blandamura, S.; et al. Profiling of expression of human papillomavirus-related cancer miRNAs in penile squamous cell carcinomas. Am. J. Pathol. 2014, 184, 3376–3383. [Google Scholar] [CrossRef] [PubMed]
- Peta, E.; Cappellesso, R.; Masi, G.; Sinigaglia, A.; Trevisan, M.; Grassi, A.; Di Camillo, B.; Vassarotto, E.; Fassina, A.; Palu, G.; et al. Down-regulation of microRNA-146a is associated with high-risk human papillomavirus infection and epidermal growth factor receptor overexpression in penile squamous cell carcinoma. Hum. Pathol. 2017, 61, 33–40. [Google Scholar] [CrossRef]
- Kuasne, H.; Barros-Filho, M.C.; Busso-Lopes, A.; Marchi, F.A.; Pinheiro, M.; Munoz, J.J.; Scapulatempo-Neto, C.; Faria, E.F.; Guimaraes, G.C.; Lopes, A.; et al. Integrative miRNA and mRNA analysis in penile carcinomas reveals markers and pathways with potential clinical impact. Oncotarget 2017, 8, 15294–15306. [Google Scholar] [CrossRef]
- Tie, J.; Pan, Y.; Zhao, L.; Wu, K.; Liu, J.; Sun, S.; Guo, X.; Wang, B.; Gang, Y.; Zhang, Y.; et al. MiR-218 inhibits invasion and metastasis of gastric cancer by targeting the Robo1 receptor. PLoS Genet. 2010, 6. [Google Scholar] [CrossRef]
- Labbaye, C.; Testa, U. The emerging role of MIR-146A in the control of hematopoiesis, immune function and cancer. J. Hematol. Oncol. 2012, 5, 13. [Google Scholar] [CrossRef] [Green Version]
- Wu, D.W.; Cheng, Y.W.; Wang, J.; Chen, C.Y.; Lee, H. Paxillin predicts survival and relapse in non-small cell lung cancer by microRNA-218 targeting. Cancer Res. 2010, 70, 10392–10401. [Google Scholar] [CrossRef]
- Taniguchi, S.; Furukawa, M.; Kutsuna, H.; Sowa, J.; Ishii, M. Squamous cell carcinoma of the scrotum. Dermatology 1996, 193, 253–254. [Google Scholar] [CrossRef]
- Guimera, N.; Alemany, L.; Halec, G.; Pawlita, M.; Wain, G.V.; Vailen, J.S.S.; Azike, J.E.; Jenkins, D.; de Sanjose, S.; Quint, W.; et al. Human papillomavirus 16 is an aetiological factor of scrotal cancer. Br. J. Cancer 2017, 116, 1218–1222. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hayes, D.N.; Van Waes, C.; Seiwert, T.Y. Genetic Landscape of Human Papillomavirus-Associated Head and Neck Cancer and Comparison to Tobacco-Related Tumors. J Clin Oncol 2015, 33, 3227–3234. [Google Scholar] [CrossRef] [Green Version]
- Spence, T.; Bruce, J.; Yip, K.W.; Liu, F.F. HPV Associated Head and Neck Cancer. Cancers 2016, 8. [Google Scholar] [CrossRef]
- de Martel, C.; Plummer, M.; Vignat, J.; Franceschi, S. Worldwide burden of cancer attributable to HPV by site, country and HPV type. Int. J. Cancer 2017, 141, 664–670. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hui, A.B.; Lin, A.; Xu, W.; Waldron, L.; Perez-Ordonez, B.; Weinreb, I.; Shi, W.; Bruce, J.; Huang, S.H.; O’Sullivan, B.; et al. Potentially prognostic miRNAs in HPV-associated oropharyngeal carcinoma. Clin. Cancer Res. 2013, 19, 2154–2162. [Google Scholar] [CrossRef] [PubMed]
- Chapman, B.V.; Wald, A.I.; Akhtar, P.; Munko, A.C.; Xu, J.; Gibson, S.P.; Grandis, J.R.; Ferris, R.L.; Khan, S.A. MicroRNA-363 targets myosin 1B to reduce cellular migration in head and neck cancer. BMC Cancer 2015, 15, 861. [Google Scholar] [CrossRef]
- Sun, Z.; Hu, W.; Xu, J.; Kaufmann, A.M.; Albers, A.E. MicroRNA-34a regulates epithelial-mesenchymal transition and cancer stem cell phenotype of head and neck squamous cell carcinoma in vitro. Int. J. Oncol. 2015, 47, 1339–1350. [Google Scholar] [CrossRef] [PubMed]
- Kumar, B.; Yadav, A.; Lang, J.; Teknos, T.N.; Kumar, P. Dysregulation of microRNA-34a expression in head and neck squamous cell carcinoma promotes tumor growth and tumor angiogenesis. PLoS ONE 2012, 7, e37601. [Google Scholar] [CrossRef]
- Hu, J.; Ge, W.; Xu, J. HPV 16 E7 inhibits OSCC cell proliferation, invasion, and metastasis by upregulating the expression of miR-20a. Tumour Biol. 2016, 37, 9433–9440. [Google Scholar] [CrossRef]
- Sannigrahi, M.K.; Sharma, R.; Singh, V.; Panda, N.K.; Rattan, V.; Khullar, M. Role of Host miRNA Hsa-miR-139-3p in HPV-16-Induced Carcinomas. Clin. Cancer Res. 2017, 23, 3884–3895. [Google Scholar] [CrossRef]
- Kobayashi, K.; Hisamatsu, K.; Suzui, N.; Hara, A.; Tomita, H.; Miyazaki, T. A Review of HPV-Related Head and Neck Cancer. J. Clin. Med. 2018, 7. [Google Scholar] [CrossRef] [PubMed]
- Costa, N.R.; Gil da Costa, R.M.; Medeiros, R. A viral map of gastrointestinal cancers. Life Sci. 2018, 199. [Google Scholar] [CrossRef] [PubMed]
- Araldi, R.P.; Sant’Ana, T.A.; Modolo, D.G.; de Melo, T.C.; Spadacci-Morena, D.D.; de Cassia Stocco, R.; Cerutti, J.M.; de Souza, E.B. The human papillomavirus (HPV)-related cancer biology: An overview. Biomed. Pharmacother. 2018, 106, 1537–1556. [Google Scholar] [CrossRef] [PubMed]
- de Freitas, A.C.; Gurgel, A.P.; de Lima, E.G.; de Franca Sao Marcos, B.; do Amaral, C.M. Human papillomavirus and lung cancinogenesis: An overview. J. Cancer. Res. Clin. Oncol. 2016, 142, 2415–2427. [Google Scholar] [CrossRef] [PubMed]
- de Oliveira, T.H.A.; do Amaral, C.M.; de Franca Sao Marcos, B.; Nascimento, K.C.G.; de Miranda Rios, A.C.; Quixabeira, D.C.A.; Muniz, M.T.C.; Silva Neto, J.D.C.; de Freitas, A.C. Presence and activity of HPV in primary lung cancer. J. Cancer Res. Clin. Oncol. 2018, 144. [Google Scholar] [CrossRef] [PubMed]
- Zhai, K.; Ding, J.; Shi, H.Z. HPV and lung cancer risk: A meta-analysis. J. Clin. Virol. 2015, 63, 84–90. [Google Scholar] [CrossRef]
- Guo, L.; Liu, S.; Zhang, S.; Chen, Q.; Zhang, M.; Quan, P.; Sun, X.B. Human papillomavirus-related esophageal cancer survival: A systematic review and meta-analysis. Medicine (Baltimore) 2016, 95, e5318. [Google Scholar] [CrossRef]
- Liyanage, S.S.; Segelov, E.; Garland, S.M.; Tabrizi, S.N.; Seale, H.; Crowe, P.J.; Dwyer, D.E.; Barbour, A.; Newall, A.T.; Malik, A.; et al. Role of human papillomaviruses in esophageal squamous cell carcinoma. Asia Pac. J. Clin. Oncol. 2013, 9, 12–28. [Google Scholar] [CrossRef]
- Syrjanen, K.; Pyrhonen, S.; Aukee, S.; Koskela, E. Squamous cell papilloma of the esophagus: A tumour probably caused by human papilloma virus (HPV). Diagn. Histopathol. 1982, 5, 291–296. [Google Scholar]
- Cui, X.; Chen, X.; Wang, W.; Chang, A.; Yang, L.; Liu, C.; Peng, H.; Wei, Y.; Liang, W.; Li, S.; et al. Epigenetic silencing of miR-203 in Kazakh patients with esophageal squamous cell carcinoma by MassARRAY spectrometry. Epigenetics 2017, 12, 698–707. [Google Scholar] [CrossRef]
- Akhtar, N.; Bansal, J.G. Risk factors of Lung Cancer in nonsmoker. Curr. Probl. Cancer 2017, 41, 328–339. [Google Scholar] [CrossRef] [PubMed]
- Li, G.; He, L.; Zhang, E.; Shi, J.; Zhang, Q.; Le, A.D.; Zhou, K.; Tang, X. Overexpression of human papillomavirus (HPV) type 16 oncoproteins promotes angiogenesis via enhancing HIF-1alpha and VEGF expression in non-small cell lung cancer cells. Cancer Lett. 2011, 311, 160–170. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Huang, B.; Xiu, Z.; Zhou, Z.; Liu, J.; Li, X.; Tang, X. PI3K/Akt/HIF-1alpha signaling pathway mediates HPV-16 oncoprotein-induced expression of EMT-related transcription factors in non-small cell lung cancer cells. J. Cancer 2018, 9, 3456–3466. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.L.; Hsu, N.Y.; Cheau-Feng Lin, F.; Lee, H.; Cheng, Y.W. MiR-30c-2* negative regulated MTA-1 expression involved in metastasis and drug resistance of HPV-infected non-small cell lung cancer. Surgery 2016, 160, 1591–1598. [Google Scholar] [CrossRef] [PubMed]
| Cancer | MiRNA | Expression | Type of Samples | Targets | Biological Significance |
|---|---|---|---|---|---|
| CERVICAL CANCER | miR-21 | Upregulated [35,36,37] | Serum [37] Tissue [36] HeLa cell line [35,36,37] SiHa cell line [36] CaSki cell line [36] | RASA1 [37] | Promoted cell migration and invasion [35,37] Associated with lymph node metastases [37] |
| miR-155 | Upregulated [38] | Tissue [38] HeLa cell line [38] | --- | Correlated with lymph nodes metastases and vascular invasion [38] Promoted cell migration and invasion [38] | |
| Upregulated [39] | CaSki cell line [39] | SMAD2 [39] CCND1 [39] | Decreased proliferation [39] Inhibited EGF-induced EMT, migration and invasion [39] Increased chemo-sensitivity [39] | ||
| miR-218 | Downregulated [40,41] | Tissue [40,41] CaSki cell line [40] ME180 cell line [40] SiHa cell line [41] HeLa cell line [40,41] | LAMB3 [40] SFMBT1 [41] DCUN1D1 [41] | Associated with cell migration and invasion [40,41] Induction of EMT and invasion [41] | |
| miR-195 | Downregulated [42] | Tissue [42] HeLa cell line [42] SiHa cell line [42] | DCUN1D1 [42] | Associated with lymph node metastases [42] Mediated cell proliferation, migration and invasion [42] | |
| miR-375 | Downregulated [43,44] | Tissue [43] SiHa cell line [43,44] CaSki cell line [43,44] | SP1 [43] LncRNA MALAT1 [44] | Correlated with pelvic lymph node metastases [43] Promoted cell proliferation, migration and invasion [43] Modulation of EMT [44] | |
| miR-124 | Downregulated [45] | Tissue [45] HeLa cell line [45] CaSki cell line [45] SiHa cell line [45] | GRB2 [45] | Increased cell invasion [45] | |
| miR-34a | Transfection with pre-miR-34a [46] | HeLa cell line [46] | NOTCH1 [46] JAGGED1 [46] | Repression of invasion [46] | |
| miR-23b | Downregulated [47] | SiHa cell line [47] CaSki cell line [47] | uPA [47] | Increased cell migration [47] | |
| miR-214 | Downregulated [48] | Tissue [48] HeLa cell line [48] | PLXNB1 [48] | Promoted cell proliferation, migration and invasion [48] | |
| miR-205 | Upregulated [50] | Tissue [50] HeLa cell line [50] CaSki cell line [50] | CYR61 [50] CTGF [50] | Promoted cell proliferation and migration [50] | |
| miR-133b | Upregulated [51] | Tissue [51] CaSki cell line [51] SiHa cell line [51] Female SCID mice [51] | MST2 [51] CDC42 [51] RHOA [51] | Enhanced cell proliferation and colony formation [51] In mice increased the number of lung metastatic foci [51] | |
| miR-125a | Downregulated [52] | Tissue [52] SiHa cell line [52] HeLa cell line [52] | STAT3 [52] | Correlated with preoperative metastases [52] Modulation of EMT [52] | |
| miR-20b | Upregulated [53] | Tissue [53] HeLa cell line [53] SiHa cell line [53] CaSki cell line [53] | TIMP2 [53] | Induced EMT, migration and invasion [53] | |
| miR-146a-5p | Downregulated [54] | Primary HFKs cell line [54] HeLa cell line [54] SiHa cell line [54] CaSki cell line [54] | KDM2B [54] | Promoted cell proliferation and migration [54] | |
| miR-27b | Upregulated [55,56] | Tissue [55,56] CaSki cell line [55,56] SiHa cell line [55,56] | PPARG [56] PLK2 [55] | Promoted cell proliferation and invasion [55,56] | |
| miR-106b-5p | Upregulated [57] | In silico studies [57] | GSK3B [57] VEGFA [57] PTK2 [57] | Correlated with the number of metastatic lymph nodes [57] |
| Cancer | MiRNA | Expression | Type of Samples | Targets | Biological Significance |
|---|---|---|---|---|---|
| HEAD AND NECK CANCER (HPV-POSITIVE) | miR-151 | Upregulated [105] | Tissue (oropharyngeal cancer biopsies) [105] | - | Associated with distant metastases [105] |
| miR-152 | Downregulated [105] | Tissue (oropharyngeal cancer biopsies) [105] | - | Associated with distant metastases [105] | |
| miR-324-5p | Upregulated [105] | Tissue (oropharyngeal cancer biopsies) [105] | - | Associated with distant metastases [105] | |
| miR-361 | Upregulated [105] | Tissue (oropharyngeal cancer biopsies) [105] | - | Associated with distant metastases [105] | |
| miR-492 | Downregulated [105] | Tissue (oropharyngeal cancer biopsies) [105] | - | Associated with distant metastases [105] | |
| miR-363 | Upregulated [106] | Tissue (head and neck squamous cell carcinoma) [106] | MYO1B [106] | Reduced cell migration [106] | |
| miR-34a | Transfection of mir-34a mimics [107] | Spheroid head and neck cancer cell lines [107] | - | Reduced invasion capacity [107] | |
| Downregulated [108] | Tissues (head and neck squamous cell carcinoma) [108] UM-SCC-74A cell line [108] UM-SCC-74B cell line [108] SCID mice [108] | E2F3 [108] | Promotes cell proliferation, migration and angiogenesis | ||
| miR-20a | Upregulated [109] | Tissue (oral squamous cell carcinoma) [109] Cal27 cell line [109] | - | Inhibited cell proliferation, migration and invasion [109] | |
| miR-139-3p | Downregulated [110] | Head and neck cancer tissues [110] UPCI-SCC-090 cell line [110] | HPV16-E1 [110] | Induced cell proliferation and migration [110] |
| Cancer | MiRNA | Expression | Type of samples | Targets | Biological Significance |
|---|---|---|---|---|---|
| OTHER HPV-RELATEDCANCERS | miR-203 | Downregulated [120] | Tissue (Esophageal squamous cell carcinoma) [120] | - | Promoted lymph node metastases [120] |
| miR-30c-2* | Downregulated [124] | Tissue (NSCLC) and TL1 cell line [124] | MTA-1 [124] | Correlated with tumor stage and lymph node metastases [124] |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Santos, J.M.O.; Peixoto da Silva, S.; Costa, N.R.; Gil da Costa, R.M.; Medeiros, R. The Role of MicroRNAs in the Metastatic Process of High-Risk HPV-Induced Cancers. Cancers 2018, 10, 493. https://doi.org/10.3390/cancers10120493
Santos JMO, Peixoto da Silva S, Costa NR, Gil da Costa RM, Medeiros R. The Role of MicroRNAs in the Metastatic Process of High-Risk HPV-Induced Cancers. Cancers. 2018; 10(12):493. https://doi.org/10.3390/cancers10120493
Chicago/Turabian StyleSantos, Joana M.O., Sara Peixoto da Silva, Natália R. Costa, Rui M. Gil da Costa, and Rui Medeiros. 2018. "The Role of MicroRNAs in the Metastatic Process of High-Risk HPV-Induced Cancers" Cancers 10, no. 12: 493. https://doi.org/10.3390/cancers10120493





