Survival Analysis in Patients with Pancreatic Ductal Adenocarcinoma Undergoing Chemoradiotherapy Followed by Surgery According to the International Consensus on the 2017 Definition of Borderline Resectable Cancer
Abstract
:1. Introduction
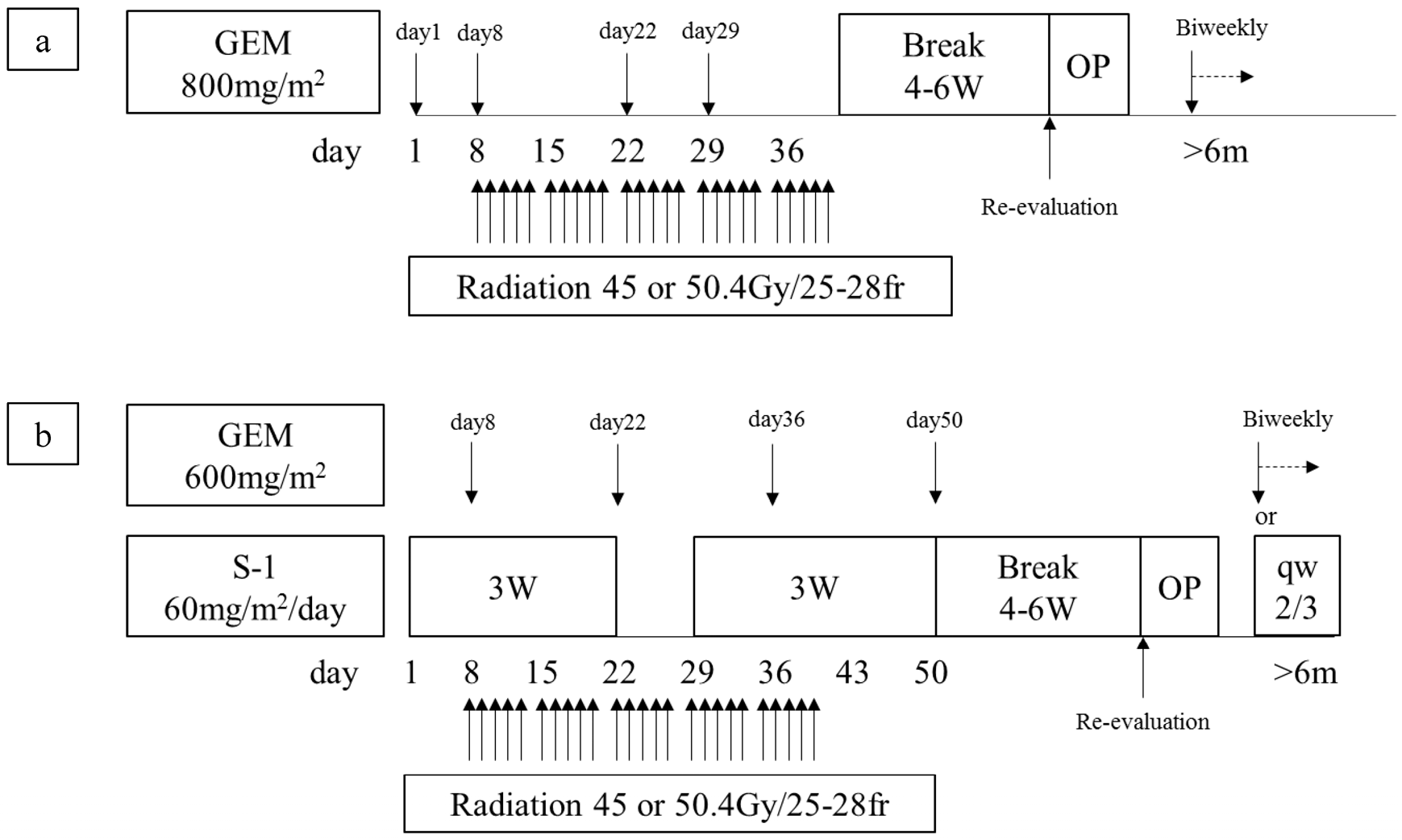
2. Materials and Methods
3. Evaluation of Biological and Conditional Factors
4. Indication of Curative-Intent Resection, Surgical Procedure, and Postoperative Complications
5. Statistical Analyses
6. Results
6.1. Clinical and Surgical Outcomes According to Anatomical Classification of Resectability
6.2. Survival Analysis According to Major Vascular Invasion on CT
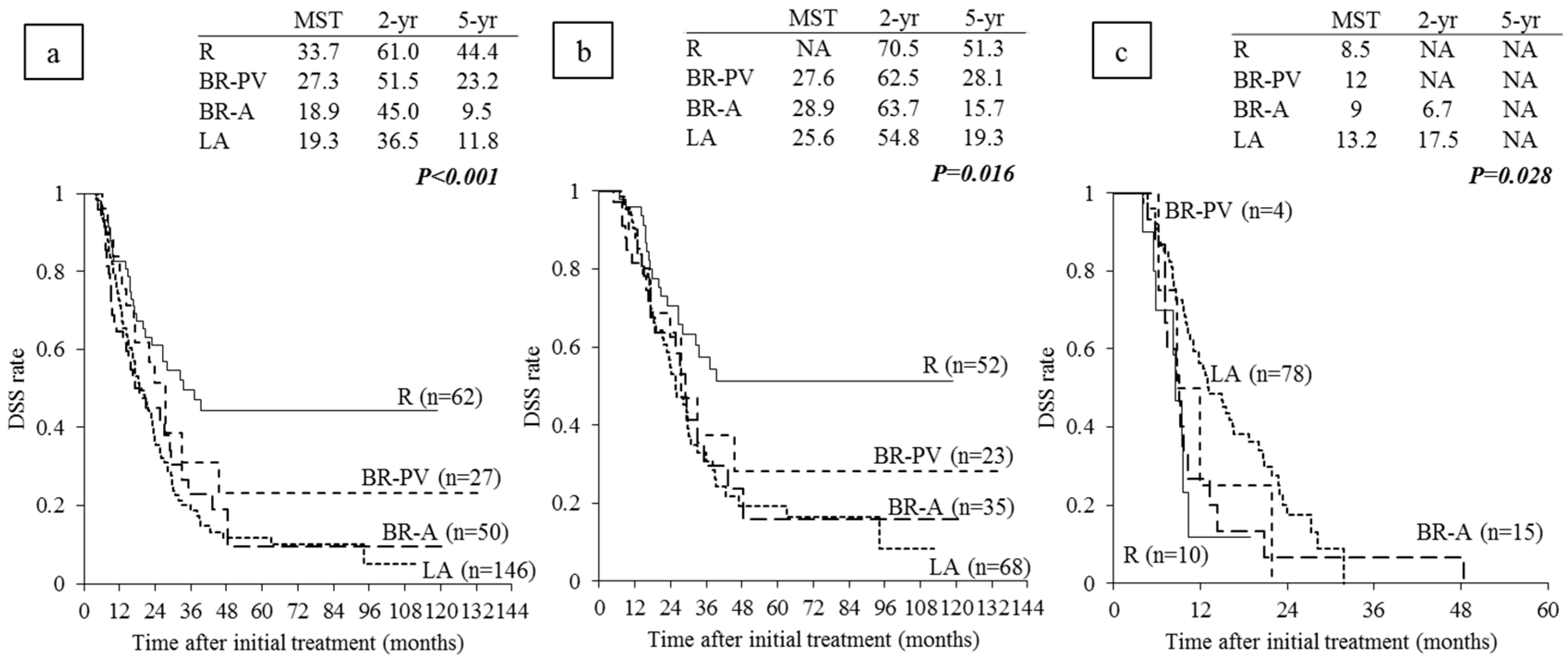
6.3. Survival Analysis According to Anatomical Resectability Classification
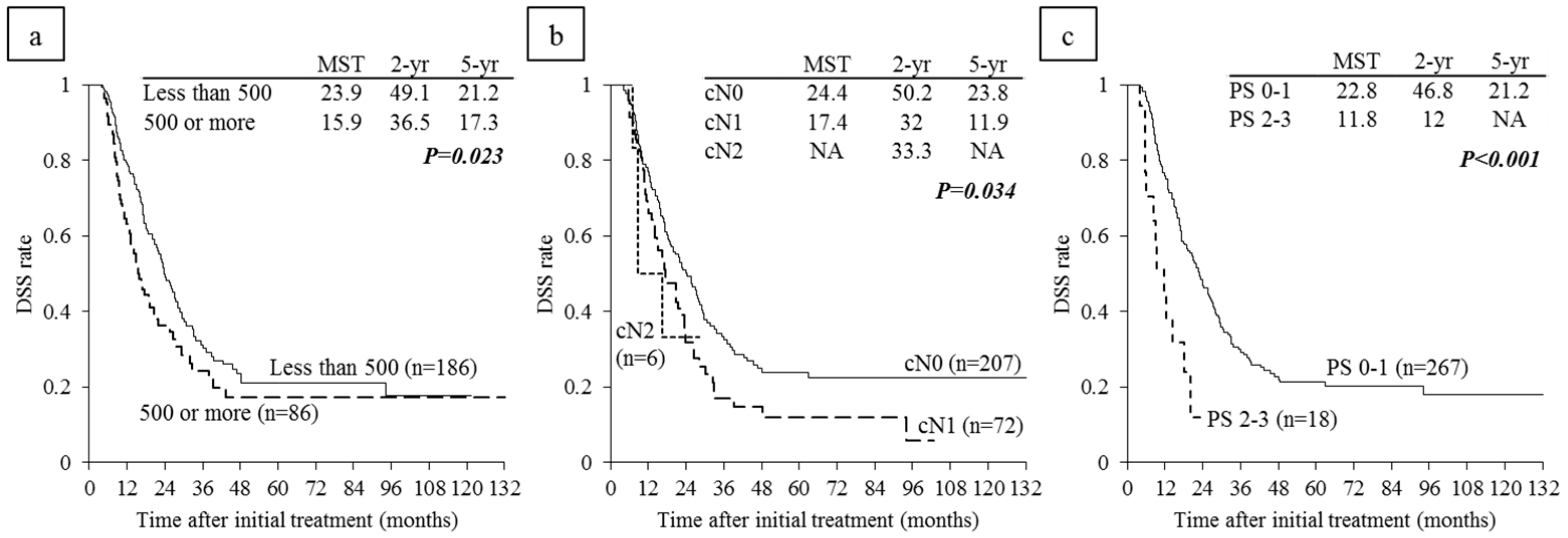
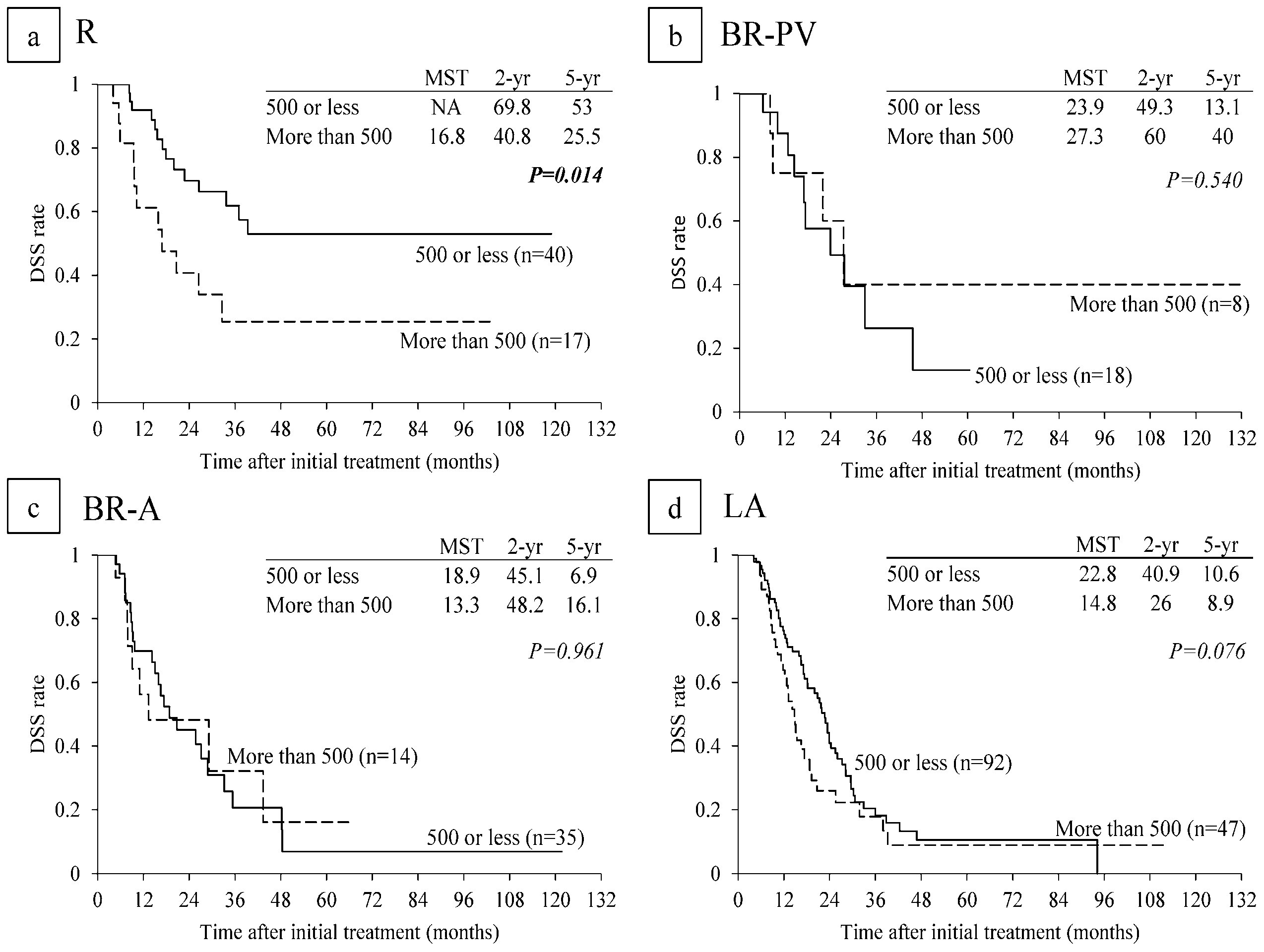
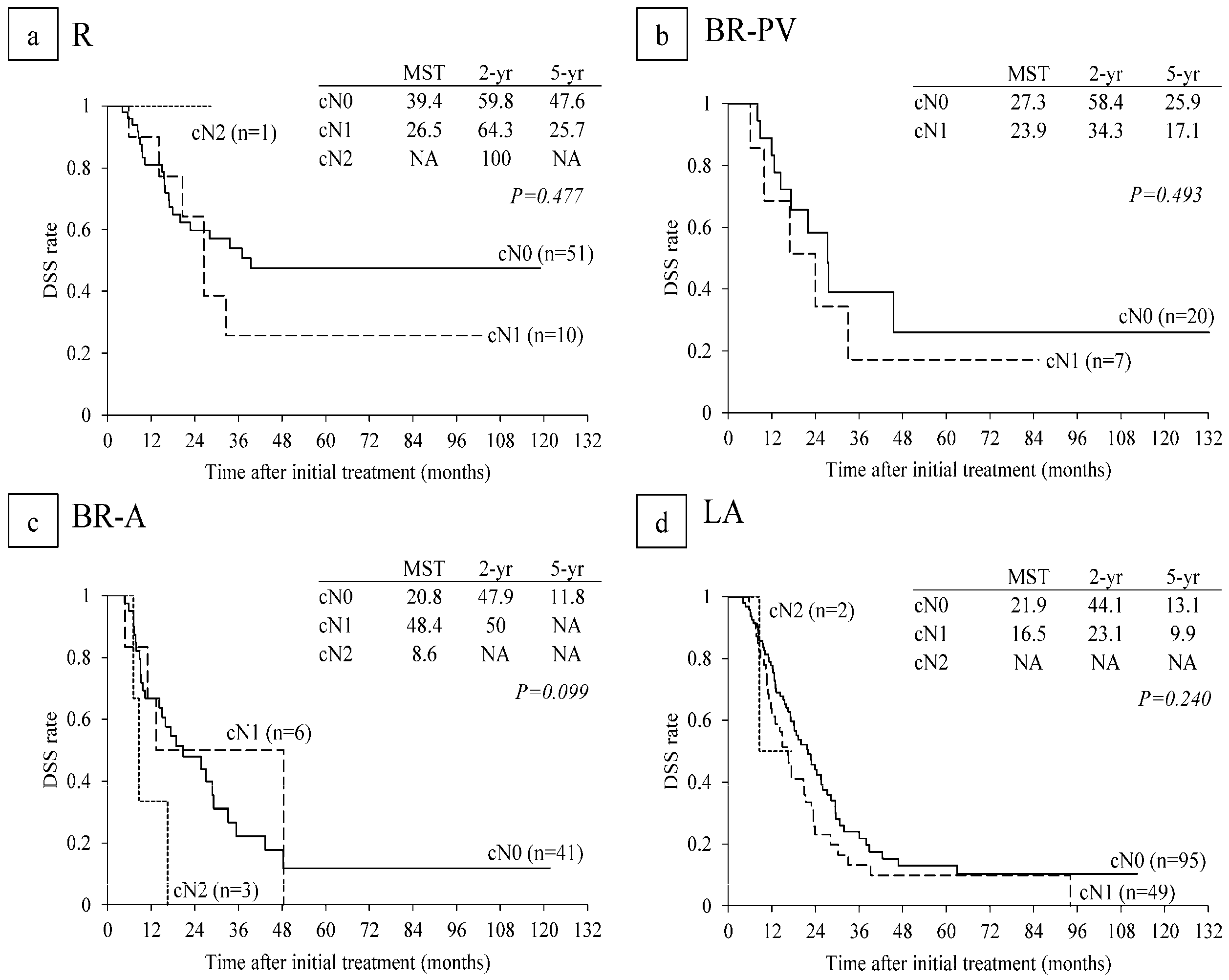
6.4. Survival Analysis According to Serum CA 19-9 Levels and Lymph Node Metastasis on CT before CRT
6.5. Survival Analysis According to Performance Status
7. Discussion
8. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Oettle, H.; Post, S.; Neuhaus, P.; Gellert, K.; Langrehr, J.; Ridwelski, K.; Schramm, H.; Fahlke, J.; Zuelke, C.; Burkart, C.; et al. Adjuvant chemotherapy with gemcitabine vs observation in patients undergoing curative-intent resection of pancreatic cancer: A randomized controlled trial. JAMA 2007, 297, 267–277. [Google Scholar] [CrossRef] [PubMed]
- Barugola, G.; Partelli, S.; Marcucci, S.; Sartori, N.; Capelli, P.; Bassi, C.; Pederzoli, P.; Falconi, M. Resectable pancreatic cancer: Who really benefits from resection? Ann. Surg. Oncol. 2009, 16, 3316–3322. [Google Scholar] [CrossRef] [PubMed]
- Kennedy, E.P.; Yeo, C.J. The case for routine use of adjuvant therapy in pancreatic cancer. J. Surg. Oncol. 2007, 95, 597–603. [Google Scholar] [CrossRef] [PubMed]
- Burris, H.A. Recent updates on the role of chemotherapy in pancreatic cancer. Semin. Oncol. 2005, 32, S1–S3. [Google Scholar] [CrossRef] [PubMed]
- Conroy, T.; Desseigne, F.; Ychou, M.; Bouché, O.; Guimbaud, R.; Bécouarn, Y.; Adenis, A.; Raoul, J.L.; Gourgou-Bourgade, S.; de la Fouchardière, C.; et al. FOLFIRINOX versus gemcitabine for metastatic pancreatic cancer. N. Engl. J. Med. 2011, 364, 1817–1825. [Google Scholar] [CrossRef] [PubMed]
- Von Hoff, D.D.; Ervin, T.; Arena, F.P.; Chiorean, E.G.; Infante, J.; Moore, M.; Seay, T.; Tjulandin, S.A.; Ma, W.W.; Saleh, M.N.; et al. Increased survival in pan- creatic cancer with nab-paclitaxel plus gemcitabine. N. Engl. J. Med. 2013, 369, 1691–1703. [Google Scholar] [CrossRef] [PubMed]
- Tamburrino, D.; Partelli, S.; Crippa, S.; Manzoni, A.; Maurizi, A.; Falconi, M. Selectioncriteria in resectable pancreatic cancer: A biological and morphologicalapproach. World J. Gastroenterol. 2014, 20, 11210–11215. [Google Scholar] [CrossRef] [PubMed]
- Tempero, M.A.; Arnoletti, J.P.; Behrman, S.W.; Ben-Josef, E.; Benson, A.B.; Casper, E.S.; Cohen, S.J.; Czito, B.; Ellenhorn, J.D.; Hawkins, W.G.; et al. Pancreatic adenocarcinoma, version 2.2012: Featured updates to the NCCN Guidelines. J. Natl. Compr. Cancer Netw. 2012, 10, 703–713. [Google Scholar] [CrossRef]
- Katz, M.H.; Marsh, R.; Herman, J.M.; Shi, Q.; Collison, E.; Venook, A.P.; Kindler, H.L.; Alberts, S.R.; Philip, P.; Lowy, A.M.; et al. Borderline resectable pancreatic cancer: Need for standardization and methods for optimal clinical trial design. Ann. Surg. Oncol. 2013, 20, 2787–2795. [Google Scholar] [CrossRef] [PubMed]
- Japan Pancreas Society. Classification of Pancreatic Cancer, 4th ed.; Kanehara & Co., Ltd.: Tokyo, Japan, 2016. [Google Scholar]
- Katz, M.H.; Pisters, P.W.; Evans, D.B.; Sun, C.C.; Lee, J.E.; Fleming, J.B.; Vauthey, J.N.; Abdalla, E.K.; Crane, C.H.; Wolff, R.A.; et al. Borderline resectable pancreatic cancer: The importance of this emerging stage of disease. J. Am. Coll. Surg. 2008, 206, 833–846. [Google Scholar] [CrossRef] [PubMed]
- Isaji, S.; Mizuno, S.; Windsor, J.A.; Bassi, C.; Fernández-Del Castillo, C.; Hackert, T.; Hayasaki, A.; Katz, M.H.G.; Kim, S.W.; Kishiwada, M.; et al. International consensus on definition and criteria of borderline resectable pancreatic ductal adenocarcinoma 2017. Pancreatology 2018, 18, 2–11. [Google Scholar] [CrossRef] [PubMed]
- Murata, Y.; Mizuno, S.; Kishiwada, M.; Hamada, T.; Usui, M.; Sakurai, H.; Tabata, M.; Inoue, H.; Shiraishi, T.; Isaji, S. Impact of histological response after neoadjuvant chemoradiotherapy on recurrence-free survival in UICC-T3 pancreatic adenocarcinoma but not in UICC-T4. Pancreas 2012, 41, 130–136. [Google Scholar] [CrossRef] [PubMed]
- Murata, Y.; Hamada, T.; Kishiwada, M.; Ohsawa, I.; Mizuno, S.; Usui, M.; Sakurai, H.; Tabata, M.; Ii, N.; Inoue, H.; et al. Human equilibrative nucleoside transporter 1 expression is a strong independent prognostic factor in UICC T3-T4 pancreatic cancer patients treated with preoperative gemcitabine-based chemoradiotherapy. J. Hepatobiliary Pancreat. Sci. 2012, 19, 413–425. [Google Scholar] [CrossRef] [PubMed]
- Kobayashi, M.; Mizuno, S.; Murata, Y.; Kishiwada, M.; Usui, M.; Sakurai, H.; Tabata, M.; Ii, N.; Yamakado, K.; Inoue, H.; et al. Gemcitabine-based chemoradiotherapy followed by surgery for borderline resectable and locally unresectable pancreatic ductal adenocarcinoma: Significance of the CA19-9 reduction rate and intratumoral human equilibrative nucleoside transporter 1 expression. Pancreas 2014, 43, 350–360. [Google Scholar] [CrossRef] [PubMed]
- Yamada, R.; Mizuno, S.; Uchida, K.; Yoneda, M.; Kanayama, K.; Inoue, H.; Murata, Y.; Kuriyama, N.; Kishiwada, M.; Usui, M.; et al. Human equilibrative nucleoside transporter 1 expression in endoscopic ultrasonography-guided fine-needle aspiration biopsy samples is a strong predictor of clinical response and survival in the patients with pancreatic ductal adenocarcinoma undergoing gemcitabine-based chemoradiotherapy. Pancreas 2016, 45, 761–771. [Google Scholar] [PubMed]
- Isaji, S.; Kishiwada, M.; Kato, H. Surgery for borderline pancreatic cancer: The Japanese experience. In Multimodal Management of Borderline Resectable Pancreatic Cancer; Katz, M.H.G., Ahmad, S.A., Eds.; Springer International Publishing: Basel, Switzerland, 2016; pp. 265–287. [Google Scholar]
- Scarà, S.; Botton, P.; Scatena, R. CA 19-9: Biochemical and clinical aspects. Adv. Exp. Med. Biol. 2015, 867, 247–260. [Google Scholar] [PubMed]
- UICC. TNM Classification of Malignant Tumours, Eighth ed.; John Wiley & Sons, Ltd.: West Sussex, UK, 2017. [Google Scholar]
- Oken, M.M.; Creech, R.H.; Tormey, D.C.; Horton, J.; Davis, T.E.; McFadden, E.T.; Carbone, P.P. Toxicity and response criteria of the Eastern Cooperative Oncology Group. Am. J. Clin. Oncol. 1982, 5, 649–655. [Google Scholar] [CrossRef] [PubMed]
- Mizuno, S.; Isaji, S.; Tanemura, A.; Kishiwada, M.; Murata, Y.; Azumi, Y.; Kuriyama, N.; Usui, M.; Sakurai, H.; Tabata, M. Anterior approach to the superior mesenteric artery by using nerve plexus hanging maneuver for borderline resectable pancreatic head carcinoma. J. Gastrointest. Surg. 2014, 18, 1209–1215. [Google Scholar] [CrossRef] [PubMed]
- Strasberg, S.M.; Drebin, J.A.; Linehan, D. Radical antegrade modular pancreato-splenectomy. Surgery 2003, 133, 521–527. [Google Scholar] [CrossRef] [PubMed]
- Hirano, S.; Kondo, S.; Hara, T.; Ambo, Y.; Tanaka, E.; Shichinohe, T.; Suzuki, O.; Hazama, K. Distal pancreatectomy with en bloc celiac axis resection for locally advanced pancreatic body cancer: Long-term results. Ann. Surg. 2007, 246, 46–51. [Google Scholar] [CrossRef] [PubMed]
- Clavien, P.A.; Barkun, J.; de Oliveira, M.L.; Vauthey, J.N.; Dindo, D.; Schulick, R.D.; de Santibañes, E.; Pekolj, J.; Slankamenac, K.; Bassi, C.; et al. The Clavien-Dindo classification of surgical complications: Five-year experience. Ann. Surg. 2009, 250, 187–196. [Google Scholar] [CrossRef] [PubMed]
- Bassi, C.; Dervenis, C.; Butturini, G.; Fingerhut, A.; Yeo, C.; Izbicki, J.; Neoptolemos, J.; Sarr, M.; Traverso, W.; Buchler, M.; et al. Postoperative pancreatic fistula: An international study group (ISGPF) definition. Surgery 2005, 138, 8–13. [Google Scholar] [CrossRef] [PubMed]
- Ishikawa, O.; Ohigashi, H.; Imaoka, S.; Teshima, T.; Inoue, T.; Sasaki, Y.; Iwanaga, T.; Nakaizumi, A. Concomitant benefit of preoperative irradiation in preventing pancreas fistula formation after pancreatoduodenectomy. Arch. Surg. 1991, 126, 885–889. [Google Scholar] [CrossRef] [PubMed]
- Cheng, T.Y.; Sheth, K.; White, R.R.; Ueno, T.; Hung, C.-F.; Clary, B.M.; Pappas, T.N.; Tyler, D.S. Effect of neoadjuvant chemoradiation on operative mortality and morbidity for pancreaticoduodenectomy. Ann. Surg. Oncol. 2006, 13, 66–74. [Google Scholar] [CrossRef] [PubMed]
- Tran Cao, H.S.; Balachandran, A.; Wang, H.; Nogueras-González, G.M.; Bailey, C.E.; Lee, J.E.; Pisters, P.W.; Evans, D.B.; Varadhachary, G.; Crane, C.H.; et al. Radiographic tumor-vein interface as a predictor of intraoperative, pathologic, and oncologic outcomes in resectable and borderline resectable pancreatic cancer. J. Gastrointest. Surg. 2014, 18, 269–278. [Google Scholar] [CrossRef] [PubMed]
- Yamada, S.; Fujii, T.; Takami, H.; Hayashi, M.; Iwata, N.; Kanda, M.; Tanaka, C.; Sugimoto, H.; Nakayama, G.; Koike, M.; et al. Evaluation and proposal of novel resectability criteria for pancreatic cancer established by the Japan Pancreas Society. Surgery 2017, 162, 784–791. [Google Scholar] [CrossRef] [PubMed]
- Kato, H.; Usui, M.; Isaji, S.; Nagakawa, T.; Wada, K.; Unno, M.; Nakao, A.; Miyakawa, S.; Ohta, T. Clinical features and treatment outcome of borderline resectable pancreatic head/body cancer: A multi-institutional survey by the Japanese Society of Pancreatic Surgery. J. Hepatobiliary Pancreat. Sci. 2013, 20, 601–610. [Google Scholar] [CrossRef] [PubMed]
- Hartwig, W.; Strobel, O.; Hinz, U.; Fritz, S.; Hackert, T.; Roth, C.; Büchler, M.W.; Werner, J. CA19-9 in potentially resectable pancreatic cancer: Perspective to adjust surgical and perioperative therapy. Ann. Surg. Oncol. 2013, 20, 2188–2196. [Google Scholar] [CrossRef] [PubMed]
- Yamada, S.; Fujii, T.; Shimoyama, Y.; Kanda, M.; Nakayama, G.; Sugimoto, H.; Koike, M.; Nomoto, S.; Fujiwara, M.; Nakao, A.; et al. SMAD4 expression predicts local spread and treatment failure in resected pancreatic cancer. Pancreas 2015, 44, 660–664. [Google Scholar] [CrossRef] [PubMed]
- Hayasaki, A.; Murata, Y.; Usui, M.; Hibi, T.; Ito, T.; Iizawa, Y.; Kato, H.; Tanemura, A.; Azumi, Y.; Kuriyama, N.; et al. Clinical significance of histological effect and intratumor stromal expression of Tenascin-C in the resected specimens after chemoradiotherapy for initially locally advanced unresectable pancreatic ductal adenocarcinoma. Pancreas. in press.
- Tas, F.; Sen, F.; Odabas, H.; Kılıc, L.; Keskın, S.; Yıldız, I. Performance status of patients is the major prognostic factor at all stages of pancreatic cancer. Int. J. Clin. Oncol. 2013, 18, 839–846. [Google Scholar] [CrossRef] [PubMed]
- Tzeng, C.W.; Katz, M.H.; Fleming, J.B.; Lee, J.E.; Pisters, P.W.; Holmes, H.M.; Varadhachary, G.R.; Wolff, R.A.; Abbruzzese, J.L.; Vauthey, J.N.; et al. Morbidity and mortality afterpancreaticoduodenectomy in patients with borderline resectable type C clinical classification. J. Gastrointest. Surg. 2014, 18, 146–155. [Google Scholar] [CrossRef] [PubMed]
- Imrie, C.W. Host systemic inflammatory response influences outcome in pancreatic cancer. Pancreatology 2015, 15, 327–330. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.H.; Chung, M.J.; Kim, B.; Lee, H.S.; Lee, H.J.; Heo, J.Y.; Kim, Y.J.; Park, J.Y.; Bang, S.; Park, S.W.; et al. The significance of the prognostic nutritional index for all stages of pancreatic cancer. Nutr. Cancer 2017, 69, 512–519. [Google Scholar] [CrossRef] [PubMed]
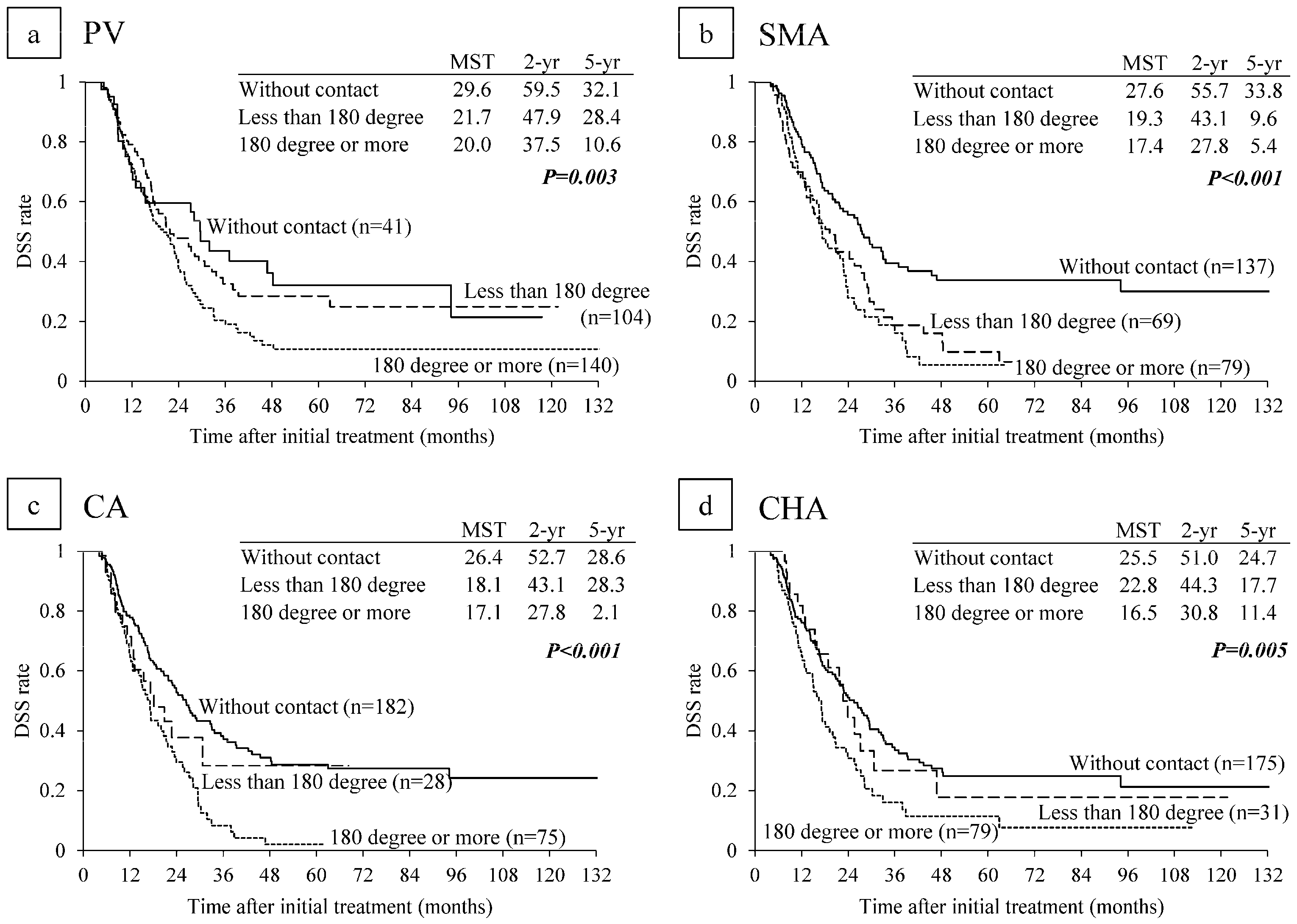

| Characteristic | R n = 69 | BR-PV n = 31 | BR-A n = 52 | LA n = 155 | All n = 307 |
|---|---|---|---|---|---|
| Age (year old) | 68 (47–85) | 68 (41–84) | 65 (49–86) | 68 (42–84) | 68 (41–86) |
| Gender (male/female) | 42/27 | 21/10 | 31/21 | 96/59 | 190/117 |
| PS (0/1/2/3) | 30/31/7/1 | 15/14/1/1 | 36/14/1/1 | 94/52/5/4 | 175/111/14/7 |
| CA 19-9 before CRT (U/mL) | 167 (1–10,093) | 286 (1–4687) | 139 (1–7054) | 187 (0–17,269) | 177 (0–17,269) |
| Tumor size (mm) | 27 (12–60) | 32 (16–52) | 30 (7–76) | 37 (13–91) | 33 (7–91) |
| Tumor location (Ph/Pb/Pt) | 50/4/15 | 25/6/0 | 41/5/6 | 90/43/22 | 206/58/43 |
| Chemotherapy (GEM/S-1+GEM) | 35/34 | 14/17 | 17/35 | 62/93 | 128/179 |
| R n = 62 | BR-PV n = 27 | BR-A n = 50 | LA n = 146 | All n = 285 | |
|---|---|---|---|---|---|
| Metastasis within 3 months from initial treatment (among 285 re-evaluated patients) | 9.7 (6/62) | 11.1 (3/27) | 10.0 (5/50) | 11.0 (16/146) | 10.5% (30/285) |
| Non-resection at re-evaluation (among 285 re-evaluated patients) | 11.3 (7/62) | 14.8 (4/27) | 22.0 (11/50) | 44.5 (65/146) | 30.5% (87/285) |
| Non-resection at laparotomy (among 194 operable patients at re-evaluation) | 5.7 (3/53) | 0 (0/23) | 10.8 (4/37) | 16.0 (13/81) | 10.3% (20/194) |
| Resection (among 285 re-evaluated patients) | 83.9 (52 */62) | 85.2 (23 */27) | 70.0 (35 */50) | 46.6 (68 */146) | 62.5% (178 */285) |
| R0 resection (among resected patients) | 98.0 (48 +/49) | 95.5 (21/22) | 84.8 (28/33) | 62.5 (40 +/64) | 81.5% (137 +/168) |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hayasaki, A.; Isaji, S.; Kishiwada, M.; Fujii, T.; Iizawa, Y.; Kato, H.; Tanemura, A.; Murata, Y.; Azumi, Y.; Kuriyama, N.; et al. Survival Analysis in Patients with Pancreatic Ductal Adenocarcinoma Undergoing Chemoradiotherapy Followed by Surgery According to the International Consensus on the 2017 Definition of Borderline Resectable Cancer. Cancers 2018, 10, 65. https://doi.org/10.3390/cancers10030065
Hayasaki A, Isaji S, Kishiwada M, Fujii T, Iizawa Y, Kato H, Tanemura A, Murata Y, Azumi Y, Kuriyama N, et al. Survival Analysis in Patients with Pancreatic Ductal Adenocarcinoma Undergoing Chemoradiotherapy Followed by Surgery According to the International Consensus on the 2017 Definition of Borderline Resectable Cancer. Cancers. 2018; 10(3):65. https://doi.org/10.3390/cancers10030065
Chicago/Turabian StyleHayasaki, Aoi, Shuji Isaji, Masashi Kishiwada, Takehiro Fujii, Yusuke Iizawa, Hiroyuki Kato, Akihiro Tanemura, Yasuhiro Murata, Yoshinori Azumi, Naohisa Kuriyama, and et al. 2018. "Survival Analysis in Patients with Pancreatic Ductal Adenocarcinoma Undergoing Chemoradiotherapy Followed by Surgery According to the International Consensus on the 2017 Definition of Borderline Resectable Cancer" Cancers 10, no. 3: 65. https://doi.org/10.3390/cancers10030065





